With the advent of biologic drugs in the management of moderate to severe psoriasis, there may have been a shift in therapeutic approach from rotational strategies to a unidirectional progression from topical treatments to the highest rung of the therapeutic ladder. We studied the frequency of switching from classic to biologic therapy and vice versa in a cohort of patients with psoriasis over a period of up to 5 years.
MethodsPatients are included in the BIOBADADERM prospective registry when they are first prescribed any specific conventional or biologic systemic treatment. The data for each patient refer to the follow-up period from the time they entered the cohort until October 2013. To describe the pattern of switches from classic to biologic therapy and vice versa, we used the data in the registry on the first day of every 365-day period following the date each patient was included in the cohort.
ResultsIn total, 47.3% of the patients (926/1956) were prescribed a classic systemic drug and 52.7% (1030/1956) a biologic agent on entry into the study. Of the 741 patients who accumulated 5 years of follow-up, 21.9% (155) were receiving nonbiologic drugs and 78.1% (553) were on biologic therapy on the first day of their 5th year of follow-up.
ConclusionsThe proportion of patients receiving biologic therapy increased with longer follow-up.
Con el advenimiento de fármacos biológicos en el manejo de la psoriasis moderada a grave, es probable que haya habido un cambio en la actitud terapéutica desde estrategias de rotación a una progresión unidireccional desde tratamientos tópicos al escalón más alta de la escalera terapéutica. Evaluamos la frecuencia del cambio desde el tratamiento clásico al biológico y vice-versa en una cohorte de pacientes con psoriasis durante un periodo de hasta 5 años.
MétodosLos pacientes fueron incluidos en el registro prospectivo de Biobadaderm cuando se les fueron prescritos por primera vez cualquier tratamiento convencional o biológico sistémico. Los datos para cada paciente se refieren al período de seguimiento desde la hora de su inclusión en la cohorte hasta octubre de 2013. Para describir el patrón de cambio desde el tratamiento clásico al biológico y vice-versa, utilizamos los datos en el registro en el primer día de cada periodo de 365 días después de la fecha de inclusión de cada paciente en la cohorte.
ResultadosEn total, 47,3% de los pacientes (926/1956) fueren prescritos un medicamento sistémico clásico y 52,7% (1030/1956) un biológico al entrar en el estudio. De los 741 pacientes que acabaron 5 años de seguimiento, 21,9% (155) recibieron medicamentos no biológicos y 78,1% (553) recibieron tratamiento biológico en el primera día del quinto año de seguimiento.
ConclusionesLa proporción de pacientes recibiendo tratamiento biológico aumento con el seguimiento más prolongado.
The introduction of biologic therapy has changed the management of moderate to severe psoriasis, with these agents now representing the highest rung of the therapeutic ladder that starts with topical treatments and phototherapy.1 However, we do not know whether, in clinical practice, this therapeutic progression is always unidirectional and progressive or whether biologic therapy will eventually become just another option in the rotational strategies used, with patients switching back and forth between biologic therapy and classic treatments. The answer to this question is important because of its relevance to safety (in terms of cumulative exposure), efficacy (which may be affected by immunogenicity induced by intermittent use), and cost of treatment.2,3
We report on the switch from classic to biologic drugs and vice versa during the specified follow-up period in a cohort of patients with psoriasis.
Materials and methodsBIOBADADERM is a prospective cohort of patients with moderate to severe psoriasis receiving systemic therapy. Patients from 12 Spanish hospitals have been enrolled in the cohort since 2008. The present study is based on data from patients enrolled before the end of October 2013. A more detailed description of 632 patients from this cohort has been published elsewhere.4 Patients are included when they are first prescribed any specific conventional or biologic systemic treatment, and they are followed up continuously thereafter. Participating centers undertake to include all patients starting biologic treatment for the first time who meet the inclusion criteria. A control receiving nonbiologic systemic therapy is enrolled for each patient added to the biologic group. These criteria should produce an initial population in which 50% of patients are starting biologic therapy and 50% classic systemic therapy. The present study describes the evolution of treatment in this population, focusing on switches between classic and biologic therapy. The data for each patient refer to the follow-up period from the time of entry into the cohort until October 2013. The data were processed as follows: year 1 refers to the data from the first year of follow-up for all the patients in the cohort, year 2 refers to the second year of follow-up, and so on. To describe the changes, we have used the data in the registry on the first day of each 365-day period following each patient's inclusion in the cohort.
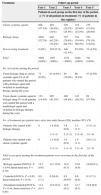
ResultsThe proportion of patients receiving biologic and nonbiologic treatment varies over time. In total, 47.3% of the patients (926/1956) were prescribed a classic systemic treatment and 52.7% (1030/1956) a biologic agent on entry into the study. Of the 741 patients who accumulated 5 years of follow-up, 21.9% (155) were receiving nonbiologic drugs and 78.1% (553) were on biologic therapy on the first day of their 5th year of follow-up (Table 1).
The proportion of patients receiving biologic and nonbiologic treatment over time.
| Treatment | Follow-up period | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Patients in each group on the first day of the period, n (% of all patients in treatment) (% of patients in the registry) | |||||
| Classic systemic agents | 926 (47.3%) | 494 (36.8%) (31%) | 329 (29.2%) (25.9%) | 241 (25.6%) (23.5%) | 155 (21.9%) (20.9%) |
| Biologic drugs | 1030 (52.7%) | 848 (63.2%) (53.4%) | 797 (70.8%) (62.7%) | 701 (74.4%) (68.5%) | 553 (78.1%) (74.6%) |
| Not receiving treatment | 0 (0%) | 245(15.%) | 146 (11.4%) | 82 (8%) | 33 (4.5%) |
| Total | 1956 (100%) | 1587 (100%) | 1272 (100%) | 1024 (100%) | 741 (100%) |
| No. of switches during the period | |||||
| From biologic drug to classic systemic agent (% of all patients who started the period with a biologic agent and switched to nonbiologic therapy during the year) | 71 (6.9%) | 83 (9.8%) | 80 (10.0%) | 66 (9.4%) | 47 (8.5%) |
| From classic systemic agent to biologic drug (% of all patients who started the period with a nonbiologic agent and switched to biologic therapy during the year) | 133 (14.4%) | 205 (41.5%) | 180 (54.8%) | 143 (59.3%) | 89 (57.4%) |
| No. of treatments per patient since entry into study,amean (SD), median (95% CI) | |||||
| Patients who started with biologic therapy | 0 | 1.4 (0.6) | 1.9 (0.9) | 2.1 (1.1) | 2.3 (1.2) |
| 1 (1–3) | 2 (1–3) | 2 (1–4) | 2 (1–4) | ||
| Patients who started with classic systemic agents | 0 | 1.3 (0.6) | 1.6 (0.9) | 1.9 (1.1) | 2.0 (1.2) |
| 1 (1–3) | 1 (1–3) | 2 (1–4) | 2 (1–5) | ||
| PASI score upon starting the treatment patients were receiving on the first day of the period | |||||
| Biologic agents(ANOVA, P=0.44, linear trend test, P=0.19) | 15.7 (9.6) | 14.7 (9.8) | 14.0 (9.7) | 14.0 (9.9) | 14.0(10.1) |
| Controls(ANOVA, P=0.63, linear trend test, P=0.19) | 9.9 (7.1) | 9.2(6.6) | 8.9 (6.6%) | 8.6 (6.8) | 8.3 (7.1) |
| All patients treated(ANOVA, P=0.44, linear trend test, P=0.94) | 13.4 (9.1) | 12.8 (9.2) | 12.6 (9.2) | 12.7 (9.5) | 12.9 (9.9) |
As the length of follow-up increased, so did the likelihood that a patient's therapy would be modified (from classic to biologic therapy or vice versa). However, more patients switched from a classic systemic agent to a biologic drug than the reverse. Per year of follow-up, the percentage of patients who switched from classic to biologic therapy ranged from 14.4% to 59.3%, while that of patients switching from a biologic agent to a classic systemic treatment ranged from 6.9% to 10% (Table 1).
The number of different treatments per patient was similar in both groups (classic and biologic therapy) and increased progressively with the length of follow-up (with a mean of 2.0 for classic and 2.3 for biologic therapy).
For the first year of accumulated follow-up, the mean Psoriasis Area and Severity Index (PASI) score at start of treatment was higher in the patients on biologics (mean 15.7 vs. 9.9), and no significant change was observed in this tendency during follow-up.
The most common cause for switching cited in the database was inefficacy or loss of response, followed by adverse events and clinical remission. However, the percentage of patients switching from conventional to biologic therapy due to inefficacy or loss of efficacy was higher than the number switching from a biologic therapy to a classic systemic drug. By contrast, clinical remission was more frequently cited as a reason for switching in patients who were in the biologic cohort at recruitment. The percentage of patients who switched treatments because of adverse events was similar in both groups (Table 2).
Reasons for switching therapy in both cohorts.a
| Reason for switching | Biologic to conventional | Conventional to biologic | Total |
|---|---|---|---|
| Inefficacy or loss of efficacy | 68 (35.4%) | 169 (56%) | 237 (48%) |
| Adverse event | 33 (17.2%) | 63 (21%) | 96 (19.4%) |
| Pregnancy or intention to become pregnant | 3 (1.6%) | 4 (1.3%) | 7 (1.42%) |
| Loss of patient | 3 (1.6%) | 1 (0.3%) | 4 (0.8%) |
| Remission | 37 (19.2%) | 17 (5.63%) | 54 (11%) |
| Others | 48 (25%) | 48 (15.9%) | 96 (19.4%) |
| Total | 192 (100%) | 302 (100%) | 494 (100%) |
In a subset of the initial cohort of patients, all systemic treatment (nonbiologic and biologic) was discontinued. The percentage of patients who discontinued systemic therapy decreased over time, from 15.4% among patients in their second year of follow-up to 4.5% between the fourth and fifth years of treatment.
DiscussionIn the BIOBADADERM cohort, patients with moderate to severe psoriasis on systemic treatment with classic or biologic drugs were frequently switched from one treatment to another. We observed that the proportion of patients receiving biologic therapy increased with longer follow-up, whereas the percentage of patients on classic treatment decreased over time. As the severity of psoriasis can vary over time in any given patient, this finding may indicate a tendency among dermatologists to prescribe biologic drugs to patients who have been receiving treatment for some time with a conventional therapy and present an exacerbation. After switching, the patient is more likely to remain in the biologic group. The reasons for this finding were not investigated in this study, but possible causes include the organ-specific toxicity associated with prolonged use of certain conventional systemic drugs, relapses, and the prospects of a better outcome with biologic agents in patients whose response to conventional treatment is inadequate.5
The number of different treatments prescribed to each patient during follow-up increased over time similarly in both groups – conventional and biologic therapy – with each group having a median of two changes by the beginning of the 4th year of follow-up. This finding may indicate that all the patients who enter the cohort are likely to be switched from one treatment to another, irrespective of the initial treatment, and that the likelihood of a switch increases the longer the patient is followed up.
The reasons recorded in the BIOBADADERM registry for discontinuing therapy or switching are predefined. However, the actual decisions are taken in routine clinical practice at each center and do not necessarily coincide with the predefined criteria or with those applied in other centers. It is interesting to note, however, that inefficacy or loss of efficacy was the most common reason for switching in all cases, although this cause was cited more frequently in patients switching from conventional to biologic therapy than vice versa. This finding suggests that efficacy remains an important limitation, even with biologic therapy. Overall, adverse events were the second most frequent cause cited, but in the subgroup of patients switching from biologic to conventional therapy the second most frequent cause was remission. It can, therefore, be speculated that the strategy of rotating therapies for various reasons (i.e., therapeutic holidays, cost-effectiveness, etc.) continues to be used in some scenarios, even in the biologic era.
An analysis of changes in therapy motivated by a variety of reasons in a cohort of patients with moderate to severe psoriasis monitored over a considerable period reveals a progressive increase in the proportion of patients on biologic therapy, a finding that may reflect the fact that these patients quite quickly move beyond the initial steps of the therapeutic ladder (treatment with classic systemic drugs) to biologic therapy – the latest addition to the therapeutic arsenal for the treatment of psoriasis. In any case, our findings suggest that a significant proportion of patients with moderate to severe psoriasis will eventually be treated with biologics, an eventuality that should be taken into account when estimating the overall cost of treating psoriasis. In the rotational strategies used before the advent of biologic agents, the main motive for switching treatments was to reduce the risk of adverse effects due to cumulative toxicity.6 However, in the case of biologic therapy, as seen in our series, patients may more often be switched because of lack of efficacy (primary treatment failure) or loss of efficacy (secondary failure) given the good safety profile and limited specific organ toxicity associated with these drugs.5,7 In some studies of patients with moderate to severe psoriasis, survival of treatment with biologic agents has been shown to be relatively short. For instance, an earlier study based on data from the Biobadaderm registry found the average survival of biologic therapy to be 1.5 years.4 Other authors, including Esposito,8 Clemmensen,9 and Gniadecki,10 have observed a progressive loss over time in the number of patients treated with each biologic drug. Thus, the promise of long standing continuous therapy with the same drug appears to be unrealistic even in the biologic era.
It is of interest to note that a percentage of patients (between 4.5% and 15.7%) discontinued all systemic therapy. Although withdrawal might be temporary, this finding contradicts the idea that all patients should be on treatment all the time. In practice, periods without treatment occur for a number of reasons (remission, concomitant conditions, etc.).
The present study has certain limitations. Although the population studied was large and representative of routine clinical practice, the results reflect the specific situation and prescribing habits of the environment in which the patients were recruited. However, given that the conditions and restrictions regulating prescription are stipulated by the European Medicines Agency, it is probable that the results are applicable within the European context. Ultimately, the assessment over time of the treatment received by patients with moderate to severe psoriasis suggests that a growing percentage of these patients receive biologic therapy at some point. This finding may have implications not only in terms of safety – cumulative exposure – but also with respect to the impact of the use of these more expensive treatments on the economic burden associated with the management of psoriasis.
Funding sourcesThe BIOBADADERM project is financed by the Fundación Academia Española de Dermatología y Venereología (FAEDV), which receives support from the Spanish Medicines and Health Products Agency (AEMPS) and from pharmaceutical companies (Abbott, PfizerMSD and Janssen). Role of the Sponsors: The FAEDV provided administrative support and the AEMPS reviewed the study protocol. Apart from these contributions, none of the sponsors participated in the design or conduct of the study, in the collection, analysis, or interpretation of data, or in the preparation, review, or approval of the manuscript.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Conflicts of interestDr Carrascosa served as a consultant and participated in speakers’ bureaus for Abbvie Laboratories, Janssen Pharmaceuticals Inc., MSD, Pfizer-Wyeth, Lilly, Novartis and Celgene. Dr Carretero served as a consultant for Abbott Laboratories, Janssen-Cilag Pty Limited, MSD, and Pfizer Inc.; gave expert testimony for Abbott Laboratories, MSD, and Pfizer Inc.; received grants from Abbott Laboratories, Janssen Pharmaceuticals Inc., MSD, and Pfizer Inc. and equipment from MSD and Pfizer Inc. Dr Vanaclocha participated in speakers’ bureaus for Abbott Laboratories, Pfizer Inc., MSD, and Janssen Pharmaceuticals Inc. Dr Daudén served as a consultant for Abbott Laboratories, Amgen, Astellas, Celgene, Centocor Ortho Biotech Inc., Galderma, Glaxo, Jannsenn-Cilag, Leo Pharma, MSD, Novartis, and Pfizer Inc.; received honoraria from Abbott Laboratories, Amgen, Celgene, Janssen-Cilag Pty Ltd, Leo Pharma, MSD, Novartis, and Pfizer Inc.; participated in speakers’ bureaus for Abbott Laboratories, Janssen Pharmaceuticals Inc., MSD, and Pfizer Inc.; and received grants from Abbott Laboratories, Janssen Pharmaceuticals Inc., MSD, and Pfizer Inc. Dr Gómez-García declares no conflicts of interest. Dr Herrera-Ceballos served as a consultant and participated in speakers’ bureaus for Abbott Laboratories, Janssen Pharmaceuticals Inc., MSD, and Pfizer-Wyett. Dr De la Cueva acted as a consultant for Janssen-Cilag, Abbott, MSD, Pfizer, Leo-Pharma and Novartis. Dr Belinchón acted as a consultant for Abbott Laboratories, Janssen Pharmaceuticals Inc., MSD, and Pfizer-Wyeth. Dr Sánchez-Carazo acted as a consultant for Abbott Laboratories, Janssen Pharmaceuticals Inc., MSD, and Pfizer-Wyeth. Dr Alsina gave expert testimony for Abbott Laboratories and Merck/Schering-Plough. Dr López-Estebaranz served as a consultant for Abbott Laboratories, Janssen Pharmaceuticals Inc., MSD, and Pfizer-Wyeth; and participated in speakers’ bureaus for Abbott Laboratories, Janssen Pharmaceuticals Inc., MSD, and Pfizer-Wyeth. Dr Ferrán acted as a consultant for Abbott Laboratories, Janssen Pharmaceuticals Inc., MSD, and Pfizer-Wyeth; participated in speakers’ bureaus for Janssen Pharmaceuticals Inc. and MSD; and received grants from Serono. Dr Ferrandiz served as a consultant for Abbott Laboratories, Janssen Pharmaceuticals Inc., and Almirall SA; received honoraria from Abbott Laboratories, Almirall SA, Janssen Pharmaceuticals Inc., and Pfizer Inc.; participated in a speaker's bureau for Abbott Laboratories, Almirall SA, and Janssen Pharmaceuticals Inc.; and received grants from Abbott Laboratories.
The authors wish to thank all the participating hospitals, especially the dermatologists and collaborators who participated in the creation of BIOBADADERM, for their dedication, effort and enthusiasm in this project. BIOBADADERM Study Group Members: Cristina Carazo, José Bañuls, Juan Francisco Silvestre, Pilar Albares, Isabel Betlloch, Monserrat Hernández, Diana Ruiz-Genao, Almudena Nuño, Patricia Guillem, Begoña Echeverría, Esther Margarit, Carlos Muñoz-Santos, Sara Pedregosa, Lara Ferrándiz, and Beatriz Pérez Zafrilla.