Cutaneous melanoma (CM) causes more deaths than any other skin tumor, and incidence and mortality rates have risen in recent years, especially in patients of advanced age. There are differences in the biological behavior of CM tumors in the elderly as well as differential management of the disease, evidently influenced by such factors as limited life expectancy, the high incidence of concomitant conditions in older patients, and issues of quality of life unrelated to CM itself. We review relevant current literature on the epidemiology, etiology, pathogenesis, and immunology of CM as well as research on the clinical features, prevention, and management of these tumors in the elderly.
El melanoma cutáneo (MC) es el tumor cutáneo que más muertes provoca, con un aumento importante de la incidencia y la mortalidad en las últimas décadas, especialmente en el paciente anciano. Existen evidencias del diferente comportamiento biológico, así como de las diferencias en el manejo del MC en este subgrupo de pacientes con respecto al resto de otras franjas de edad, evidentemente condicionadas por unas limitadas expectativas de supervivencia y calidad de vida ajenas al melanoma y una elevada incidencia de comorbilidades. El presente artículo revisa los datos actuales más relevantes de la epidemiología, etiopatogenia e inmunología, clínica, prevención y manejo del MC en el anciano.
The Spanish population, like other populations in the Western world, is getting older. Old age is associated with a higher incidence of melanoma and a higher disease-related mortality.1 Improvement in screening and treatment of melanoma in elderly patients is therefore essential.2 Moreover, the biological behavior of cutaneous melanoma is different in elderly individuals. This may lead to differences in the management and treatment of this group, for which life expectancy and quality of life are limited by causes unrelated to melanoma and a high incidence of comorbidities. This review will focus on the most relevant aspects of epidemiology, pathogenesis and immune system, clinical characteristics, surgical management, and systemic treatment of cutaneous melanoma in elderly patients.
Materials and MethodsA literature review was undertaken in Pubmed, EMBASE, and Scholar Google. The search terms used were (“elderly” OR “older age” OR “aged” OR “aged 80 and over”) AND “cutaneous melanoma,” adding different terms according to the subsection under study (Table 1 of the supplementary material). The reference lists of the selected articles were also reviewed to identify additional relevant articles.
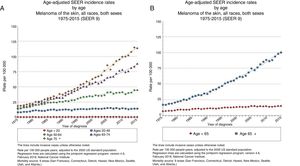
EpidemiologyAdvanced Age and Frequency of MelanomaThe largest epidemiological registry in existence, the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER), reported in 2015 an incidence of melanoma of 14.4 cases/100 000 inhabitants for patients under 65 years of age, 101.7/100 000 inhabitants for those over 65 years, and 114.7 cases/100 000 inhabitants for those over 75 years (Fig. 1),3 with a larger yearly percentage increase in men over 65 years.4
In Spain, a recent meta-analysis by Tejera-Vaquerizo et al.5 reported a raw overall incidence of 8.82 (95% confidence interval [CI], 7.59-10.04)/100 000 person-years, with differences between studies conducted several decades ago (3-4/100 000 person-years) and those conducted from the 1990s onwards, with rates greater than 7/100 000 person-years, reflecting the possible increase in melanoma incidence.
Advanced Age and Melanoma PrognosisElderly patients are more likely to die from melanoma than young ones,6 with an annual increase in incidence rate of 1.7% (Fig. 2).4 Although melanomas in elderly individuals account for 40% of such tumors diagnosed, they are cause of 60.2% of melanoma-related deaths.7
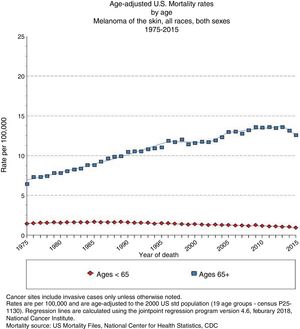
In Spain, the ARIADNA Interactive Epidemiology Information System, managed by the Instituto de Salud Carlos III,8 shows an increase in mortality for men and women, both in terms of raw rates and those adjusted to the world population (Fig. 3).9
The SEER data suggest that the raw incidence of melanoma is significantly greater in patients aged over 60 years and that mortality is higher than in other age groups. The age group with highest percentage of deaths due to melanoma corresponds to patients between 75 and 84 years.10 A study that analyzed 3 different cohorts, including the SEER cohort, with more than 300 000 patients, found that age is a predictor of worse melanoma-specific survival (MSS).11
In a multicenter study, which analyzed more than 7000 patients with cutaneous melanoma, age was identified as an independent prognostic factor in patients with stage i-iii disease.12 In elderly patients, melanoma was more frequently located on the head and neck, and had a greater thickness, mitotic rate, and ulceration. In patients with regional lymph node involvement (stage iii), age was still an important prognostic factor when variables such as number of positive sentinel lymph nodes, tumor burden, and ulceration of the primary tumor were included. Moreover, a progressive decrease in overall survival at 5 years was observed, such that survival in patients aged 60-70 years was 20% greater than those aged 80-90 years.
In a retrospective study of 4785 patients, increased age and male sex was associated with greater tumor thickness and ulceration. MSS at 10 years was 10% lower in patients over 65 years.13 The fact that advanced age was maintained as an independent factor of poor prognosis after adjusting for histological characteristics of the tumor, socioeconomic level, and comorbidity suggest that the differences observed in overall survival do not depend solely on delayed diagnosis (Table 1).14
Studies With Multivariate Analyses That Included Age as a Prognostic Factor in Cutaneous Melanoma.
| Reference | Stage/N/Type of Sample/Country | Measurement of Age as Prognostic Factor | Method of Outcome Assessment | Other Independent Prognostic Factors |
|---|---|---|---|---|
| Kemeny et al.15 | All stages/N=23 341/population/US | m ≤ 45 vs f ≤ 45, HR: 1.9 (1.6-2.3), P<.0001 | Cox/DFS | S, H, A |
| f ≥ 55 vs m ≤ 45, HR: 2.8 (2.3-3.3), P <.0001 | ||||
| m ≥ 55 vs f ≤ 45, HR: 3.6 (3.0-4.2), P <.0001 | ||||
| Balch et al.16 | I, II /N=13 581/hospital/international | Decades of increasing age, RR: 1.1 (1.07-1.13), P <.00001 | Cox/DFS | T, U, A, G, C |
| Azzola et al.17 | I, II/N=3661/hospital/Australia | Decades of increasing age, RR: 1.15 (1.07-1.2), P <.0001 | Cox/DFS | T, U, A, G, IM |
| Leiter et al.18 | Breslow ≤ 1 mm/N=11 927/hospital/Germany, Austria, Switzerland | > 50 vs ≤ 60, HR: 1.6 (1.1-1.2), P=.0075 | Cox/DFS | T, H, A |
| Lindholm et al.19 | I, II/N=6191/population/Sweden | ≥ 70 vs <50,HR: 1.59 (1.23-2.06), P=.0005 | Cox/DFS | T, U, A, G, C, H, DM |
| Caracò et al.20 | I, II referred for SLNB/N=399/hospital/Italy | > 50 vs <50, OR: 1.95 (1.13-3.39), P=.01 | Cox/DFS | T, U, G, SLNB |
| Reyes-Ortizet al.21 | All/N=23 068/population/US | 70-74 vs 65-69, HR: 1.15 (1.01-1.3), P=.04 | Cox/DFS | T, A, G, S, H, income, civil status, race, year of diagnosis, comorbidities |
| 75-79 vs 65-69, HR: 1.24 (1.08-1.3), P=.001 | ||||
| ≥ 80 vs 65-69, HR: 1.48 (1.3-1.68), P <.001 | ||||
| Downing et al.22 | All/N=3127/population/United Kingdom | Increasing age in years, HR: 1.04 (1.04-1.05), P: n.a. | Cox/DFS | T, A, G, H, socioeconomic status |
| Lasithiotakis et al.13 | I, II, IIIA/N=4785/population/Germany | Increasing age in years, HR: 1.01 (1.003-1.013), P=.005 | Cox/DFS | T, U, A, G, H, C, SLNB, year of diagnosis |
| De Vries et al.23 | All/N=10 538/population/Netherlands | 65-74 vs <45, RER: 1.37 (1.15-1.64), P=n.a.75-84 vs <45, RER: 2.2 (1.8-2.7)≥ 85 vs <45, RER: 2.18 (1.39-3.4) | Multivariate model/DFS | T, A, G, H, N, geographic region |
Abbreviations: A, anatomic site; C, Clark level; Cox, Cox proportional risks survival analysis; DFS, disease-free survival; G, sex; H, histologic subtype; HR, hazard ratio (95% confidence interval); f, female; m, male; M, presence of distant metastasis; N, presence of lymph node metastasis; n.a. not available; RER, relative excess risk (95% CI); RR, relative risk; S, stage; SLNB, sentinel lymph node biopsy; T, Breslow tumor thickness; U, ulceration.
Adapted from Lasithiotakis et al.14
Baltch et al.16 observed that sentinel lymph node involvement occurred less frequently in elderly patients, even in cases with more aggressive phenotypes. This observation has also been made in other studies.24–28 It is believed that atrophy of cutaneous lymphatic vessels may contribute to a decrease in immune response and explain the low rate of positive sentinel lymph node dissection.14 Conway et al.29 demonstrated that lymphatic function, as measured by radiocolloid transit to and uptake within the sentinel lymph node, decreased with age. Some authors concluded that this lymphatic dysfunction might have an impact on metastatic spread, with predominance of hematogenous dissemination.30
Role of the Immune System in Elderly Patients With Cutaneous MelanomaWith age, immune system function changes, resulting in a different response to infections and tumors, with decreased defense against infections and tumors.31,32 Tumor infiltrating lymphocytes (TIL), a marker of host immune response, are considered an indicator of good prognosis. Weiss et al.11 observed that the intensity of TIL in the primary tumor was positively correlated with MSS and that this effect appeared to be greater in patients aged more than 45 years.
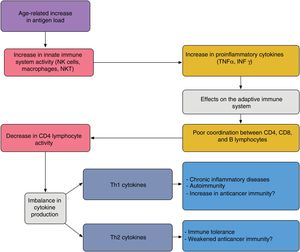
In elderly patients, imbalances between the effector and regulatory components of immune response are present; this state is known as immunosenescence32 and arises because of chronic antigen stimulation and oxidative stress during the lifetime of the individual.33 The increase in proinflammatory lymphokines due to chronic antigen stimulation leads to an increase in Th1 response and tumor cell death. This effect is amplified when tumor antigens are generated by cell death caused by chemotherapy (Fig. 4).
Proposed interaction between the innate and adaptive immune system in elderly patients; the age-related increase in antigen load leads to overstimulation of the innate immune system thereby increasing proinflammatory cytokines. This has an impact on the acquired immune system, giving rise to poor coordination between CD4, CD8, and B lymphocytes, and an imbalance between Th1 and Th2 cytokine production. The activity of cytotoxic T lymphocytes under Th1 conditions favors autoimmunity and chronic inflammatory diseases; under Th2 conditions, immune tolerance is favored.
Adapted from Hegde et al.33
Although the same clinical presentation of cutaneous melanoma occurs in elderly patients and their younger counterparts, melanomas in elderly patients are diagnosed in more advanced stages. This can be explained by multiple factors.
Superficial-spreading melanoma is the most common histological subtype, but in elderly patients, thicker and more ulcerated tumors tend to be diagnosed compared with younger patients, due to the higher proportion of nodular clinicopathologic subtypes.34,35
Furthermore, in elderly patients, there is a higher proportion of fast-growing melanomas,36,37 many of which are nodular and amelanotic.38 This hinders early diagnosis, as the lesions do not follow the classic clinical description of asymmetry, borders, color, and diameter (ABCD rule). Thus, there are suggestions to add the term E to this classic diagnostic mnemonic, which refers not only to the elevation of the lesion but also to evolving lesions during follow-up.39 Other authors propose adding the acronym EFG34 (elevated, firm, and growing) to help identify these lesions (Figs. 5 and 6).
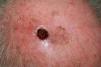
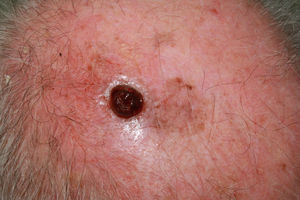
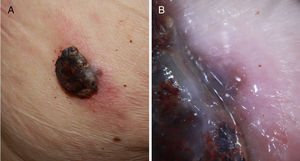
Ulcerated, fast-growing nodular amelanotic melanoma of 4 months standing on the left temple of an 87-year-old man, with a Breslow thickness of 7mm and 8 mitoses per mm.2
A, Fast-growing nodular melanoma of 3 months standing in the right scapular region on a prior flat lesion of several years standing. The Breslow thickness was 4mm, the lesion was not ulcerated, and there were 5 mitoses per mm.2 Presence of perilesional in situ melanoma in the histopathological study. B, Detail of the lesion base where pigmentation is observed, corresponding to the in situ component of the prior lesion.
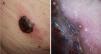
Clinically, these nodular lesions have been described in dermoscopy as typical multiple and irregular peripheral dots and globules, with blue-white veil, homogeneous blue pigmentation, more than 5 colors, and black color.40 Often, these melanomas are completely amelanotic on clinical examination. To assist with diagnosis, dermoscopy has been reported to feature the presence of milky-red areas and an atypical vascular pattern, but these are criteria that at times are insufficient for diagnosis of nodular amelanotic melanoma (Fig. 7).41 The fast-growing variant appears to be more likely to present with the above findings simultaneously.42
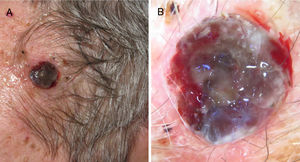
Ulcerated, fast-growing nodular melanoma on the left temple with a Breslow thickness of 4mm and 10 mitoses por mm.2 B, Dermoscopy of the lesion in which several colors and small milky areas are observed with atypical vascularization.
In elderly patients, the lentigo maligna melanoma histological subtype is more common, with a predilection for the head and neck.43 The dermoscopic criteria described for diagnosis include presence of grey dots, isobar sign (circle-within-a-circle structure), pigmented rhomboidal structures, target-like patterns, follicular occlusion, and grey-white scar-like areas.44 Lentigo maligna lesions on the cheeks occur more frequently in women whereas lesions on the nose and scalp are more frequent in men. But the most notable difference with respect to age is that in the eldest patients, lentigo maligna lesions are located on areas with lesions of chronic sun damage, unlike the case in younger individuals, who do not show such an extent of skin damage.44
Another characteristic described recently is the low frequency of association of melanoma with nevus, whether common or atypical.45
Possible Causes of Delayed Diagnosis in Elderly PatientsIn addition to the characteristic phenotypic features of melanoma in elderly patients described above, there are other possible causes for the delay in melanoma diagnosis (Table 2) and these may contribute to the increased thickness of melanomas observed in this population.
Causes of Delay in Diagnosis of Melanoma in Elderly Patients.
| Cause of Delay | Remarks |
|---|---|
| Aspects pertaining to melanoma | |
| Increased frequency of fast-growing melanoma | Increase in nodular subtypes34 which do not follow the classic ABCD rule and which are hypomelanotic or amelanotic38 |
| Increased frequency of lentigo maligna melanoma | Very slow-growing lesions on photoaged skin44 |
| Site | More frequent location of melanomas in elderly patients in areas difficult to observe, particularly in men23 |
| Aspects pertaining to the patient | |
| Low socioeconomic status | Low income has been associated with thicker melanomas21,46,47 |
| Marital status | Single, separated, or widowed patients have thicker melanomas than married patients47 |
| Level of education | The stage on diagnosis bears an inverse relationship with level of education of the patient46,48 |
| Whole-body skin self-examination and participation in screening campaigns | Less frequent in elderly patients49,50 |
| Aspects pertaining to the physician | |
| Whole-body skin examination | Elderly patients undergo fewer routine whole-body skin examinations51 |
| Level of training of the physician | Longer delay when the lesion is seen by a primary care physician than by a dermatologist52 |
Adapted from Lasithiotakis et al..14
In the case of site, there are some relevant characteristics. One of these is that melanoma may present in anatomical sites with low visibility. Thus, a Dutch epidemiological study reported a greater propensity, almost double, for melanoma to present on the trunk in men compared with women, and this may contribute to a greater thickness.23 The scalp is also a more frequent site in this risk group of elderly men, with the same characteristics as the more aggressive phenotype.53
There are a series of demographic factors related to a longer delay in diagnosis. The fact that elderly patients have lower income has been independently associated with diagnosis of thicker melanoma.21,46,47,54
Marital status has also been associated with thickness of melanomas. Thus, patients who are single, separated, or widowed, with predominance for males, have thicker melanomas compared with married ones. It seems that the partner contributes to recognition of suspected lesions that would otherwise not be noticed.47
Among the patient-dependent causes, elderly patients are less likely to participate in prevention campaigns,50 or conduct whole-body skin self-examinations.49
Finally, possible causes related to quality of health care have also been described as a possible reason for delay in diagnosis. Data are contradictory in terms of frequency of whole-body skin examinations by the primary care physician. Some studies have reported that fewer whole-body skin examinations are performed in older patients than younger ones,51,54 whereas another study of the population in Queensland, Australia, did not observe this difference.55 Moreover, up to a third of the population over 50 years of age had had a partial skin examination in the past year.
Surgical Locoregional Management of Melanoma in Elderly PatientsTreatment of the Primary LesionPrimary excision of melanoma is considered a minor surgical procedure that can generally be performed under local anesthetic.56 However, elderly patients are often not considered candidates for surgical treatment, resulting in lower rate of excision of suspected pigmented lesions and failure to comply with recommendations for tumor management.6
Thus, Marks et al.57 showed that the ratio between nevus and melanoma in excised pigmented lesions was 27:2 in patients between 21 and 40 years of age, and 1:4 in those aged over 60 years.
There is also a greater tendency to perform incisional biopsy in large pigmented lesions that are often found on elderly patients, but this technique complicates histopathological study and should be avoided, unless, as for other age groups, diagnosis is uncertain and excisional biopsy requires complex reconstruction.58
Finally, elderly patients have a higher proportion of head and neck melanoma,59,60 with a functional and esthetic impact on complex areas, such as the nose and eyelids. The tendency to reduce the surgical margin, along with the difficulty in establishing margins for lentiginous lesions, which are more frequent in elderly individuals, is responsible for a higher proportion of peritumoral resections or resections with inadequate margins.14 Although this does not have an impact on overall survial,61 it could be significant for determining the risk of local recurrence.
On analyzing more than 18 000 patients with melanoma in the SEER,62 it was found that in patients aged 65 years or more, excision with inadequate margins was more frequent than in those under 65 years (risk ratio, 1.37), and this difference was even greater for those aged 75 years or more (risk ratio, 2.38). In a retrospective study conducted in France, in which variations in treatment of patients with stage i-iii melanoma were assessed, it was found that the factors associated with excision with inadequate margins as defined by the recommendations of the clinical guidelines were age greater than 60 years, greater tumor thickness, and site on the head and neck.63 These latter 2 factors are, furthermore, more frequent in elderly patients.
Selective Sentinel Lymph Node BiopsyWith regards SLNB, although a previous study suggested that age did not influence whether one was performed,63 other studies have found that the procedure is indicated less frequently in patients aged 75 years or more,59,64,65 regardless of their comorbidities.
Moreno-Ramírez et al.66 showed that the main deciding factor for performing SLNB was Breslow thickness, such that 71.6% of patients with tumors with a thickness of 1.01-4.00mm underwent SLNB. In this group, the Karnofsky performance status and age were the most significant deciding factors in patients with tumors thicker than 4mm, while age was the most relevant determinant for lack of indication of SLNB, performed in 64.1% of patients under 70 years of age and only in 8.7% of those over 70 years.
Unlike for excision of the primary tumor, SLNB may require spinal or general anesthesia, and so, in these cases, the procedure is associated with anesthetic risk. This risk can be calculated with general comorbidity scales or more specific scales, such as the American Society of Anesthesiologists Physical Status System classification system.67 These patients require a preoperative study (that includes analysis with coagulation, plain chest X-ray, and electrocardiogram); detailed knowledge of the patient́s general clinical condition, cardiorespiratory function, and usual medications; meticulous surgical planning; intraoperative monitoring; and appropriate postoperative follow-up.56,67 The most important clinical trial of SLNB in melanoma, the Multicenter Selective Lymphadenectomy Trial-I (MSLT-I), excluded patients over 75 years of age68; however, other studies have shown the undoubted prognostic value of this test in elderly individuals and its feasibility in patients with a reasonable life expectancy.69
LymphadenectomyLymphadenectomy after positive SLNB (immediate complete lymphadenectomy [ICL]) is also indicated less frequently in elderly patients.70,71 Moreover, age greater than 75 years has been identified as a predictive factor for not complying with the recommendations in terms of performing ICL, with a lower mean number of lymph nodes dissected during the procedure in older patients.71
Some authors consider that this lower level of intervention in elderly patients is a possible explanation for the greater mortality observed in this age group.1 However, the lower frequency of metastasis in SLNB and the results of the Multicenter Selective Lymphadenectomy Trial-II (MSLT-II),72 which show a lack of survival benefit in patients with positive SLNB and ICL (compared with observation and therapeutic lymph node dissection once the patient develops identifiable lymph node metastasis), would not support a possible association between undertreatment and mortality. In any case, of note is that the age range established as an inclusion criterion in the MSLT-II was 18 to 75 years. Although the consistency of the results of the trial suggest that they could be extrapolated to elderly patients, we still lack high quality evidence to support ICL in these patients. Confirmation of the regional control observed in patients treated with ICL in the MSLT-II would, moreover, have been of great interest to guide decisions in elderly patients.1
Treatment of Advanced Locoregional and Metastatic DiseaseAdvanced Locoregional DiseaseSeveral studies have shown that the efficacy of intraarterial chemotherapy with melphalan (with or without tumor necrosis factor alfa or actinomycin) administered by hyperthermic isolated limb perfusion in the treatment of locally advanced malignant melanoma (unresectable lesions, with in-transit metastasis) was similar in patients aged 75 years or more than in younger patients.73–76 Moreover, perioperative mortality does not increase with increasing age and most events were of locoregional toxicity.
Systemic TreatmentThe elderly population has certain characteristics (greater presence of other diseases, several concomitant pharmacological treatments with the potential for drug-drug interactions, possibility of cognitive decline, and general state of the patient) that make it particularly important to assess the benefit-risk of each treatment.77 There is evidence that geriatric assessment prior to an oncological therapeutic plan could help achieve more satisfactory outcomes in terms of survival, quality of life, functional status, and risk of hospitalization in elderly patients with cancer.78
Before 2010, treatment of metastatic melanoma was limited to classic chemotherapy with dacarbazine or the use of high-dose interleukin 2. Both treatments had low efficacy and a high toxicity that limited their use in elderly patients.14 In 2010, the results of the first clinical trials with vemurafenib and ipilimumab were published, and treatment of advanced melanoma entered a new era. Information on the usefulness of these new therapies in elderly patients is derived mainly from subgroup analyses of this population who participated in the clinical trials, with the associated limitations of such an approach.
Therapeutic TargetThe clinical utility of treatment with BRAF inhibitors (vemurafenib and dabrafenib) alone or, as currently employed, in combination with MEK inhibitors (cobimetinib or trametinib) is limited to melanomas carrying the BRAF kinase mutation. Several studies suggest that the frequency of appearance of BRAF mutations is inversely correlated with age.79,80 In an Australian cohort of more than 300 patients with metastatic melanoma, all patients under 30 years of age had the BRAF mutation, whereas only 25% of those over 70 years did.80 Interestingly, in elderly patients, the proportion of individuals with the most frequent BRAF mutation, V600E, decreases whereas other less common BRAF mutations, such as the V600K BRAF mutation, increase in frequency.
Although the low number of elderly patients recruited to clinical trials is a global problem in oncology,81 in trials involving this therapeutic target, the decrease in BRAF mutation frequency with age has surely also contributed to their underrepresentation.
Currently, the regimen most widely used for this therapeutic target is a combination of a BRAF inhibitor with a MEK inhibitor, as this not only offers greater efficacy but also limits adverse cutaneous effects. In the analysis by age subgroups, no differences in efficacy were observed.82,83
With regards the safety of these treatments in the elderly population, it seems that the overall frequency of adverse effects is similar to the younger population. However, the most severe adverse effects (grade iii-iv), as well as the risk of withdrawing treatment, are greater in the elderly population.84
ImmunotherapyIpilimumab, a CTLA-4 inhibitor, was the first immunotherapy agent to be approved for metastatic melanoma. A response rate of 10% to 15% was achieved with its use.85 Subsequently, in 2015, anti-PD-1 agents (nivolumab and pembrolizumab) became available, with better efficacy and safety profiles than ipilimumab. Anti-PD-1 agents in monotherapy can achieve response rates of between 33% and 40%.86 The combination of ipilimumab with an anti-PD-1 agent is the most effective immunotherapy regimen, with a response rate of 61%, although this combination is the one that generated greatest toxicity.87
There is currently some debate as to whether the elderly population is particularly sensitive to immunotherapy. While some studies have found differences between the efficacy of immunotherapy in different age groups,88 others have even pointed to a better response, particularly for anti-PD1 agents in elderly patients. In a recent retrospective cohort study, in which all patients treated with new immunotherapy agents in the Hospital of Lyon, France, were reviewed, the authors reported longer disease-free survival in patients aged over 65 years compared with those under 65 years.89 Another recent multicenter study found that the risk of progression under treatment with pembrolizumab decreased by 13% for every decade of life of the patient on starting treatment.90 The mechanisms that might explain this possible benefit are not yet understood, but they focus on the potential of immunotherapy for reverting changes in the immune system that arise during old age.91
Given the particular mechanism of action of immunotherapy, deterioration in the function of several organs —characteristic of aging —is of greater relevance. For example, there is no contraindication for use of immunotherapy in patients with renal or heart failure. Nevertheless, it is of vital importance that patients and their caregivers are aware of potential unwanted effects of immunotherapy, particularly those such as asthenia and arthralgia, which could be attributed to aging. The best option for minimizing immunotherapy toxicity is one centered on diagnosis and early management of adverse effects. Toxicity associated with immunotherapy does not appear to increase with increasing age.85,89,90
In Spain, the only approved adjuvant for high-risk melanoma is high-dose interferon alfa-2b. Given the substantial toxicity and limited benefit, this treatment is not usually used in elderly patients.92
Currently, treatment of metastatic disease with targeted therapy and immunotherapy is thought to have a comparable effect on overall survival in elderly patients, without a substantial increase in toxicity in elderly patients.93 Nevertheless, it is necessary to perform studies in every-day clinical practice in elderly patients treated with these new drugs, given that these patients, who are increasingly numerous, are excluded from clinical trials.
Table 3 shows the level of evidence for each therapeutic procedure in patients with melanoma.
Staging and Treatment of Cutaneous Melanoma in Elderly Patients.
| Intervention | Remarks | Level of Evidence and Strength of Recommendation (USPSTF) |
|---|---|---|
| Primary excision | Same recommendation as for other age groupsLMM requires adequate margins to be established around the lesion, ideally through Mohs micrographic surgery | III A |
| SLNB | Staging, no therapeutic benefit.Lower rate of positive findings (lower sensitivity, rate of micrometastasis, or lymphatic spread?)Assess anesthetic risk | II-2 B |
| Lymphadenectomy | Morbidity (lymphedema, nerve damage, surgical wound complications)No impact on survival demonstrated.Palliative treatment if clinically relevant lymph node metastasis | III C |
| Adjuvant treatment | Little information available on benefit-riskFavorable response to immunotherapy due to imbalance in immune system | III I |
| Intraarterial chemotherapy via hyperthermic isolated limb perfusion | Assess in locally advanced melanoma (unresectable, in transit metastasis) | III-2 B |
| Treatment of metastatic melanoma (immunotherapy, targeted therapy) | Same therapeutic approach as in young patientsAssess prior geriatric assessment | III B |
Levels of evidence (USPSTF): ii, at least one randomized, controlled clinical trial with appropriate design; ii-1, well-designed, controlled clinical trials, but not randomized; ii-2, well designed cohort studies or case-control studies, preferably multicenter; ii-3, multiple series compared over time, with or without intervention, and surprising results in uncontrolled studies; iii opinions based on clinical experience, descriptive studies, clinical observations, or expert committee reports.
Strength of Recommendation: A, extremely recommended (good evidence that the measure is effective and that the benefits easily outweigh the harms); B, recommended (at least moderate evidence that the measure is effective and that the benefits outweigh the harms); C, not recommended or unadvised (at least moderate evidence that the measure is effective but the benefits are similar to the harms and cannot justify a general recommendation); D, unadvised (at least moderate evidence that the measure is ineffective or that the harms exceed the benefits); I, insufficient evidence, of poor or contradictory quality, and the balance between benefits and harms cannot be determined.
Abbreviations: LMM, lentigo maligna melanoma; SLNB, selective sentinel lymph node biopsy; USPSTF, United States Preventive Services Task Force
Adpated from Lasithiotakis et al.14
The authors declare that they have no conflicts of interest.
Please cite this article as: Iglesias-Pena N, Paradela S, Tejera-Vaquerizo A, Boada A, Fonseca E. Melanoma cutáneo en el anciano: revisión de un problema creciente. Actas Dermosifiliogr. 2019;110:434–447.