Psoriasis is a chronic inflammatory disease that has been linked to increased cardiovascular risk. The glycoprotein clusterin (apolipoprotein J) is a component of high-density lipoproteins and has a protective role in atherosclerosis. The aim of the present study was to evaluate the plasma levels of clusterin and the proinflammatory cytokine macrophage migration inhibitory factor (MIF) in patients with severe psoriasis, comparing groups of patients with different risks of cardiovascular disease.
Material and methodsTwenty-one patients with severe psoriasis (psoriasis area severity index and body surface area>10) and 11 healthy controls with no dermatologic disease were studied. Cardiovascular risk factors were assessed according to the Adult Treatment Panel (ATP) III criteria. Subclinical carotid atheromatosis was assessed by Doppler ultrasonography of the carotid arteries. Plasma clusterin and MIF levels were measured by enzyme-linked immunosorbent assay.
ResultsATP-III criteria for metabolic syndrome were met by 47% of the patients, and 33% had carotid atheromatous plaque. Mean (SD) clusterin plasma levels were significantly lower in patients with psoriasis compared with controls (81.39 [27.30] μg/mL for the 21 patients vs 117 [21.6] μg/mL for the 11 controls; P=.0017). MIF plasma levels (ng/ml) were significantly higher in patients with atheromatous plaque compared with controls (53.22 [29.02] for the 6 patients with plaque vs 34.21 [9.65] for the 11 controls; P=.0394).
ConclusionsThe decreased plasma levels of clusterin in psoriatic patients suggested an association with the disease and might be an indicator of systemic inflammatory activity. Increased levels of MIF appear to be associated with cardiovascular risk factors and carotid atheromatous plaque.
La psoriasis es una enfermedad inflamatoria crónica que se ha asociado a un incremento del riesgo cardiovascular. La clusterina (apolipoproteína J) es un componente de las lipoproteínas de alta densidad (HDL) y tiene un papel protector de la ateroesclerosis. El objetivo del estudio ha sido evaluar la clusterina y el factor inhibitorio de la migración del macrófago (MIF) plasmáticos en pacientes con psoriasis grave comparando grupos de pacientes con distintos riesgos cardiovasculares asociados.
Material y métodosSe estudiaron 21 pacientes con psoriasis grave (Psoriasis Area Severity Index [PASI] y Body Surface Area [BSA]>10) y 11 controles sin enfermedad dermatológica. Se evaluaron los factores de riesgo cardiovascular según criterios del síndrome metabólico del Adult Treatment Panel III (ATP-III) y la ateromatosis carotídea subclínica mediante ecografía doppler de carótidas. La clusterina y MIF plasmáticos se midieron mediante Enzyme-Linked ImmunoSorbent Assay (ELISA).
ResultadosEl 47% de los pacientes con psoriasis presentaba criterios de síndrome metabólico y el 33% presentó placa de ateroma carotídea. Se observó una disminución significativa de la clusterina plasmática (μg/ml) en pacientes con psoriasis respecto a controles (81,39±27,30; n=21, versus 117±21,6, n=11; p=0,0017). El MIF plasmático (ng/ml) estaba aumentado significativamente en los pacientes con psoriasis y placa de ateroma carotídea respecto a los controles (53,22±29,02; n=6, versus 34,21±9,65; n=11; p=0,0394).
ConclusionesLa disminución de clusterina en pacientes con psoriasis sugiere una relación con la enfermedad y con la situación inflamatoria sistémica. El aumento de MIF en pacientes parece relacionarse con la presencia de factores de riesgo cardiovascular asociados y placa de ateroma carotídea.
Psoriasis is a chronic inflammatory disease of the skin that can also affect joints. Current evidence indicates that patients with psoriasis have an increased frequency of cardiovascular risk factors, metabolic syndrome, obesity, dyslipidemia, diabetes, and hypertension.1–4 Carotid intima-media thickness (CIMT) is considered a useful, sensitive marker of atherosclerosis, even when this is not clinically evident.5 Several studies have shown higher CIMT values in patients with psoriasis compared with controls.6,7
Clusterin, also known as apolipoprotein J, is a heterodimeric glycoprotein with a molecular weight of approximately 70 to 80 kDa. It is encoded by a single gene. It has several isoforms, which are referred to as nuclear or secreted, depending on their location. Secreted clusterin is the most common isoform. Clusterin is constitutively expressed in many mammalian tissues.8,9 It has been implicated in a wide range of biological processes, including regulation of apoptosis, attenuation of complement activation, response to damage and stress, autoimmune damage, elimination of toxic substrates, and interaction with lipids.10 More recently, it has been proposed as a possible biomarker of senescence and oxidative stress in erythrocytes11 and it has also been linked to diabetes and metabolic syndrome.12,13 Clusterin, like apolipoprotein A-I, is a component of high-density lipoproteins (HDLs). Evidence that it can bind lipids has led to the suggestion that it may have a protective role against atherosclerosis through its involvement in the transport of cholesterol to the liver. Low levels of circulating HDL-clusterin may also be significantly associated with metabolic syndrome. Yu et al.14 also recently reported that low plasma levels of clusterin in patients with Kawasaki disease appeared to be significantly associated with the presence of coronary artery lesions and suggested that this protein could be a useful biomarker for Kawasaki disease. Kujiraoka et al.,15 in turn, described significantly higher serum levels of clusterin in healthy individuals than in patients with diabetes, myocardial infarction, or coronary artery disease. Furthermore, underexpression of the clusterin gene in synovial tissue has been observed in patients with rheumatoid arthritis (an autoimmune chronic inflammatory disease) compared with patients with osteoarthritis and healthy individuals.16
Migration inhibitory factor (MIF) is a proinflammatory cytokine, identified in activated T cells, that inhibits the migration of macrophages in vitro. The monomeric form of MIF has a molecular weight of approximately 12.5 kDa and its active form is homotrimeric. MIF is produced by a wide variety of cells, including monocytes and macrophages, T and B cells, and endocrine, endothelial, and epithelial cells.17 It has been implicated in innate and acquired immune responses, immune regulation, and inflammation.17–19 MIF regulates macrophage function by suppressing the anti-inflammatory effects of corticosteroids,18 and has been reported to favor the development and progression of autoimmune diseases such as rheumatoid arthritis.18 It has also been implicated in the clonal expansion and increased survival of inflammatory cells, promoting migration and stimulating the production of inflammatory mediators such as interleukin (IL) 1, tumor necrosis factor α, IL-6, IL-17, and nitric oxide.18,19 Increased levels of MIF have been described in patients with systemic lupus erythematosus,20,21 systemic scleroderma, Wegener granulomatosis, atopic dermatitis, psoriasis,22,23 and inflammatory diseases in general.24 This cytokine has also been associated with cardiovascular disease, type 2 diabetes mellitus, metabolic syndrome, and atherosclerosis.24 It has also been postulated that MIF might have a role in various skin disorders and in wound healing time.25
Clusterin levels have not been analyzed in patients with psoriasis. The aim of this study was to evaluate plasma levels of clusterin and MIF in patients with severe psoriasis and to compare results in patients with different levels of cardiovascular risk.
Materials and MethodsPatientsWe studied 21 patients with severe psoriasis diagnosed on the basis of clinical criteria.26 The inclusion criteria were age of over 18 years and the presence of severe plaque psoriasis (Psoriasis Area Severity Index [PASI] and body surface area [BSA] scores of > 10). The patients were consecutively enrolled at the dermatology office of Hospital Clínico Universitario San Cecilio in Granada, Spain. During the same period, 11 unmatched healthy volunteers, with no skin disorders, were enrolled as controls. The exclusion criteria for both patients and controls were the presence of cutaneous lymphomas or other cancers and established cardiovascular disease (myocardial infarction or stroke). The study protocol was approved by the research commissions and ethics committees of Hospital Clínico Universitario San Cecilio and Instituto de Parasitología y Biomedicina “López-Neyra”, which belongs to the Spanish National Research Council (CSIC). All the patients and controls signed an informed consent form in accordance with the principles of the Declaration of Helsinki.26
Clinical and Laboratory VariablesPsoriasis severity was determined by PASI and BSA scores. The following variables were recorded for each patient: weight, height, abdominal circumference, body mass index (kg/m2), and systolic and diastolic blood pressures (mean of 3 readings taken after 10minutes of rest). Serum levels of HDL cholesterol (HDL-C) and triglycerides were measured in patients and controls. A diagnosis of metabolic syndrome was established using the Adult Treatment Panel III (ATP-III) criteria, whereby patients are considered to have metabolic syndrome if they have 3 or more of the following: abdominal circumference >102cm in men and >80cm in women, serum triglycerides >150mg/dL, HDL-C <40mg/dL in men and <50mg/dL in women, blood pressure >130/85mm Hg, and fasting glucose >110mg/dL.27
Blood and Plasma SamplesBlood samples were collected following standard procedures (K2 EDTA tubes, Vacutainer BD system; BD Diagnostics) in the hospital laboratory. Plasma was obtained by Ficoll gradient centrifugation,26 and the aliquots were stored at –80°C until their analysis.
Plasma Clusterin and MIF LevelsPlasma levels of clusterin and MIF were measured in patients and controls by enzyme-linked immunosorbent assay (ELISA), which was performed in duplicate using the corresponding ELISA kits (No. RD194034200R, BioVendor and No. DMF00B; R&D Systems, respectively), in accordance with the manufacturers’ instructions.
UltrasoundPatients underwent color Doppler ultrasound examination of the carotid artery (10-5MHz transducer with imaging software to visualize the supra-aortic vessels), with evaluation of flow (mL/s) and CIMT (mm). CIMT was measured by calculating the distance from the inner hyperechogenic line of the vessel (intima) to the outer hypoechogenic line (media). Carotid atheromatous plaque was considered to be present when the CIMT was greater than 1.5cm. CIMT was measured on the posterior wall of the common carotid artery, 1cm proximal to the bifurcation.5
Statistic AnalysisData were expressed as means (SD). The nonparametric Mann-Whitney U test was used to analyze between-group differences, while the Fisher test was used to analyze qualitative variables. Correlations were calculated using the nonparametric Spearman rank correlation coefficient. Results were considered to be statistically different at a P value of less than .05 and to be close to significance up to P≤.1 (GraphPad Prism version 5.01; GraphPad Software, Inc).
ResultsClinical Data and Lipid ProfilesOf the 21 patients studied, 9 (43%) were men (age, 44.2 [7.4] years) and 12 (57%) were women (age, 43.3 [19.1] years).26 In the control group, there were 5 men (45%) and 6 women (age, 56.2 [15.5] and 52.0 [13.9] years, respectively). No significant differences were observed between patients and controls for sex (P=1.0, Fisher test) or age (P=.059 [men] and P=.303 [women], Mann-Whitney U test).26 All the patients and controls were white. The mean PASI and BSA scores in the patient group were 17.24 (10.42) and 14.33 (6.06), respectively, and the mean CIMT was 0.743 (0.21).26 Ten patients (47.62%) met the ATP-III criteria for metabolic syndrome and 7 (33.3%) had carotid plaque. CIMT values were significantly higher in patients with plaque (n=7) than in those without (n=14) (0.97 [0.17]mm vs 0.63 [0.11] mm], P=.001).26 At the time of blood collection, only 12 of the 21 patients were receiving treatment (methotrexate in 10 cases and acitretin in 2). None of the patients were on ciclosporin, corticosteroids, or biologic therapy.26
HDL-C levels were significantly lower in patients with metabolic syndrome or carotid plaque than in controls (Table 1). There were no significant differences between patients (n=21) and controls (n=7) for triglycerides, (144.4 [98.7] vs 123.7[7.04]; P=.791) or for HDL-C (45.8 [13.5] vs 54 [4.3]; P=.124).26 It should be noted that our results might have been influenced by the dispersion of data and by our small sample size.
Serum Triglyceride and High-Density Lipoprotein Cholesterol (HDL-C) Levels.
| Patients | Patients | Controls | |||
|---|---|---|---|---|---|
| MetSyn (+) (n=10) | MetSyn (–) (n=11) | CAP (+) (n=7) | CAP (–) (n=14) | (n=7)a | |
| Triglycerides,b mean (SD), mg/dL | 182,9 (13.02) | 109.5 (37.51) | 197.7 (152.6) | 117.8 (44.23) | 123.7 (7.04) |
| P valuec | .187 | .469 | .201 | .737 | |
| HDL-C, mean (SD), mg/dL | 37.5 (10.88) | 53.27 (11.23) | 39.14 (10.88) | 49.07 (13.75) | 53.86 (4.26) |
| P valuec | .001 | .751 | .004 | .654 | |
Abbreviations: CAP, carotid atheromatous plaque; MetSyn, metabolic syndrome.
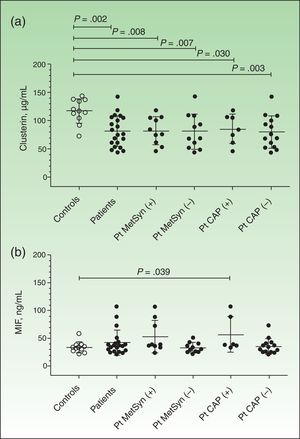
Plasma clusterin levels were significantly lower in patients (n=21) than in controls (n=11) (81.39 [27.30] μg/mL vs 117 [21.6] μg/mL, P=.002) (Fig. 1A). The difference remained significant when the results were analyzed according to the concomitant presence of metabolic syndrome or carotid plaque (Fig. 1A). However, no significant differences were observed between patients with metabolic syndrome (n=10) and those without (n=11) (85.45 [24.39] μg/mL vs 80.43 [30.88] μg/mL, P=.751). Similar results were seen when the patients were analyzed according to the presence (n=7) or absence of carotid plaque (n=14) (85.03 [26.1] μg/mL vs 79.58 [28.66] μg/mL, P=.628), and according to whether the patients were receiving treatment (n=12) or not (n=9) (85.84 [28.48] μg/mL vs 75.47 [26.05] μg/mL, P=.414). Finally, no significant correlations were observed between plasma clusterin levels and the different variables analyzed (age, blood glucose, PASI, BSA, HDL-C, and triglycerides).
Plasma levels of clusterin and macrophage migration inhibitory factor (MIF). Results are expressed as means (SD). Levels were measured by enzyme-linked immunosorbent assay, as described in the Material and Methods section. A, Plasma clusterin levels. B, Plasma MIF levels. Between-group differences were analyzed using the nonparametric Mann-Whitney U test Results were analyzed using GraphPad Prism, version 5.01. Pt indicates patients; MetSyn, metabolic syndrome; CAP, carotid atheromatous plaque.
Plasma MIF concentrations were higher in patients (n=20 because 1 out-of-range result was excluded from the analysis) than in controls (n=11), but the differences were not significant (42.19 [22.39] vs 34.21 [9.654], P=.171) (Fig. 1B). MIF concentrations were, however, significantly higher in controls than in patients with carotid plaque (57.30 [32.11], P=.039) and showed a tendency towards significance in patients with metabolic syndrome (53.22 [29.02]) compared with controls (P=.058). No significant correlations were observed between plasma MIF levels and age, blood glucose, PASI, BSA, HDL-C, or triglycerides.
DiscussionThe main finding of the present study is that plasma clusterin levels were decreased in patients with psoriasis compared with controls. It has been postulated that clusterin, like other components of HDL, may have a protective role against atherosclerosis in humans through its involvement in the transport of circulating cholesterol to the liver. Yuet al.14 recently reported that low plasma clusterin levels were significantly associated with the presence of coronary artery lesions in patients with Kawasaki disease. Clusterin is a heterodimeric glycoprotein and may be sequestered by HDL, of which it is a component. In our series, patients with psoriasis and either metabolic syndrome or carotid plaque had significantly lower levels of HDL-C than controls (Table 1). Low HDL-C levels, which are one of the hallmarks of metabolic syndrome, have also been described in patients with coronary aneurysms.29,30 Furthermore, we observed no differences in clusterin levels between patients with metabolic syndrome and those with carotid plaque, or any correlation between clusterin and HDL-C levels in patients with psoriasis.
MIF plays a key role in various serious chronic inflammatory diseases (e.g., autoimmune and metabolic disorders) and other disease states,17,19,24 and significantly increased serum levels have been described in patients with psoriasis compared with controls.23 In our series, plasma MIF levels were significantly increased in patients with psoriasis and carotid plaque (Fig. 1B). Recent findings reported by our group indicate that there may be an association between psoriasis, carotid plaque, and metabolic syndrome.28
In another study by our group, we observed that patients with severe psoriasis had significantly increased expression of the toll-like receptor (TLR) genes TLR4 and TLR2 in peripheral blood mononuclear cells.26 The detection of correlations between TLR4 and TLR2 overexpression and regulatory and proinflammatory cytokines and acute phase proteins such as S100A9 highlights the role played by the innate immune response in psoriasis. We also observed significantly elevated gene expression of helper T (Th) cells with elevated Th1/Th2 and Th17/Th2, ratios, indicating activation of Th1 and Th17 cells. Furthermore, systemic inflammation in patients with psoriasis may give rise to or aggravate other associated inflammatory diseases such as atherosclerosis.1–3,28
Despite the limitations of the present study, we obtained significant results that suggest that there are certain biologic functions and associations of clusterin and MIF that warrant further study. Our patient and control groups were small and we did not perform a prior sample size calculation as our aim was to conduct a pilot study and there are no published data on clusterin and MIF levels in psoriasis patients. The preliminary results reported in this article should be validated in studies with larger samples.
In conclusion, our observation of decreased plasma levels of clusterin in patients with severe psoriasis suggests that clusterin is associated with psoriasis and also possibly with a systemic inflammatory state. Furthermore, increased plasma levels of MIF in psoriasis patients appear to be associated with the presence of cardiovascular risk factors (carotid plaques). Plasma measurements of both clusterin and MIF could contribute to a better understanding of systemic inflammation in psoriasis.
Ethical DisclosuresProtection of humans and animals, The authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
FundingEuropean Social Fund (European-Commission–European Regional Development Fund [ERDF]); Instituto de Carlos III Health Research Fund (ISCIII-FIS06/1502) (MZ); Spanish National Research Council (CSIC-PI 200820I216) (MZ); Junta de Andalucía, Departments of Innovation-Science-Business and Education-Science (CVI 226, CVI 908/2006 y PC08-CTS-04046) (JS and MZ); Ministry of Education-Ministry of Science and Education (SAF-2008-03685) (JS and MZ) and SAF-2011- 27261 (JS); and CSIC JAE-Doc program-CSIC-ERDF (contract SGR).
Conflicts of InterestThe authors declare that they have no conflicts of interest.