Topical therapy is key to the successful management of psoriasis, and patient adherence to treatment contributes to its effectiveness in the long-term.
ObjectivesTo establish consensus on adherence to topical treatment in psoriasis, draw up recommendations on how adherence could be improved, and evaluate the properties of the main vehicles used.
MethodWe designed a questionnaire on adherence to topical treatments in psoriasis and another on the properties of the main vehicles used; the 2 questionnaires were evaluated using the Delphi method by a panel of experts and members of the Psoriasis Group of the Spanish Academy of Dermatology and Venereology, respectively.
ResultsConsensus was reached on the following statements: a) treatment adherence increases the effectiveness of topical treatments in psoriasis; b) to improve adherence, it is necessary to improve communication between patients and health care staff, provide written instructions, and simplify treatment with easy-to-use, pleasant products that are preferably applied only once a day; c) treatment satisfaction increases adherence and tends to improve the health-related quality of life of the patient. Ointment was rated the worst vehicle, while foams and solutions were rated the best. Creams and lipophilic gels were considered to be better than ointment in several respects.
ConclusionTo improve adherence to topical regimens in psoriasis and the effectiveness of such therapy, we need to give patients more information, simplify treatment regimens, and prescribe easy-to-use products that will ensure satisfaction.
El tratamiento tópico es fundamental en el manejo de la psoriasis y la adherencia al mismo contribuye a su eficacia en el tratamiento prolongado.
ObjetivosEstablecer un consenso sobre la adherencia al tratamiento tópico en la psoriasis y recomendaciones para mejorarla, así como evaluar las características de los diferentes vehículos.
MétodoSe elaboró un cuestionario sobre adherencia al tratamiento tópico de la psoriasis que se sometió a Consenso Delphi por un panel de expertos, al igual que un cuestionario sobre las características de los principales vehículos utilizados, que también fue sometido a consenso por los miembros del Grupo de Psoriasis de la Academia Española de Dermatología y Venereología.
ResultadosSe alcanzó consenso en que: a) la adherencia al tratamiento tópico aumenta su eficacia en la psoriasis; b) para aumentar la adherencia es necesario mejorar la comunicación con el personal sanitario, proporcionar instrucciones escritas, simplificar el tratamiento, con preparados de aplicación cómoda, preferiblemente diaria, y agradables; y c) la satisfacción con el tratamiento aumenta la adherencia y tiende a mejorar la calidad de vida relacionada con la salud de los pacientes. La pomada fue el vehículo peor valorado, mientras que los mejor considerados fueron las espumas y las soluciones. Las cremas y el gel lipofílico tuvieron valoraciones superiores a la pomada en diversos parámetros.
ConclusiónPara aumentar la adherencia al tratamiento tópico y la eficacia del mismo en la psoriasis hay que proporcionar más información, simplificar el tratamiento y prescribir preparados cómodos de aplicar y que aseguren la satisfacción del paciente.
Psoriasis has an estimated prevalence of 1.4% in the Spanish population and is one of the most common reasons why patients consult a dermatologist; approximately 70% of psoriasis patients, generally those who have mild to moderate disease, continue to be treated with topical therapy alone.1
Patients consider topical therapy to be one of the most negative aspects of this disease and their satisfaction is significantly lower with this form of therapy than with oral or parenteral treatments (injections or infusions).2 At this time, however, topical therapy remains a pillar of psoriasis management.1 Adherence to topical therapy (which implies a voluntary decision to continue to apply the medication rather than merely comply with the physician's prescription) is probably the determining factor in ensuring efficacy in long-term use (initially for several weeks and later for as-needed or maintenance therapy).
Adherence can be improved in a variety of ways: through greater interaction between the patient and health care givers (physicians or nurses) and by providing more information about the disease and its management, simplifying therapy, giving written instructions, and establishing ways to contact the nurse or physician.3 These measures also increase patient satisfaction.3
However, the most important factors related to satisfaction with topical therapy and adherence are probably the following: objective, evidence-based efficacy; ease and convenience of application (the less often, the better); and acceptance of the physical properties of the formulation, particularly of the vehicle. Other factors may intervene in forming the patient's opinion of the product. Personal characteristics are relevant (psychological traits, type of clothing worn, etc.), and climate is also an issue.3 Although oil-based vehicles, for example, may stain clothing when they are spread on the skin and liquify in warm conditions, they may be preferred in cool climates or in winter. Cultural contexts, with all their variety, also play a role.3 The best excipient, or vehicle, is the one the patient prefers and can use with greatest satisfaction.
The aims of this Delphi study by dermatologists who are experts in psoriasis was to reach a consensus about adherence to topical therapy and make recommendations to improve it. The study also aimed to evaluate the perceived physical qualities and ease of use of topical formulations available in the Spanish market, including their different vehicles and presentations.
MethodsThe Delphi process4 began with the creation of a panel of 10 experts in psoriasis (the authors of this article). In April 2012 the panel drafted 14 questionnaire items in the form of statements related to the vehicles used in topical treatments for psoriasis and the factors that influence effectiveness and adherence (Table 1). Nine other items were presented in the form of a list of physical properties of topical preparations and the ease and convenience of application of the 8 vehicles that are most often used. Nine members of the panel (excluding the coordinator) then responded to the questionnaire online, using a Likert-type scale with a range of values between 1 (strongly disagree) and 7 (strongly agree). An item was considered to have gained positive consensus (agreement with the statement) when at least 75% of the respondents (7 of the 9 panelists) marked 6 or 7 on the Likert scale (25th and 75th percentiles, both ≥6). An item was considered to have elicited negative consensus (disagreement with the statement) when at least 75% of the respondents (7 of the 9 panelists) marked 1 or 2 on the Likert scale (25th and 75th percentiles, both ≤2). During the annual conference of the Spanish Academy of Dermatology and Venereology (AEDV) in Oviedo, on May 7, 2012, the panelists revised the statements in the questionnaire and again responded anonymously online.
Statements Related to the Use of Different Vehicles in Topical Treatments for Psoriasis and Factors That Affect Efficacy and Adherence: Items in the Delphi Consensus Exercisea
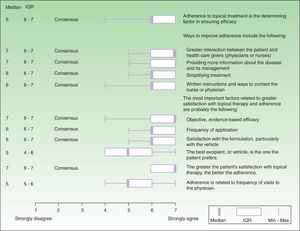
| Adherence to topical treatment (meaning the patient's voluntary decision to continue to apply the medication rather than merely comply with the physician's prescription) is probably the determining factor in ensuring efficacy in long-term use (initially for several weeks and later for as-needed or maintenance therapy). |
| Ways to improve adherence include the following: |
| 1) Greater interaction between the patient and health care givers (physicians or nurses) |
| 2) Providing more information about the disease and its management |
| 3) Simplifying treatment |
| 4) Provision of written instructions and ways to contact the nurse or physician, which leads to greater patient satisfaction |
| The most important factors related to greater satisfaction with topical therapy and adherence are probably the following: |
| 1) Objective, evidence-based efficacy |
| 2) Ease and convenience of application (the less often, the better) |
| 3) Satisfaction with the physical properties of the formulation, particularly of the vehicle |
| The best excipient, or vehicle, is the one the patient prefers and can use with the greatest satisfaction. |
| The greater the patient's satisfaction with topical therapy, the better the adherence, which tends to improve health-related quality of life. |
| Attempting to use topical treatments over large areas with multiple lesions is doomed to failure. |
| Tachyphylaxis, or loss of response after long-term use of topical corticosteroids is a real problem in clinical practice |
| If loss of response does occur with long-term use, it might be because of poor adherence in many cases. |
| Adherence to therapy is partly dependent on age and sex. |
| Adherence is related to frequency of visits to the physician. |
Ninety dermatologists who are members of the Psoriasis Group of the AEDV and work in different areas of Spain were later asked to give responses on items referring to the properties of the various vehicles (presented in list form). These dermatologists were also sent a list of 95 topical medications routinely used to treat psoriasis and asked to describe the physical properties of the ones they prescribed and how they were used. The same statistical criteria for positive or negative consensus described above were also applied in this part of the survey.
Among the attributes proposed for consideration were the following positive ones: ease of application, good absorption, and a pleasant consistency. The negative attributes proposed were the following: causes stains, oily consistency, unpleasant smell, causes itching or burning, leaves residue on hands after application, and time-consuming application.
Descriptive statistics were compiled. The median and interquartile range are reported along with the actual range (minimum and maximum scores). The Kruskal-Wallis test was used to analyze differences between scores for different vehicles. The Mann-Whitney U test was used to compare differences between the responses of the expert panel and participants from the AEDV's Psoriasis Group. Two-tailed tests (α=.05) were used.
ResultsIn the first part of the questionnaire the dermatologists on the expert panel answered general questions about adherence and satisfaction with topical therapy for psoriasis in their routine practice. In the second round for that part of the questionnaire, a positive Delphi consensus was achieved on 9 items (Fig. 1) related to the participants’ observations of routine clinical practice:
- 1.
Adherence to topical therapy is probably the determining factor in ensuring efficacy in long-term use.
- 2.
Ways to improve adherence include the following: greater interaction between the patient and health care givers (physicians or nurses), more information about the disease and its management, simplifying treatment, and providing written instructions and ways to contact the nurse or physician.
- 3.
The most important factors related to greater satisfaction with topical therapy and adherence were the following: a) efficacy, b) application frequency, and c) satisfaction with the physical properties of the formulation, especially the vehicle.
- 4.
The greater the patient's satisfaction with topical therapy, the better the adherence, which tends to improve health-related quality of life.
Expert panel's Delphi consensus on factors that affect adherence to topical therapy and patient satisfaction, showing the medians, interquartile ranges (IQR), and actual ranges (minimum [Min] and maximum [Max] scores on a scale of 1 to 7). The 9 panelists did not reach a consensus on the following items:
- Attempting to use topical therapy to manage large areas of affected skin, with multiple lesions, is doomed to failure.
- Loss of response after long-term use of topical corticosteroids is a real problem in clinical practice
- Adherence to therapy is partly dependent on age and sex.
The responses for items referring to the physical properties and use of products with different vehicles are shown in Table 2. The expert panel reached consensus on 20 items in this section. The panel expressed the highest level of agreement on formulations in solutions, foams and lotions, and although the criteria for consensus were not reached on ointments, 6 of the 9 experts did agree that they are not easy to apply (median, 6 [2-3]), are not well absorbed (median 2 [2-4), and are oily (median, 6 [5-6]).
Expert Panel and Psoriasis Group Dermatologists’ Evaluations of the Physical Properties and Use of Vehicles in Topical Treatments of Psoriasisa
| Product | No. of Responses | Ease of Application | Absorbs Well | Causes Stains | Oily Consistency | Smells Bad | Pleasant | Causes Itching, Burning | Leaves a Residue on Hands | Application Is Time-Consuming |
|---|---|---|---|---|---|---|---|---|---|---|
| Ointments | ||||||||||
| Expert panel | 9 | 2 (2–3) | 2 (2–4) | 6 (5–6) | 6 (5–6) | 3 (2–4) | 2 (2–3) | 2 (2–2) | 6 (5–6) | 5 (4–6) |
| Psoriasis Group | 26 | 2.5 (2–5) | 4 (3–5) | 4 (3–5) | 5 (5–6) | 2 (1–3) | 3 (2–4) | 2 (2–3) | 4 (3–5) | 4 (2.2–5) |
| Creams | ||||||||||
| Expert panel | 9 | 4 (4–6) | 4 (4–5) | 3 (3–5) | 4 (3–5) | 2 (2–4) | 5 (4–5) | 2 (2–2) | 4 (3–4) | 4 (3–4) |
| Psoriasis Group | 35 | 5 (4.6–6) | 5 (4–6) | 2 (1–3) | 2.5 (1–4) | 1 (1–2) | 5 (2–6) | 2 (1–2.75) | 2 (1–3) | 2 (2–3.75) |
| Lipophilic gels | ||||||||||
| Expert panel | 9 | 5 (5–6) | 6 (5–6) | 3 (3–4) | 3 (2–4) | 2 (1–3) | 5 (4–6) | 2 (1–2) | 3 (2–4) | 3 (2–3) |
| Psoriasis Group | 14 | 5 (5–6.75) | 6 (5–6) | 2 (2–3) | 3 (2–4) | 1 (1–2) | 5 (4–6) | 2 (1–2) | 2 (1.25–3) | 2 (2–3.75) |
| Foams | ||||||||||
| Expert panel | 9 | 6 (6–6) | 6 (6–7) | 1 (1–1) | 1 (1–1) | 1 (1–2) | 5 (5–6) | 2 (1–4) | 2 (1–2) | 2 (1–2) |
| Psoriasis Group | 8 | 6 (6–7) | 6.5 (6–7) | 2 (1–2.25) | 2 (1–2) | 1 (1–2) | 5 (2.75–6) | 2.5 (1–4.25) | 1.5 (1–2) | 1.5 (1–2.2) |
| Solutions in suspension/lotions | ||||||||||
| Expert panel | 9 | 6 (5.5–6.5) | 6 (6–6.5) | 2 (1.5–3) | 1.5 (1.5–2) | 2 (1–3) | 5.5 (4–6) | 3 (2–3.5) | 2 (1–2) | 2 (1–3) |
| Psoriasis Group | 11 | 6 (5–6.5) | 6 (5–6) | 2 (1.25–3) | 2 (1.25–3) | 2 (1–3) | 6 (2–6) | 4 (2–5) | 2 (1–2) | 2 (2–3) |
| Emulsions | ||||||||||
| Expert panel | 9 | 5 (5–6) | 5 (4–6) | 3 (2–3) | 3 (2–3) | 2 (1–4) | 5 (4–5) | 2 (2–2) | 3 (2–3) | 3 (3–3) |
| Psoriasis Group | 8 | 7 (6–7) | 7 (5.7–7) | 1.5 (1–2) | 1 (1–2.25) | 1 (1–2) | 6 (5–6) | 1 (1–2) | 2 (1–3) | 2 (1.7–5) |
Data are shown as median (interquartile range). Variables in bold face are those for which consensus was reached, as shown by responses of 6–7 or 1–2 given by 75% of the respondents in each group or more; that is to say, both the 25th and 75th percentiles were ≥6 or ≤2, respectively. Lotions and solutions in suspension were assessed together.
The panel's assessments of the various vehicles showed significant differences with respect to the following attributes: ease of application (P<.0001), absorption (P<.0001), tendency to cause stains (P<.0001), oily consistency (P<.0001), residue left on skin (P<.0001), and time required for application (P=.0011) based on a multiple-comparison statistical procedure. Specifically, ointments had significantly lower evaluations than emulsions and solutions or lotions on all the previously mentioned attributes; ointments were also considered worse than creams because of their tendency to cause stains and their oily consistency, and worse than lipophilic gels with respect to ease of application and absorption.
Thirty-seven of the 90 members of the Psoriasis Group also completed this section of the questionnaire (response rate, 41%). Like members of the expert panel, the larger group of dermatologists thought ointments had significantly less appealing physical properties than the other vehicles (P<.05). No significant differences in the group's views of the other vehicles were observed.
The Psoriasis Group respondents expressed a greater range of opinions than the expert panel concerning the physical properties of the vehicles in the topical treatments they routinely prescribed.
No differences were observed between the opinions of the 2 groups as regards ease of application, except in the case of emulsions, about which the Psoriasis Group informants expressed more favorable opinions than the expert panel (median, 5 [5-6] vs. 7 [6-7], respectively; P=.046) (Table 2). The expert panel tended to have lower opinions of ointments than the Psoriasis Group dermatologists, but there was greater agreement with regard to the attributes of lotions and lipophilic gels. Between the 2 groups, the greatest differences in opinion concerned emulsions and creams.
DiscussionTopical therapy is the mainstay of the management of mild psoriasis, defined by the National Psoriasis Foundation as involving less than 3% of the body surface.5 Some 80% of patients with psoriasis have this form. Topical therapy is also a fundamental resource in the management of moderate forms of the disease, as shown by the fact that 70% of patients with mild to moderate disease use only this form of treatment.2 In patients with very severe psoriasis, topical therapy has also been used in some studies to encourage faster clearing of lesions or to enable prescription of a lower dose of a systemic drug.6
Moisturizers, humectants, and emollients are essential components of topical treatments and although they are considered secondary active ingredients, their beneficial effects when used alone have been attributed to their ability to restore hydration and the water barrier function to the area of the psoriatic plaque.7 This effect has been confirmed in studies showing that application of the vehicle alone can reduce the psoriasis area and severity index (PASI) by around 20%.7,8
Topical corticosteroids and vitamin D analogs, alone or in combination, are presently the principal evidence-based topical treatments for psoriasis.9 Ointments are conventionally believed to be more effective than other vehicles in plaque psoriasis because lesions are dry and scaly. Ointments facilitate hydration and repair of the skin's barrier function. This potential benefit, however, is not always observed in routine practice, very possibly because the physical properties of ointments interfere with adherence, as suggested by patients’ expression of a preference for other formulations.10 When a series of 150 German, Spanish, and British patients were interviewed to elicit their opinions on the application of 3 excipients, the cream and the gel received more positive evaluations for the item “stickiness” and the ointment was considered the oiliest of the 3 products.11 A product with fixed doses of calcipotriol and betamethasone dipropionate in a new vehicle (a lipophilic gel) has been developed to improve patient acceptance of and adherence to therapy.12
Well designed trials comparing formulations of the same active ingredients in different vehicles are lacking. Since psoriasis is a chronic disease, it is likely that trials lasting only 1 or 2 months would not reflect the real efficacy of a preparation in routine use. One study, however, compared 4 formulations applied to a test plaque.13 Two of the products contained calcipotriol plus betamethasone (one in an ointment and the other in a lipophilic gel), and 2 contained only calcipotriol (one in a cream and the other in a lipophilic gel). This trial showed that both the formulations with the drug combination had a comparable antipsoriatic effect.
The Spanish Psoriasis Group's consensus statements on topical therapy in plaque psoriasis1 and scalp psoriasis14 specify topical corticosteroids and vitamin D analogs—alone or in combination—as first-line choices during the induction phase. These statements place considerable emphasis on the importance of the formulation used to manage scalp psoriasis.
The variety of topical treatments available reflects the difficulty of finding the ideal vehicle for ensuring patient adherence and satisfaction with a product's convenience or pleasantness of application as well as efficacy.15 As a result, the physician must take care that a prescribed therapy be not only effective but also have satisfactory aesthetic attributes and minimal local adverse effects.
The success of topical therapy in psoriasis depends on a high level of patient motivation and cooperation, a requirement considered one of this disease's problematic aspects.3 In fact, poor adherence places a major constraint on therapeutic efficacy. Nearly 50% of patients with psoriasis do not even purchase the prescribed product,16 and up to 70% do not use their medication according to instructions,17 which are often inadequate, confusing or difficult to follow.18 The main reasons patients give to explain their nonadherence to topical corticosteroid therapy have been found to be frustration with the medication's efficacy, inconvenience, and fear of possible adverse effects.19 Paradoxically, adherence is lower when the physical and emotional effects of the disease are greater,20,21 even though severity ought to increase motivation. A vicious circle is created as a result of a patient's feelings of hopelessness, helplessness and depression. On the other hand, the greater the patient's satisfaction with topical therapy, the better the adherence, which tends to improve health-related quality of life.22
Attempting to use topical therapy to manage large areas of affected skin, with multiple lesions, is evidently impractical.3 That many clinical trials of topical treatments use PASI goals as a measure of efficacy is surprising: the floor effect of the PASI and its low sensitivity in patients with psoriasis on less than 10% of the skin surface, corresponding to a low PASI score (<5), make this index inappropriate for assessing change in this context.23
Tachyphylaxis, or loss of response after long-term use of topical corticosteroids, is assumed by many practicing dermatologists. Such loss has not been demonstrated in regular applications for 12 weeks,24 however, and when tachyphylaxis does occur, it may be a consequence of poor adherence in many cases.
One clinical trial found that adherence decreased as much as 40% over the course of only 8 weeks of therapy, even in a context in which patients knew their medication containers were fitted with electronic monitoring caps.25 Presumably, the rate of adherence in routine clinical contexts is even lower. The same study found that adherence was associated with patient age and female sex (the rate being 5% higher in women) and that more medication gaps tended to occur on weekends. Adherence is also known to increase on the days before a clinical visit26,27; real adherence is probably lower.
Several recently published systematic reviews of the literature on adherence to psoriasis treatment (usually with systemic agents) have demonstrated both the importance of psychosocial factors28 and the methodological limitations of published studies29; recommendations for future research in this area have been made.30
Four factors have been linked to poor adherence to topical psoriasis therapy.31 The first is patient dissatisfaction with efficacy, which leads to frustration, a feeling that is further influenced by the chronic, incurable nature of the disease. Appropriate patient education about psoriasis may therefore be very important. The second is the development of such adverse effects as irritation, atrophy, telangiectasias and striae, or else the fear of these effects. Corticophobia is more marked in the treatment of atopic dermatitis and in pediatrics, but patient education about treatment regimens is also essential in psoriasis if risk is to be minimized. The third is a therapeutic regimen's complexity and inconvenience. Problems begin with an aspect as basic as dosing (approximately 500mg of the active ingredient will fit on the volar aspect of a finger tip, providing a sufficient amount to spread over an area that could be covered by 2 hands). Other problematic aspects concern the appropriate way to eliminate residues with soap or water, combining treatments (short-term dithranol or tar derivatives and corticosteroids), and the use of occlusive methods. The fourth and possibly most important factor affecting adherence is a patient's preference for one vehicle over another. Product formulations should allow the active ingredient to be spread easily over the affected area. Patients reject products that feel dirty or have an unpleasant smell as well as those that are difficult or time-consuming to apply. They ask for a medication that works fast and spreads easily.
Programs to educate patients are clearly necessary and should include techniques for applying medications, information on the rarity of adverse effects, and ways to minimize the risk of such effects. We continue to need new formulations with low oil content, given that the oil component liquifies in hot climates (the main reason for stained clothes and bed linen). In addition to developing more potent products, the pharmaceutical industry should make an effort to develop new vehicles that have better physical properties and are more convenient to use. Foam and gel preparations represent a market gap that remains to be filled, once it has been demonstrated that greater potency does not necessarily require occlusion of the lesion or an oil-based excipient (an ointment), as has traditionally been assumed.32
This article summarizes the opinions of dermatologists who are specialists in the treatment of psoriasis. Additionally, it offers consensus-based definitions and practical recommendations for improving the psoriasis patient's adherence to topical therapy and, therefore, ways to improve treatment efficacy (Table 3).
Summary of the Delphi Consensus-Based Practice Guidelines for Improving Adherence to Topical Therapy for Psoriasis.
| 1 Adherence to topical therapy for psoriasis increases its efficacy. |
| 2 The following actions are necessary for improving adherence: |
| a) Enhance interaction between the patient and the health care giver. |
| b) Give better information about the nature of the disease and its management. |
| c) Simplify the therapy. |
| d) Provide written instructions and ways to contact the nurse or physician. |
| e) Prescribe evidence-based therapy. |
| f) Prescribe products that are easy to apply conveniently (preferably once a day). |
| g) Make sure the patient is satisfied with the physical properties of the formulation and the vehicle. (Ointments were least liked by the expert panel and the Spanish Psoriasis Group respondents. The differences between the evaluations of ointments and the other vehicles were statistically significant.) |
| 3 The greater the patient's satisfaction with topical therapy, the better the adherence, which tends to improve health-related quality of life. |
The results of this Delphi consensus process indicate the need to increase adherence to topical therapy in psoriasis, improve the information provided to patients, and improve their satisfaction with the physical properties and ease of application of existing medications. We believe it would be useful to have more information about the degree of adherence to topical therapy. Such information should take into consideration demographic and psychosocial variables that have been linked to adherence. In the eventual development of new preparations, patient opinions about the physical properties of the products and users’ degree of satisfaction should be consulted.
In summary, consensus-based recommendations for the use of topical therapy for psoriasis in Spain as well as the present study lead us to conclude that alternatives must be found for ointments. Our conclusion is based on patients’ opinions of these formulations and our finding that dermatologists gave ointments the lowest scores on all attributes in the present study. Alternatives are needed in order to improve adherence as much as possible.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no private patient data are disclosed in this article.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
FundingLaboratorios LEO Pharma, S.A. sponsored the Delphi process that led to this consensus statement by funding the online questionnaire (Bioclever), the meetings, and telephone costs. The sponsor also paid consulting fees to members of the expert panel. LEO Pharma personnel did not participate in any of the expert panel's discussions or in the writing of the paper.
Conflicts of InterestDr Lluís Puig has received consultancy fees and speaker's fees from LEO Pharma and has taken part in clinical trials sponsored by Galderma and LEO Pharma.
Dr J. M. Carrascosa has received consultancy fees, speaker's fees, or research grants from Pfizer, Abbott, Serono, MSD, Janssen, Amgen, and LEO Pharma; or he has participated in clinical trials sponsored by those laboratories.
Dr Isabel Belinchón has received consultancy or speaker's fees from Pfizer, Abbott, MSD, Janssen, and LEO Pharma; or she has participated in clinical trials they have sponsored.
Dr V. Fernández-Redondo has written a monograph funded by Laboratorios LEO Pharma and has participated in this Delphi process, also funded by Laboratorios LEO Pharma.
Dr G. Carretero declares that he has no conflicts of interest.
Dr J. C. Ruiz-Carrascosa has participated in research projects, conferences, and courses sponsored by Abbott, Janssen-Cilag, MSD, Pfizer, LEO Pharma, and Novartis.
Dr J. M. Careaga has served as a consultant or received speaker's fees from the following pharmaceutical companies: Viñas, Pfizer, Abbott, MSD, Janssen, and LEO Pharma; or he has participated in clinical trials they have sponsored.
Dr Pablo de la Cueva has been a consultant for the following pharmaceutical companies: Abbott, Janssen-Cilag, LEO Pharma, MSD, Novartis, and Pfizer.
Dr M. T. Garate declares that he has no conflicts of interest.
Dr M. Ribera declares that he has received research grants and payments, consultancy fees, and training fees from the following companies: Abbott, Janssen, LEO Pharma, MSD, Novartis, and Pfizer.
We wish to express our thanks to all the members of the Psoriasis Group of the Spanish Academy of Dermatology and Venereology (AEDV) who responded to the survey in various rounds.
Please cite this article as: Puig L, et al. Adherencia y satisfacción del paciente y características organolépticas y de uso de los tratamientos tópicos utilizados para la psoriasis. Actas Dermosifiliogr. 2013;104:488–96.