Regesmohs registry is a nationwide registry including patients evaluated for Mohs surgery in 17 Spanish centres since July 2013. Given that Mohs surgery is the therapy with best results for high risk basal cell carcinoma (BCC) and other skin tumours, we wanted to describe the reasons that lead to some patients being excluded from this therapy and the alternative treatments that they received. These data may be useful to avoid excluding patients for Mohs surgery use, to estimate the healthcare demand of these patients and the demand for Hedgehog inhibitors therapy in this group.
ObjectiveTo describe patients excluded for Mohs surgery after pre-surgical assessment, and the treatments that they received.
MethodsRegesmohs includes all consecutive patients assessed for Mohs surgery in the participating centres, collecting data on patient characteristics, intervention, and short and long-term results. Patients excluded for Mohs surgery after pre-surgical evaluation were described.
Results3011 patients were included in Regesmohs from July 2013 to October 2016. In 85, Mohs surgery was not performed as they were considered inadequate candidates. 67 had BCC. Reasons for exclusion were: medical contraindication (27.1%, n=23) low-risk tumour in (18.8%, n=16) and giant tumour and bone invasion (15.3%, n=13). Only 1 patient (1.2%) showed lymph node involvement and no patients had visceral metastases. Of the 85 excluded patients, 29 (34.1%) were treated with conventional surgery, 24 (28.3%) with radiotherapy, 4 (4.7%) with inhibitors of the Hedgehog pathway (only indicated for BCC), and 2 (2.4%) received palliative care. We had no follow-up data on 14 patients (16.5%).
ConclusionMedical comorbidities were the most common reason for withholding Mohs surgery. Withholding therapy on the basis of distant extension is uncommon. Most excluded patients received simpler therapies: conventional surgery or radiotherapy, with hedgehog inhibitors being a new option.
El registro Regesmohs es un registro de ámbito nacional, de pacientes evaluados y sometidos a una cirugía de Mohs, en 17 centros españoles, desde julio de 2013. Como la cirugía de Mohs es el tratamiento que mejores resultados da para el manejo del carcinoma de células basales (CCB) de alto riesgo y otros tumores de la piel, queríamos describir los motivos por los que algunos pacientes fueron considerados no aptos para ser sometidos a este tratamiento y qué tratamientos alternativos recibieron. Estos datos pueden ser útiles para evitar excluir a pacientes aptos para ser sometidos a una cirugía de Mohs, para calcular la demanda que estos pacientes generan a nivel sanitario, así como la demanda que hay de tratamientos de inhibidores de la vía de Hedgehog en dicho grupo de pacientes.
ObjetivoDescribir a aquellos pacientes que fueron considerados no aptos para ser sometidos a una cirugía de Mohs tras valoración prequirúrgica y los tratamientos que recibieron.
MétodosRegesmohs incluye a todos los pacientes consecutivos para ser sometidos a una cirugía de Mohs en los centros participantes, recogiendo datos sobre las características de los pacientes, las intervenciones y los resultados a corto y largo plazo. Se hizo una descripción de los pacientes considerados no aptos para ser sometidos a una cirugía de Mohs tras valoración prequirúrgica.
ResultadosTres mil once pacientes fueron incluidos en el registro Regesmohs entre julio de 2013 y octubre de 2016. En 85 pacientes no se realizó cirugía de Mohs porque se consideraron candidatos inadecuados. Sesenta y siete pacientes presentaban CCB. Las razones para ser considerado paciente no apto fueron: contraindicaciones médicas (27,1%, n=23), tumores de bajo riesgo (18,8%, n=16) y tumores gigantes e invasión ósea (15,3%, n=13). Solo un paciente (1,2%) reveló compromiso de ganglios linfáticos y ningún paciente metástasis visceral. De los 85 pacientes considerados no aptos 29 (34,1%) fueron sometidos a cirugía convencional, 24 (28,3%) a radioterapia, 4 (4,7%) a inhibidores de la vía de Hedgehog (solo indicado para el CCB) y 2 (2,4%) a tratamiento paliativo. No hubo datos de seguimiento de 14 pacientes (16,5%).
ConclusiónLas comorbilidades médicas fueron la razón más habitual para retener la cirugía de Mohs. Retener un tratamiento en función de una propagación a lugares distantes no es algo habitual. La mayoría de los pacientes considerados no aptos recibieron tratamientos más sencillos: cirugía convencional o radioterapia, siendo los inhibidores de la vía de Hedgehog una opción novedosa.
Mohs micrographic surgery (MMS) is a technique used to treat various types of skin cancer, performed in many public and private Spanish hospitals. The experience published in the literature related to this technique, including long series of cases and an extensive follow-up period, mainly comes from the US, Australia, Latin America and other European countries.1–5
The Spanish Registry of Mohs Surgery (Regesmohs) collects data on patients assessed for Mohs surgery in 17 national centres from July 2013 with the aim of obtaining a representative description of Mohs surgery in Spain.6 Given that Mohs surgery is probably the therapy with best results for high risk basal cell carcinoma7 and probably other skin tumours, we wanted to describe the reasons that lead to some patients being excluded from this therapeutic option and the alternative therapy that these patients received. These data will help describe the approach to complex tumours, might be useful to detect preventable factors that could increase the use of Mohs surgery, and will also be helpful to estimate the need for alternative treatments. We have not found previous studies describing these patients.
Our objective was to describe the characteristics of patients excluded for Mohs surgery after pre-surgical assessment in Regesmohs, and the treatments that they received.
Materials and methodsThe Spanish Registry of Mohs¿ Surgery (Regesmohs) is a prospective cohort study collecting data from 17 centres that perform at least one weekly MMS. These centres represent a very high percentage of those that perform MMS in Spain. Participating centres include all consecutive patients evaluated for performing MMS. In this registry, data related to patient characteristics and their intervention, short-term results (in a postsurgical visit) and long-term results are collected. Patients should have an annual postsurgical visit to describe their long-term evolution. Some patients were candidates for MMS, but were excluded on their pre-surgical visit. These are the patients that we describe in this paper.
The information is collected through an online data collection system (OpenClinica, version 3.1) of the Research Unit of the Spanish Academy of Dermatology. All investigators use a common dictionary data to ensure uniformity in the definition of the variables. The registry has a continuous online monitoring system that detects missing or inconsistent data. Regesmohs received ethical approval from Comité Etico de Investigación Clínica Universidad de Navarra (EO11/2013) and all patients gave their informed consent to participate.
Statistical analysis consisted of descriptive analysis of demographic data and clinical characteristics of patients. The qualitative variables were expressed as absolute values ??and percentages. Quantitative variables were expressed as means and standard deviations. It was performed using Stata (StataCorp. 2015 Release 14.2).
Results3011 patients have been included in the registry from July 2013 to October 2016. Of these, 85 patients were considered inadequate candidates for MMS. The median age of these patients was 83.2 years (p25=75.1; p75=88.4), with a moderate predominance of males (56.5%, n = 48). Most patients came from the catchment area of each centre (88.2%, n=75), while 11.8% (n = 10) patients were referred from other areas. Few patients 3.5% (n = 3) had some type of immunosuppression, 16.5% (n = 14) had diabetes mellitus and 4.7% (n = 4) were diagnosed with some type of multiple tumours syndrome. The median tumour size was 11mm of major axis (5-24) and 10mm of minor axis (4-18). The median time of tumour progression from diagnosis to treatment decisions was 17.8 months (P25-P75: 7.7-39.7).
Patients that received MMS (2926) were in median age 70.7 years old (58.8-79.8), with a percentage of males of 51.0% (n = 1493) and an median tumour size of 10mm (6-18). Moreover 955 (32.6%) patients received some type of previous surgery before MMS. Compared to patients that received MMS, patients excluded were older, with a higher percentage of males and a higher average tumour size.
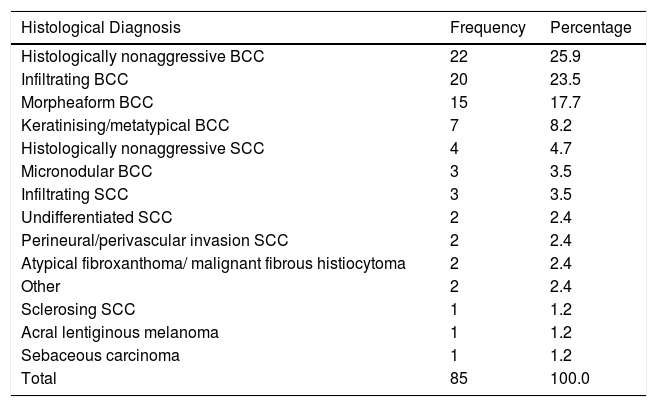
Most excluded patients had basal cell carcinoma (BCC) (78.8%, n = 67) and 14.1% had squamous cell carcinoma (n=12). Detailed histological subtypes are described in Table 1. Most of the excluded tumours were primary (i.e. non-recurrent) (67.1%, n = 57) and located on the face and scalp (91.8%, n = 78), where the nose was the most common location (37.7% n = 32).
Frequency and percentage of tumours histological subtypes.
| Histological Diagnosis | Frequency | Percentage |
|---|---|---|
| Histologically nonaggressive BCC | 22 | 25.9 |
| Infiltrating BCC | 20 | 23.5 |
| Morpheaform BCC | 15 | 17.7 |
| Keratinising/metatypical BCC | 7 | 8.2 |
| Histologically nonaggressive SCC | 4 | 4.7 |
| Micronodular BCC | 3 | 3.5 |
| Infiltrating SCC | 3 | 3.5 |
| Undifferentiated SCC | 2 | 2.4 |
| Perineural/perivascular invasion SCC | 2 | 2.4 |
| Atypical fibroxanthoma/ malignant fibrous histiocytoma | 2 | 2.4 |
| Other | 2 | 2.4 |
| Sclerosing SCC | 1 | 1.2 |
| Acral lentiginous melanoma | 1 | 1.2 |
| Sebaceous carcinoma | 1 | 1.2 |
| Total | 85 | 100.0 |
BCC: basal cell carcinoma; SCC: squamous cell carcinoma;.
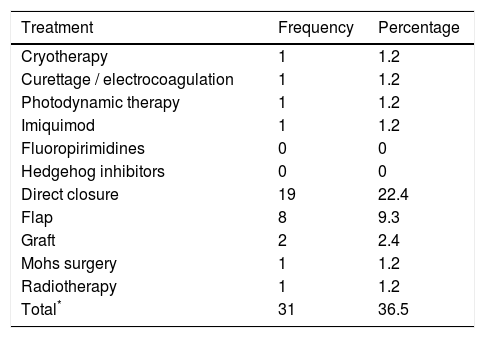
36.5% of the patients (n=31) had received a previous therapy (Table 2), most commonly surgery. The median time between the application of a previous surgical treatment (cryotherapy / curettage / Simple excision) until the new clinical assessment and exclusion from the registry was 6.5 months (P25-P75: 2.9-17.5).
Previous treatments received by patients excluded for Mohs surgery.
| Treatment | Frequency | Percentage |
|---|---|---|
| Cryotherapy | 1 | 1.2 |
| Curettage / electrocoagulation | 1 | 1.2 |
| Photodynamic therapy | 1 | 1.2 |
| Imiquimod | 1 | 1.2 |
| Fluoropirimidines | 0 | 0 |
| Hedgehog inhibitors | 0 | 0 |
| Direct closure | 19 | 22.4 |
| Flap | 8 | 9.3 |
| Graft | 2 | 2.4 |
| Mohs surgery | 1 | 1.2 |
| Radiotherapy | 1 | 1.2 |
| Total* | 31 | 36.5 |
Only 1 patient (1.2%) showed lymph node involvement and no patients had visceral metastases.
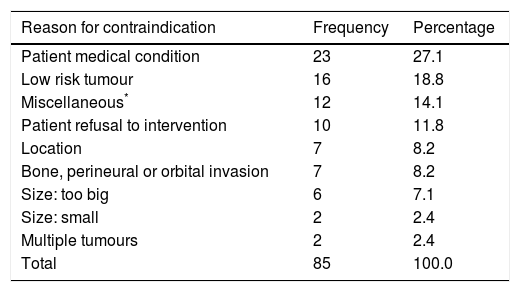
The main reasons for withholding Mohs surgery was the existence of some medical contraindication (27.1%, n = 23) and the presence of a low-risk tumour in 18.8% (n = 16). The presence of a giant tumour and deep invasion represented 15.3% (n = 13) of the total (Table 3).
Reasons for contraindicating Mohs surgery.
| Reason for contraindication | Frequency | Percentage |
|---|---|---|
| Patient medical condition | 23 | 27.1 |
| Low risk tumour | 16 | 18.8 |
| Miscellaneous* | 12 | 14.1 |
| Patient refusal to intervention | 10 | 11.8 |
| Location | 7 | 8.2 |
| Bone, perineural or orbital invasion | 7 | 8.2 |
| Size: too big | 6 | 7.1 |
| Size: small | 2 | 2.4 |
| Multiple tumours | 2 | 2.4 |
| Total | 85 | 100.0 |
Of the 85 excluded patients, 29 (34.1%) were treated with conventional surgery, 24 (28.3%) with radiotherapy, 4 (out of the 67 BCC (6.0%)) (4.7%) with inhibitors of the Hedgehog pathway (3 with locally advanced BCC and invasion of deep structures and 1 with Gorlin syndrome), and 2 (2.4%) received palliative care. We had no follow-up data on 16 patients (18.8%).
DiscussionWe have found that medical contraindications, or the overall situation of the patient, is the most common reason for withholding Mohs surgery in our patients.
Some patients (18 /3011) were considered inadequate for Mohs surgery because the tumour was too small or of low risk, probably indicating an inadequate referral. The 7 tumours excluded because of their location could also belong to this group.
8.2% (n=7) were excluded due to deep structures invasion, and 7.1% (n=6) because the tumour was too large. The presence of disseminated disease was an uncommon contraindication.
Perhaps because of these facts, most patients excluded from MMS are derived to simpler therapeutic measures, such as conventional surgery (34%) or radiotherapy (28%). Few patients received treatment with vismodegib. However, this estimation has limited validity as this drug was licensed in 2016, and it is only indicated for locally advanced and metastatic BCC
Although the use criteria for appropriate use of Mohs surgery have been described,8 we have not found in the literature previous descriptions of those patients considered inadequate for Mohs surgery and the treatments that they received.
Our registry has the advantage of being highly representative of current MMS in Spain, because it prospectively records the activity of most centres performing this technique. One limitation is that the patterns of referral, surgical ability and availability of alternative therapies might be different in different countries, so the results might not be fully generalizable to other contexts. The main limitations of our study is that we have only taken into account those patients that have been initially referred for Mohs surgery and excluded in their pre-surgical visit. Many patients could have been referred to a different therapy without this pre-surgical evaluation. However, we feel that the results are informative and relevant, and that these data might be useful for estimating the healthcare demand associated with skin tumours treated with MMS. Medical comorbidities are the main reason for contraindicating Mohs surgery, and it is possible that this limitation might be overridden by improved perioperative care.
Funding sourcesRegesmohs is promoted by the Spanish Academy of Dermatology, with the financial support of Roche Farma SA.
The following members of Regesmohs have participated in data collection: Luis Miguel Valladares (Complejo Asistencial Universitario de León), Eduardo Nagore Enguidanos, Beatriz Llombart Cussac and Celia Requena Caballero (Instituto Valenciano de Oncología) Luis Hueso Gabriel, Antonio Martorell (H. Manises), Victoriano Morales Gordillo (H. Quirón, Pozuelo, Madrid), Raquel Navarro Tejedor (H de la Princesa, Madrid) M Mayoral (Hospital La Paz, Madrid) R Suárez (H Gregorio Marañón, Madrid) Mª José Seoane Pose (CHU Santiago). We also thank other members of participating departments that have helped with the registry.
Please cite this article as: Ruiz-Salas V, Garcés JR, Alonso-Alonso T, Rodríguez-Prieto MA, Toll-Abelló A, Eusebio Murillo E, et al. Description of patients excluded for Mohs surgery after pre-surgical evaluation: data from the Regesmohs Spanish registry. Actas Dermosifiliogr. 2018;109:346–350.