Psoriatic arthritis is a common type of inflammatory arthritis found in up to 40% of patients with psoriasis. Because skin involvement usually precedes joint involvement, dermatologists play a key role in early detection. Early diagnosis is important for reducing the risk of irreversible structural damage, attenuating the deterioration of physical function, and improving patients’ quality of life. This consensus statement was drafted by a group of 9 dermatologists and 1 rheumatologist to provide simple recommendations to help dermatologists screen for psoriatic arthritis in patients with psoriasis. The experts offer consensus-based guidelines that draw on a review of available scientific evidence and on experience acquired in routine clinical practice.
La artritis psoriásica (APs) es una forma común de artritis inflamatoria que aparece hasta en el 40% de los pacientes con psoriasis. Dado que la afectación cutánea suele preceder a la afectación articular, los dermatólogos desempeñan un papel fundamental en la detección precoz de la APs. El diagnóstico precoz es importante para reducir el riesgo de daños estructurales irreversibles, limitar el deterioro de la función física y mejorar la calidad de vida de los pacientes. El presente documento ha sido elaborado por un grupo de especialistas (nueve dermatólogos y un reumatólogo) con el objetivo de proporcionar recomendaciones sencillas que ayuden a los dermatólogos en el cribado de la APs en pacientes con psoriasis. Los expertos elaboraron el presente documento ofreciendo unas recomendaciones consensuadas basadas en una revisión descriptiva de la evidencia científica disponible y en la experiencia adquirida en la práctica clínica diaria.
Psoriatic arthritis (PsA) is a chronic inflammatory disease with an estimated prevalence of 0.05% to 0.25% in the general population and 6% to 41% in patients with psoriasis.1 It is a highly heterogeneous disease with a variable course.2 In the joints, it causes inflammation of the synovial membrane and the periosteal insertions of tendons and ligaments, leading to erosions and enthesophytes, respectively.3 PsA causes joint deformity and destruction in approximately 40% to 60% of patients and is therefore associated with functional impairment, reduced quality of life, psychosocial complications, and an increased risk of death compared with the general population.4,5 The manifestations of PsA are highly variable and can affect any joint; patients may have peripheral, axial, or mixed involvement.6 Inflammatory pain, a characteristic feature of joint disease, improves with activity and worsens with rest and is usually accompanied by heat, swelling, morning stiffness, and limited movement.7,8 Other hallmark manifestations of PsA are enthesitis, dactylitis, and nail involvement.9
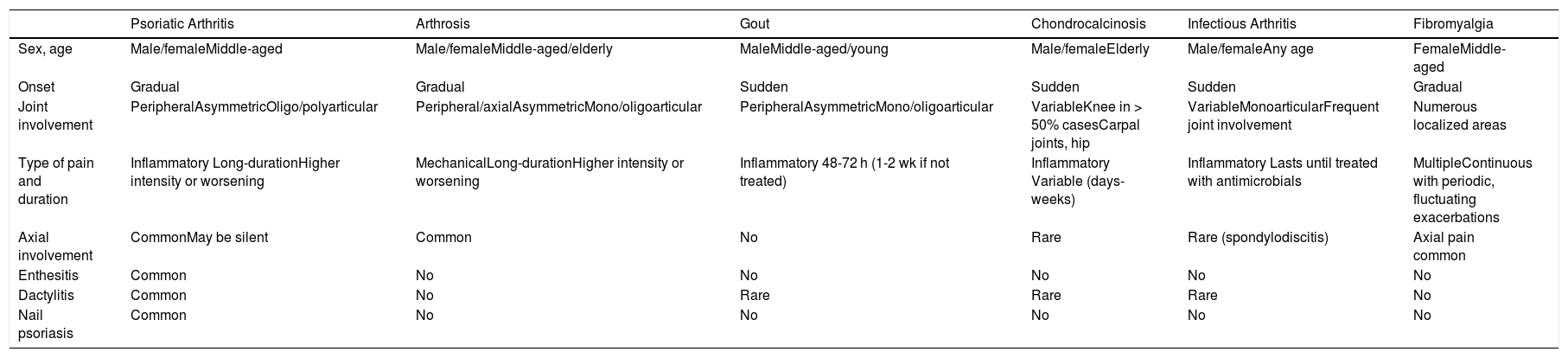
Clinical diagnosis and treatment is complex because of the heterogeneous clinical spectrum of PsA. In addition, other arthropathies must be ruled out (Table 1).
| Psoriatic Arthritis | Arthrosis | Gout | Chondrocalcinosis | Infectious Arthritis | Fibromyalgia | |
|---|---|---|---|---|---|---|
| Sex, age | Male/femaleMiddle-aged | Male/femaleMiddle-aged/elderly | MaleMiddle-aged/young | Male/femaleElderly | Male/femaleAny age | FemaleMiddle-aged |
| Onset | Gradual | Gradual | Sudden | Sudden | Sudden | Gradual |
| Joint involvement | PeripheralAsymmetricOligo/polyarticular | Peripheral/axialAsymmetricMono/oligoarticular | PeripheralAsymmetricMono/oligoarticular | VariableKnee in > 50% casesCarpal joints, hip | VariableMonoarticularFrequent joint involvement | Numerous localized areas |
| Type of pain and duration | Inflammatory Long-durationHigher intensity or worsening | MechanicalLong-durationHigher intensity or worsening | Inflammatory 48-72 h (1-2 wk if not treated) | Inflammatory Variable (days-weeks) | Inflammatory Lasts until treated with antimicrobials | MultipleContinuous with periodic, fluctuating exacerbations |
| Axial involvement | CommonMay be silent | Common | No | Rare | Rare (spondylodiscitis) | Axial pain common |
| Enthesitis | Common | No | No | No | No | No |
| Dactylitis | Common | No | Rare | Rare | Rare | No |
| Nail psoriasis | Common | No | No | No | No | No |
Early diagnosis is essential to enable early treatment, alter the natural course of disease, and prevent irreversible inflammation-induced structural damage.14,15
Several studies have shown that joint damage in patients with PsA occurs within just a few years of disease onset.16–18 One retrospective study found that a diagnostic delay of just 6 months was associated with worse long-term radiographic and functional outcomes.19
Up to 84% of patients with PsA develop skin manifestations before the joint involvement becomes clinically evident,20 meaning that dermatologists are often faced with the challenge of establishing an early diagnosis. Although dermatologists are very familiar with psoriatic skin lesions, they may have less experience with the musculoskeletal manifestations of PsA.21 In addition, most of them do not routinely screen for PsA, increasing the likelihood of diagnostic delays. The prevalence of undiagnosed PsA among psoriasis patients under treatment at dermatology practices has been estimated at between 15% and 40%.20,22,23 To ensure favorable long-term clinical outcomes, dermatologists need to systematically screen for PsA in patients with psoriasis. To do this, however, they need appropriate training and practice to detect suspicious cases and enable early referral to a rheumatologist to confirm the diagnosis and initiate treatment before permanent joint damage occurs.24,25
Adequate screening tools for use by both dermatologists and primary care physicians are also lacking. While several tools exist, their usefulness in everyday practice is limited as they are time-consuming, complex, and have a high false-positive rate.
The recommendations in this document, which build on and update previous recommendations,8 address several issues related to the early detection of PsA by dermatologists and draw on an updated review of psoriasis treatments and their efficacy in the treatment of joint involvement. We present a concise, systematic, structured overview of potentially useful and practical tools for the early detection of PsA by dermatologists and offer a series of recommendations and a simple, practical algorithm that may be useful for the clinical management of PsA in dermatology practices. Our recommendations are based on an updated review of the evidence and expert opinions.
ObjectiveThe main aim of this study was to produce a guide to help dermatologists establish an early diagnosis of PsA in routine clinical practice.
MethodsThis document was created by an expert panel formed by 9 dermatologists and 1 rheumatologist, all with extensive experience in psoriasis and PsA. The methodological approach was based on the so-called formal method for developing consensus and recommendations in which an expert panel evaluates scientific evidence and uses clinical experience (expert opinion) to achieve consensus on given clinical problems.26,27
Following the precepts of this approach, the expert panel reviewed the relevant literature and completed a previously designed questionnaire to identify important unresolved issues related to the early diagnosis of PsA and potential diagnostic tools. A face-to-face meeting was then held to analyze and discuss the results of the questionnaire, deliberate on aspects of clinical management related to the early diagnosis of PsA in dermatology practices, and achieve evidence- and experience-based consensus on discrepancies identified. Analysis of the questionnaire results and the most relevant points that emerged in the meeting formed the basis for the management algorithm and other recommendations provided in this document.
Section 5 of the results section (treatment) contains a table based on the available scientific evidence that summarizes the efficacy of different psoriasis drugs for the treatment of each of the clinical domains of PsA and axial spondyloarthritis according to clinical trial results. The table also shows the number of phase 3 clinical trials, including head-to-head trials, conducted in white adults that have analyzed the efficacy of the different treatments for each domain. The literature search, performed in clinicaltrials.gov and PubMed, targeted studies published up to June 4, 2020. The references of all the articles and trials used to prepare the table are provided in Appendix A supplementary material 1.
ResultsTo facilitate the early detection of PsA, this section presents a summary of the findings of the literature review and the opinions of the expert panel in 5 sections:
- 1.
Screening tools
- 2.
History taking and physical examination
- 3.
Additional tests
- 4.
Multidisciplinary care: referral to a rheumatologist
- 5.
Treatment
- a
Literature Review
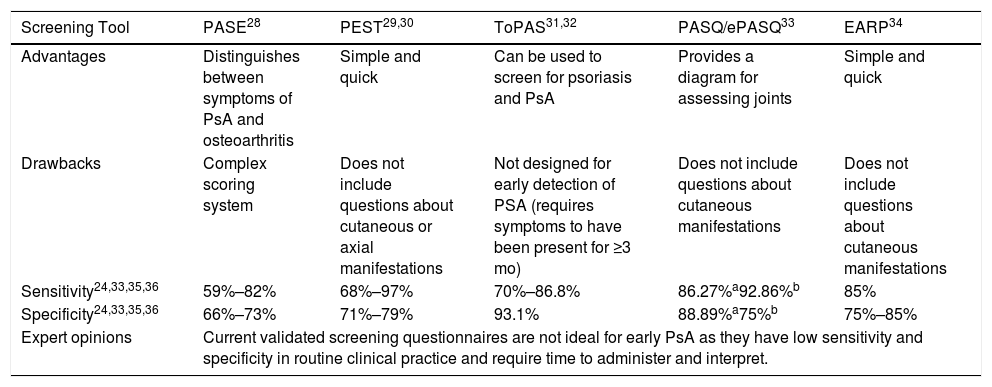
Numerous screening tools have been developed and validated to facilitate the early diagnosis and treatment of PsA (Table 2).
Characteristics of Screening Tools for the Early Detection of PsA.
| Screening Tool | PASE28 | PEST29,30 | ToPAS31,32 | PASQ/ePASQ33 | EARP34 |
|---|---|---|---|---|---|
| Advantages | Distinguishes between symptoms of PsA and osteoarthritis | Simple and quick | Can be used to screen for psoriasis and PsA | Provides a diagram for assessing joints | Simple and quick |
| Drawbacks | Complex scoring system | Does not include questions about cutaneous or axial manifestations | Not designed for early detection of PSA (requires symptoms to have been present for ≥3 mo) | Does not include questions about cutaneous manifestations | Does not include questions about cutaneous manifestations |
| Sensitivity24,33,35,36 | 59%–82% | 68%–97% | 70%–86.8% | 86.27%a92.86%b | 85% |
| Specificity24,33,35,36 | 66%–73% | 71%–79% | 93.1% | 88.89%a75%b | 75%–85% |
| Expert opinions | Current validated screening questionnaires are not ideal for early PsA as they have low sensitivity and specificity in routine clinical practice and require time to administer and interpret. | ||||
Abbreviations: EARP, Early Arthritis for Psoriatic Patients screening questionnaire; PASE, Psoriatic Arthritis screening and Evaluation questionnaire; PASQ, Psoriasis and Arthritis Screening Questionnaire; PsA; psoriatic arthritis; ToPAS, Toronto Psoriatic Arthritis Screening questionnaire.
Two recent questionnaires are the Simple Psoriatic Arthritis Screening (SiPAS) questionnaire37 and the Psoriatic arthritis UnclutteRed screening Evaluation (PURE-4) questionnaire.38 The SiPAS is a 5-item questionnaire designed to be completed quickly by the patient. It has a similar sensitivity (79%) and specificity (87%) to other questionnaires.37 The PURE-4 questionnaire has just 4 items based on the 4 domains with the highest diagnostic yield for PsA in patients with psoriasis. The initial results are promising (85.7% sensitivity and 83.6% specificity)38 and the questionnaire was recently validated in Spanish.39
- b
Expert Panel Recommendations
The expert panel concluded that currently validated screening questionnaires are not ideal for early PsA detection, mainly due to the time needed for their completion and interpretation in daily practice. Nonetheless, the recently validated Spanish version of PURE-4 is promising because it is short, has high diagnostic sensitivity, and is feasible in routine clinical practice.
Patient History and Physical Examination- a
Literature Review
Although PsA is a highly heterogeneous disease, its main clinical manifestations are inflammatory arthritis (peripheral), enthesitis, dactylitis, and inflammatory axial pain (Table 3).7,40,41
Clinical Characteristics of Inflammatory Arthritis, Enthesitis, Dactylitis, and Spondylitis or Axial Pain.7
| Type of Inflammation | Signs and Symptoms |
|---|---|
| Morning stiffness ≥ 30 min; joint swelling; pain that improves with activity and gets worse with rest; limited mobility | |
| Chronic back pain; chronic pain involving buttocks, hips, and behind the thighs; morning stiffness; limited mobility and flexibility | |
| Swelling; heat; erythema; sensitivity at inflammation site; reduced mobility; sausage-like digits | |
| Pain next to the joint; swelling at site of pain; functional limitations; history of plantar fascitis; pain involving the enthesis (Achilles tendon, plantar fascia, quadriceps tendon, patellar ligament, iliac crest); more common in the lower than upper extremities |
Nail psoriasis is also common.42 Some authors consider that patients with nail psoriasis are more likely to have enthesitis and distal interphalangeal joint disease.43,44 Dermatologists should therefore be familiar with these signs, as they may be the only manifestation of PsA during the early phase of disease.
Approximately 25% of patients with PsA have inflammatory disease involving the axial skeleton; this causes pain, mainly in the lumbar region and sacroiliac joints (sacroiliitis),45 and mobility limitations.
- b
Expert Panel Recommendations
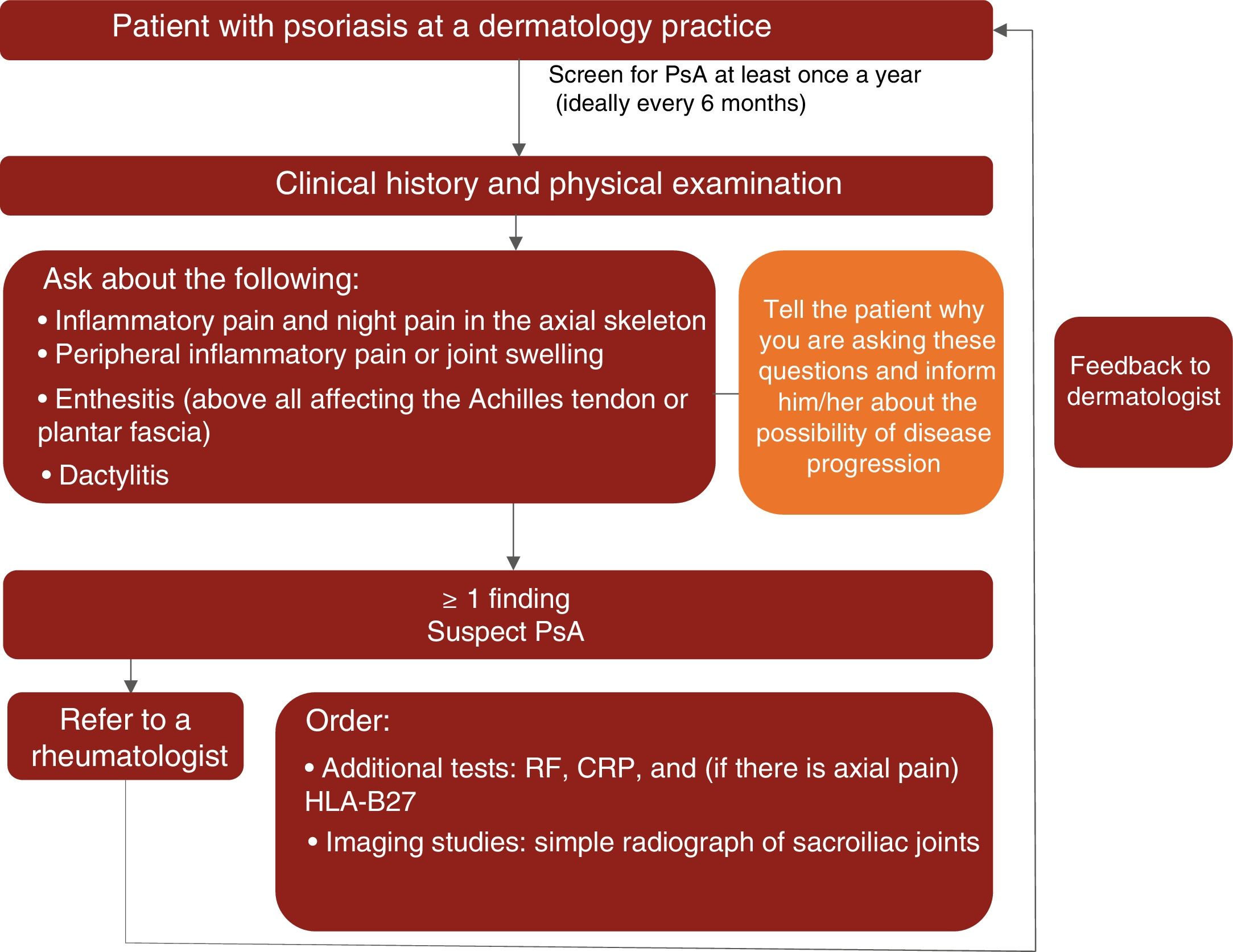
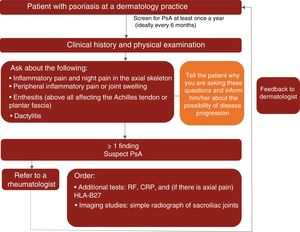
Based on the available scientific evidence and their clinical experience, the experts recommend that dermatologists should actively look for signs of PsA in patients with psoriasis at least once a year and ideally every 6 months by taking a complete history and evaluating key manifestations (inflammation of the axial skeleton, dactylitis, enthesitis, and peripheral arthritis) to check for inflammatory pain.14,15,19,46
During the physical examination, special attention should be paid to the fingers and toes (particularly the distal interphalangeal joints) to check for peripheral inflammation and dactylitis (especially in the toes); the entheses (e.g., Achilles tendon) to check for enthesitis; and the spine to check for inflammatory axial pain.
The expert panel also highlighted the importance of informing patients about the signs and symptoms of PsA to raise their awareness about the disease and the possibility of progression to joint disease. Informed patients will be better equipped to identify and consult their dermatologist about the manifestations of PsA, increasing thus the likelihood of an early diagnosis.
Additional TestsLaboratory Tests- a
Literature Review
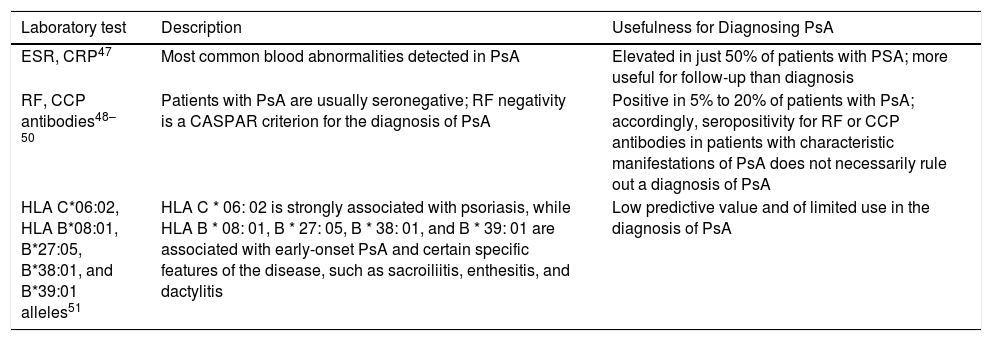
Since the diagnosis of PsA is essentially clinical, it can often be difficult to distinguish between PsA and other conditions with similar manifestations. The availability of specific biomarkers could facilitate a definitive diagnosis. The different laboratory tests and their usefulness in the diagnosis of PsA are described in Table 4.
- b
Expert Panel Recommendations
Possible Biomarkers for Diagnosing PsA.
| Laboratory test | Description | Usefulness for Diagnosing PsA |
|---|---|---|
| ESR, CRP47 | Most common blood abnormalities detected in PsA | Elevated in just 50% of patients with PSA; more useful for follow-up than diagnosis |
| RF, CCP antibodies48–50 | Patients with PsA are usually seronegative; RF negativity is a CASPAR criterion for the diagnosis of PsA | Positive in 5% to 20% of patients with PsA; accordingly, seropositivity for RF or CCP antibodies in patients with characteristic manifestations of PsA does not necessarily rule out a diagnosis of PsA |
| HLA C*06:02, HLA B*08:01, B*27:05, B*38:01, and B*39:01 alleles51 | HLA C * 06: 02 is strongly associated with psoriasis, while HLA B * 08: 01, B * 27: 05, B * 38: 01, and B * 39: 01 are associated with early-onset PsA and certain specific features of the disease, such as sacroiliitis, enthesitis, and dactylitis | Low predictive value and of limited use in the diagnosis of PsA |
Abbreviations: CASPAR, ClASsification criteria for Psoriatic ARthritis; CCP, cyclic citrullated peptide; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HLA, human leukocyte antigen; PsA, psoriatic arthritis; RF, rheumatoid factor.
The experts concluded that none of the currently available serum biomarkers provides sufficient information to establish a definitive diagnosis of PsA. Nonetheless, should a dermatologist decide to order blood tests before referring a patient to a rheumatologist, the following biomarkers may complement clinical findings and enable a faster diagnosis: rheumatoid factor (RF), C-reactive protein (CRP), and, in patients with axial pain, human leukocyte antigen B27 (HLA-B27). If it is not possible to request all 3 tests, high-sensitivity CRP is recommended as the test of choice.
Diagnostic Imaging- a
Literature Review
Conventional radiography (X-rays), ultrasound, and magnetic resonance imaging (MRI) have been widely used in the evaluation of PsA.
A radiograph is the simplest imaging test. Radiographic evidence of juxta-articular new bone formation (not including osteophytes) in the hands or feet is one of the classification criteria for PsA.52 Erosions are also a characteristic feature of joint lesions in PsA. The main purpose of radiography, however, is to identify PsA manifestations specifically present in patients with advanced disease.
In recent decades, there has been a substantial increase in the use of ultrasound and MRI for the early diagnosis of PsA. Both techniques provide useful data for determining pathogenicity and level of disease activity.53–55 The findings of several studies have suggested that by detecting enthesitis and extracapsular inflammation MRI can discriminate between PsA, rheumatoid arthritis, and osteoarthritis.56–58 Ultrasound is used to detect early lesions (mainly synovitis and enthesitis). Several studies have evaluated the role of ultrasound in patients with psoriasis but without signs of joint disease.59,60 The predictive value of subclinical enthesitis in the diagnosis of PsA, however, is still unknown.
In light of the growing evidence on the value of diagnostic imaging in the treatment of rheumatic diseases, the European League Against Rheumatism (EULAR) published the first recommendations on the use of imaging studies for the diagnosis and treatment of spondyloarthritis, including PsA.61 Radiography, ultrasound, and MRI are recommended for patients with a personal or family history of psoriasis and signs suspicious for peripheral PsA, although MRI is indicated as a second-line option due to its limited availability and relatively high cost. The procedure of choice for the diagnosis of axial PsA is radiographic examination of the pelvis to check for sacroiliitis. An MRI, however, may be required to distinguish old bone lesions from active lesions, which will show evident bone swelling.
- b
Expert Panel Recommendations
The experts consider that routine imaging tests are not feasible in daily dermatology practice. However, such tests can optimize the care process if ordered in the context of referral to rheumatology. In such cases, the recommendation is to perform a radiographic evaluation of the sacroiliac joints (if there is axial pain) and the most symptomatic joints (especially in the hands and feet). The panel also recommends MRI for patients with axial manifestations. For dermatologists skilled in interpreting images, ultrasound can be a useful tool for identifying musculoskeletal signs of PsA.
Multidisciplinary Care: Referral to a Rheumatologist- a
Literature Review
The value of multidisciplinary care is being increasingly recognized.62–65 Several models have been proposed and evaluated, including a model in which patients are seen by a rheumatologist and a dermatologist on the same day and virtual models in which they are seen by both specialists at the same clinic but at different times.62,66 Given the current coronavirus disease 2019 (COVID-19) pandemic, teledermatology models are gaining traction as a means of reducing face-to-face consultations and risk of infection.67,68 Although different teledermatology models exist in Spain, their use is not yet widespread,69 although this is likely to change with the current situation.
Multidisciplinary management models have been shown to improve patient care and treatment outcomes, mainly because they facilitate faster and more accurate diagnoses. Other advantages mentioned by specialists include better communication and collaboration and more research opportunities.70–73 Scheduling visits, however, remains a major challenge, as both rheumatologic and dermatologic evaluations are time consuming and require clinical experience.
In light of the heterogeneity of referral criteria and procedures, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has highlighted the need for clear criteria on which patients should be referred to a rheumatologist for a more detailed evaluation.74
- b
Expert Panel Recommendations
The expert panel considers that dermatologists have a key role in the early diagnosis of PsA because of their involvement in psoriasis management. However, a joint evaluation is recommended to confirm diagnosis.
In the event of multidisciplinary care, the experts recommend maintaining fluid, 2-way communication to monitor the patient’s condition, resolve diagnostic uncertainties, and choose an appropriate treatment strategy.
In line with the recommendations published by Daudén et al.8 in 2012, the experts consider that patients with psoriasis and suspected PsA should be referred to a rheumatologist when they have at least 1 of the following signs or symptoms:
- •
Inflammatory axial pain, including night pain
- •
Inflammatory peripheral pain or swelling
- •
Enthesitis or signs of enthesitis (especially in the Achilles tendon and plantar fascia)
- •
Dactylitis or signs of dactylitis
As already mentioned, the expert group recommends that dermatologists should actively look for signs or symptoms of PsA at least once a year and ideally every 6 months.
Finally, telemedicine could acquire a key role in the multidisciplinary management of patients with psoriasis, particularly in the current COVID-19 situation.67
A proposed algorithm for the clinical management of PsA in dermatology practices is shown in Fig. 1.
Treatment- a
Literature Review
Although immediate referral to a rheumatologist is recommended for patients with rapidly progressive or advanced disease or for those being treated by a dermatologist with less experience in managing PsA, the realities of the healthcare system are such that dermatologists sometimes need to take decisions regarding the treatment of a patient with psoriasis and suggestive signs of PsA.75,76
Specific treatment guidelines and recommendations for patients with early PsA are lacking. Most of the current guidelines (e.g., GRAPPA, EULAR, ACR) focus on patients with established PsA, that is patients who meet the Classification Criteria for Psoriatic Arthritis (CASPAR).77–79
Nonsteroidal anti-inflammatory drugs and other conservative strategies are usually indicated for patients with mild clinical manifestations and/or nonerosive disease. In patients with moderate to severe disease, however, first-line treatment with nonbiological disease-modifying antirheumatic drugs (conventional DMARDS) may be considered. Other options include biologic drugs (tumor necrosis factor inhibitors and interleukin [IL] 17 and IL-12/23 inhibitors) and new oral synthetic molecules (phosphodiesterase inhibitors), which have substantially improved the treatment of PsA.76
Evidence on the treatment of early PsA is scarce. Nonetheless, a recent exploratory study showed that early treatment with secukinumab for 24 weeks in psoriasis patients without PsA but with arthralgia and inflammatory joint lesions resolved inflammation and stopped progression of structural changes. These results suggest that early treatment with secukinumab is possible and may lead to improved clinical and radiographic outcomes in patients with early PsA.15 Another exploratory study showed that treatment with ustekinumab in patients with psoriasis but not PsA reduced subclinical enthesitis after 12 weeks.80
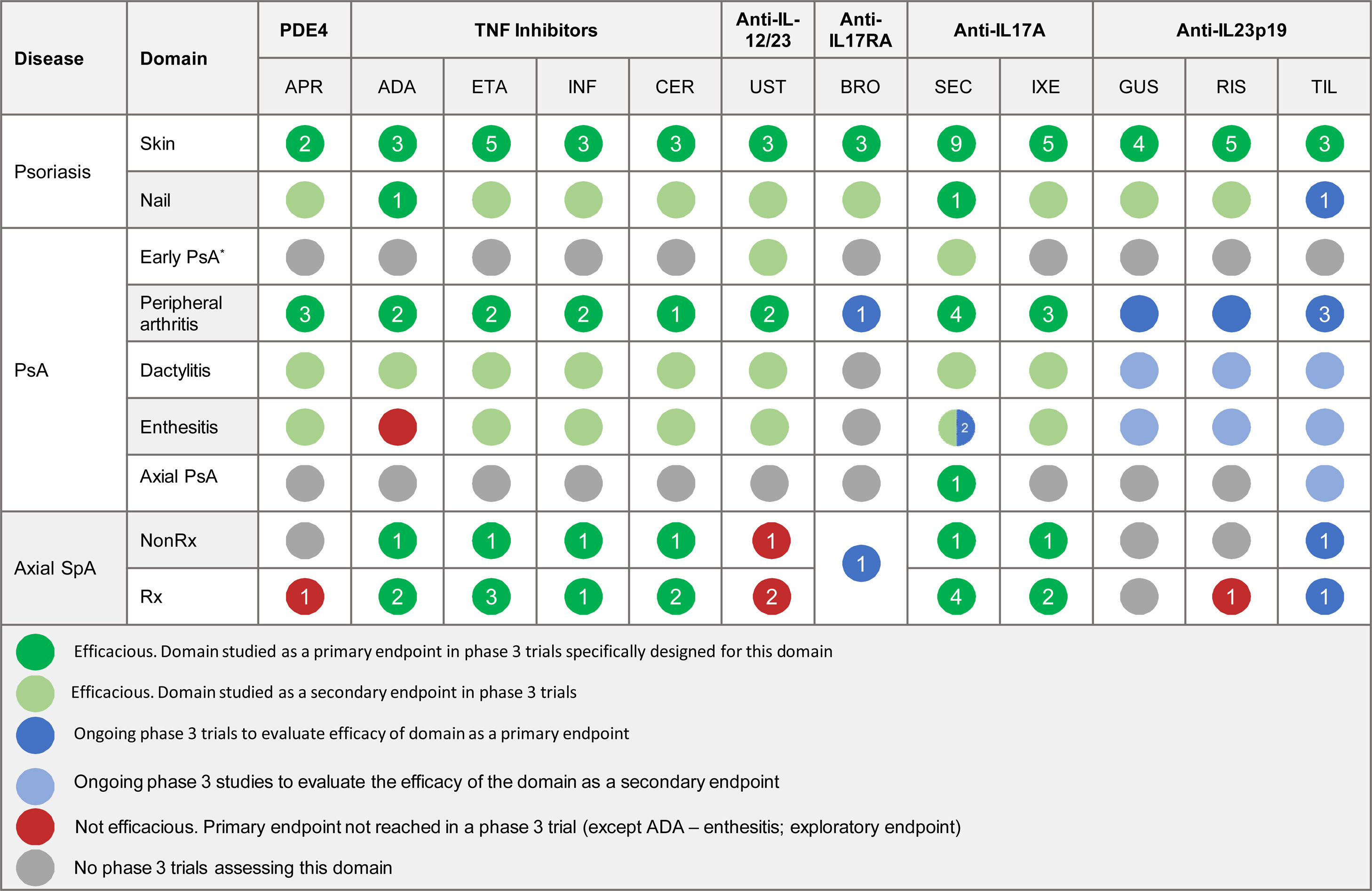
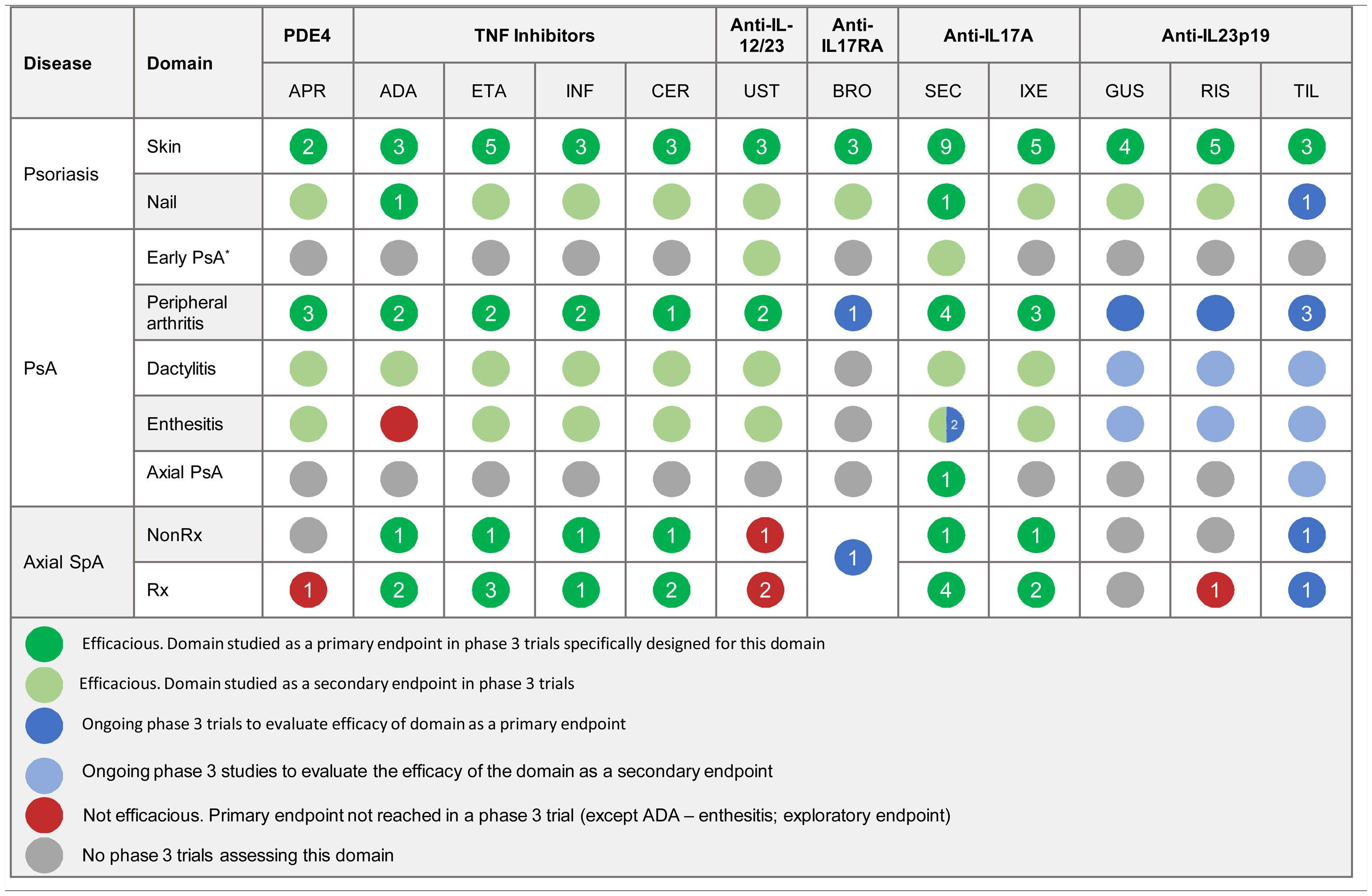
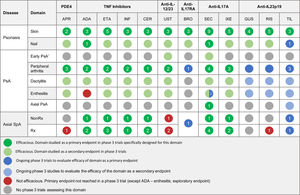
The efficacy of psoriasis drugs for the treatment of the different clinical domains of PsA and axial spondyloarthritis as shown by clinical trials is summarized in Table 5.
- b
Expert Panel Recommendations
Summary of Clinical Efficacy of Systemic Biologic Drugs and Oral Small Molecules Approved for Psoriasis in the Treatment of the Clinical Domains of Psoriatic Arthritis and Axial Spondyloarthritis. The number in each circle indicates the number of clinical trials evaluating a given domain. All the references for the articles and clinical trials used to prepare this table are provided in Appendix A supplementary material 1. Supplementary material
Abbreviations: ADA, adalimumab; APR, apremilast; BRO, brodalumab; CER, certolizumab; ETA, etanercept; GUS, guselkumab; IL, interleukin; INF, infliximab; IXE, ixekizumab; NonRx, nonradiographic; PDE4, phosphodiesterase inhibitor 4; PsA, psoriatic arthritis; RIS, risankizumab; Rx, radiographic; SEC, secukinumab; SpA, spondyloarthritis; TIL, tildrakizumab; TNF, tumor necrosis factor; UST, ustekinumab.
*The term early PsA refers to exploratory studies in which patients did not have a definitive diagnosis of PsA.
The expert panel recommends a treatment strategy based on the different clinical manifestations of PsA. Specific treatments should be chosen according to efficacy and safety data from clinical trials and real-world experience.
ConclusionsEarly diagnosis of PsA is essential, as early treatment and management can alter the natural course of PsA and prevent irreversible joint damage. The lack of adequate screening tools for this purpose, however, constitutes an unmet need addressed in this work. We have analyzed key issues, as well as potential tools and procedures that dermatologists could use for the early detection of PsA.
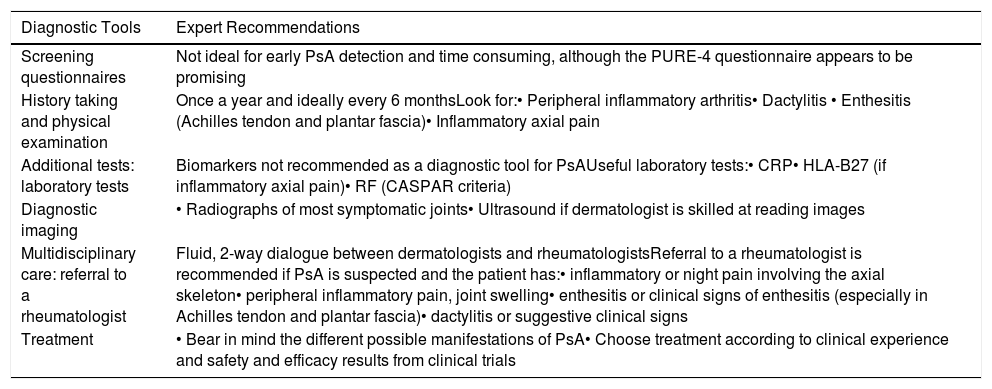
The conclusions of each of the sections are presented below and summarized in Table 6.
Summary of Recommendations.
| Diagnostic Tools | Expert Recommendations |
|---|---|
| Screening questionnaires | Not ideal for early PsA detection and time consuming, although the PURE-4 questionnaire appears to be promising |
| History taking and physical examination | Once a year and ideally every 6 monthsLook for:• Peripheral inflammatory arthritis• Dactylitis • Enthesitis (Achilles tendon and plantar fascia)• Inflammatory axial pain |
| Additional tests: laboratory tests | Biomarkers not recommended as a diagnostic tool for PsAUseful laboratory tests:• CRP• HLA-B27 (if inflammatory axial pain)• RF (CASPAR criteria) |
| Diagnostic imaging | • Radiographs of most symptomatic joints• Ultrasound if dermatologist is skilled at reading images |
| Multidisciplinary care: referral to a rheumatologist | Fluid, 2-way dialogue between dermatologists and rheumatologistsReferral to a rheumatologist is recommended if PsA is suspected and the patient has:• inflammatory or night pain involving the axial skeleton• peripheral inflammatory pain, joint swelling• enthesitis or clinical signs of enthesitis (especially in Achilles tendon and plantar fascia)• dactylitis or suggestive clinical signs |
| Treatment | • Bear in mind the different possible manifestations of PsA• Choose treatment according to clinical experience and safety and efficacy results from clinical trials |
Abbreviations: CRP, C-reactive protein; HLA, human leukocyte antigen; RF, rheumatoid factor; PSA, psoriatic arthritis; PURE-4, Psoriatic arthritis UnclutteRed screening Evaluation.
Screening tools. Although screening questionnaires are not useful for the early detection of PsA, the PURE-4 questionnaire, which was recently validated in Spanish, may be a feasible tool for the early detection of PsA by dermatologists in routine clinical practice.
Patient history and physical examination. Dermatologists should perform a targeted physical examination to look for signs of PsA at least once a year and ideally every 6 months. In particular, the key signs are inflammatory peripheral arthritis, enthesitis (Achilles tendon and plantar fascia), dactylitis, and axial inflammatory pain.
Additional tests. The use of biomarkers and imaging tests, in general, is not recommended by specialists. However, if PsA is suspected and the dermatologist decides to request laboratory tests, the experts consider that RF, CPR, and HLA-B27 could be useful. The experts also recommend requesting a radiograph of the most symptomatic joints and, if there is axial pain, the sacroiliac joints. MRI is recommended for patients with axial symptoms, and ultrasound may be useful for dermatologists who are skilled in reading images to identify musculoskeletal signs of PsA.
Multidisciplinary care and referral to a rheumatologist. Dermatologists should refer patients with axial or peripheral inflammatory pain, enthesitis, or dactylitis to a rheumatologist. Fluid communication between dermatology and rheumatology departments is essential for monitoring the status of referred patients, clarifying diagnostic doubts, and making joint treatment decisions. Finally, telemedicine could have a key role in the multidisciplinary management of patients with psoriasis, particularly in the context of the current COVID-19 pandemic.
Treatment. Treatment should be adapted to the different clinical manifestations of PsA and based on safety and efficacy findings from clinical trials and clinical experience.
FundingNovartis Farmacéutica S.A. funded the meetings and the writing and review of this manuscript.
Conflicts of InterestI. Belinchón has served as a consultant and/or speaker and/or participated in clinical trials sponsored by companies that manufacture drugs for the treatment of psoriasis, including Janssen, Almirall, Eli Lilly and Company, AbbVie, Novartis Pharmaceutica S.A., Celgene España S.L., Biogen Inc., Amgen, LEO Pharma, Pfizer-Wieth, UCB, and Merck Sharp & Dohme Española S.A.
L. Salgado-Boquete: AbbVie, Almirall, Celgene España S.L., Janssen, LEO Pharma, Eli Lilly and Company, Novartis, MSD, Pfizer, and Reig Jofre.
A. López-Ferrer: Novartis Farmacéutica S.A., Janssen, MSD, Eli Lilly and Company, Pfizer, Celgene España S.L., Almirall, LEO Pharma, AbbVie, and Amgen.
M. Ferran has served as a speaker and/or consultant and/or participated in clinical trials for Janssen, Eli Lilly and Company, Novartis, Pfizer, MSD, AbbVie, Celgene, and Almirall.
P. Coto-Segura has served on advisory committees and as a consultant, participated in clinical trials, and received grants and speaker and research support fees from the following pharmaceutical companies: AbbVie (Abbott), Janssen-Cilag, Novartis Farmacéutica S.A., Pfizer, MSD, UCB, Eli Lilly and Company, and Celgene España S.L.
R. Rivera has served as a consultant, researcher and/or speaker for AbbVie, Almirall, Celgene España S.L., GlaxoSmithKline, Janssen-Cilag, Lilly, LEO Pharma, MSD, Novartis, Pfizer, and UCB.
D. Vidal has served as a consultant, researcher, and/or speaker for AbbVie, Celgene España S.L., Eli Lilly and Company, Janssen, Novartis Farmacéutica S.A., Laboratorios Gebro Pharma S.A., LEO Pharma, and UCB.
L. Rodríguez has served as a consultant and speaker for AbbVie, Janssen, MSD, Pfizer-Wyeth, Novartis Farmacéutica S.A., Celgene España S.L., Almirall, Eli Lilly and Company, and LEO Pharma.
P. de la Cueva has served as a consultant and/or researcher, and/or speaker for AbbVie, Almirall, Astellas Pharma, Biogen Inc., Boehringer Ingelheim, Celgene España S.L., Janssen, LEO Pharma, Eli Lilly and Company, Merck Sharp & Dohme Española S.A., Novartis Farmacéutica S.A., Pfizer, and UCB.
R. Queiro has served as a consultant, researcher, and/or speaker for AbbVie, MSD, Pfizer, Novartis Farmacéutica S.A., Lilly, Janssen, UCB, and Celgene España S.L. and received unconditional research funds from AbbVie, Novartis Farmacéutica S.A., and Janssen.
The authors would like to thank Itsaso Cabezón Rodríguez (Ediciones Mayo, Spain) for support in writing and reviewing this manuscript.
Please cite this article as: Belinchón I, Salgado-Boquete L, López-Ferrer A, Ferran M, Coto-Segura P, Rivera R et al. El papel del dermatólogo en el diagnóstico precoz de la artritis psoriásica: recomendaciones de un grupo de expertos. Actas Dermosifiliogr. 2020;111:835–846.