Although the arrival of new chemotherapy drugs and combinations has brought progress in terms of cancer patient survival, they entail many adverse effects that can compromise treatment, and hence prognosis, of the disease. Cytostatic agents can cause dermatological toxicity, among other side effects. The most familiar adverse effect of chemotherapy is alopecia. Although not serious, this changes the outward appearance of cancer patients. Other adverse effects include hypersensitivity and photosensitivity reactions, hand-foot syndrome, epidermal necrolysis, recall reactions, scleroderma-like reactions, Raynaud's phenomenon, eccrine squamous syringometaplasia, neutrophilic eccrine hidradenitis, nail abnormalities, pigmentation changes and extravasation injuries. Onset of these adverse effects often causes dose reduction and/or delayed treatment, which can affect patient survival and quality of life. It is therefore important to prevent their occurrence and treat them promptly, which requires cooperation between medical oncologists and dermatologists. This article reviews chemotherapy-associated dermatological toxicity, along with its diagnosis and therapeutic management.
A pesar del avance que ha supuesto en la supervivencia de los pacientes oncológicos, la aparición de nuevos agentes quimioterápicos y nuevas combinaciones, estos han traído consigo numerosos efectos adversos que pueden llegar a comprometer el tratamiento y, por consiguiente, el pronóstico de la enfermedad. Entre otros efectos secundarios los citostáticos pueden causar toxicidad dermatológica. El efecto adverso más conocido de la quimioterapia es la alopecia que, aunque no es grave, altera la apariencia externa de los pacientes con cáncer. Otros efectos adversos que pueden observarse son las reacciones de hipersensibilidad y fotosensibilidad, el síndrome mano-pie, la necrólisis epidérmica, las reacciones de reactivación, las reacciones esclerodermiformes, el fenómeno de Raynaud, la siringometaplasia escamosa ecrina, la hidradenitis neutrofílica ecrina, las alteraciones ungueales, las alteraciones en la pigmentación y las lesiones por extravasación. La aparición de estos efectos adversos produce en muchas ocasiones una reducción de dosis y/o retraso del tratamiento, lo que puede afectar a la supervivencia y a la calidad de vida del paciente. Por ello, es importante prevenir su aparición e instaurar un tratamiento temprano, para lo que se hace imprescindible la colaboración entre oncólogos médicos y dermatólogos. En este artículo se revisa la toxicidad dermatológica asociada con la quimioterapia, así como su diagnóstico y abordaje terapéutico.
Conventional chemotherapy remains an essential mainstay of cancer treatment. Indeed, the indications for chemotherapy grow more numerous every day, in an ever-wider range of tumors. For this reason, there is a greater diversity of toxicities, which must be identified and managed. Managing them correctly means better symptom control and better quality of life for patients. Cytotoxic drugs inhibit cell division by acting at various points in the cell cycle. Therefore, as well as working on tumor cells, they also affect healthy cells that have a high rate of division, such as those found in the skin and its appendages, and therefore many structures can be affected.
Dermatological toxicities caused by chemotherapy use need to be diagnosed correctly. In many cases, therapeutic measures to reduce their severity and duration must be introduced as soon as possible. Sometimes, cutaneous toxicity means having to reduce the dose, delay cycles, or even stop cancer therapy. Preventive measures should be applied whenever they exist. Multidisciplinary assessment by dermatologists and medical oncologists is key to caring for these patients. This article reviews the main dermatological reactions to chemotherapy, focusing on their diagnosis and treatment.
Classification of chemotherapy agents and mechanism of action of cutaneous toxicityDermatological toxicity caused by chemotherapy can develop while the drug(s) are being administered, for example cutaneous manifestations of extravasation and drug hypersensitivity reactions. Alternatively, it may arise from the side effects of cytotoxicity itself. The characteristics of cutaneous reactions vary depending on the drug involved. Although no mechanisms of action triggering specific toxicities have been identified, reactions can be categorized according to the chemotherapy drug classes that caused them (Table 1) .1–9
Classification of chemotherapy drugs, mechanisms of action, and common cutaneous toxicities.
| Chemotherapy drug classes and mechanism of action | Cutaneous toxicity | |
|---|---|---|
| Alkylating agents:Form covalent bonds with DNA; cause cell death by inhibiting DNA function | Busulfan | Hyperpigmentation, mucositis, skin rash and pruritus |
| Thiotepa | Hyperpigmentation, mucositis, desquamation and allergic reactions | |
| Nitrogen mustards: cyclophosphamide, ifosfamide, chlormethine, chlorambucil and melphalan | Hyperpigmentation of skin and its appendages with cyclophosphamide and ifosfamide.Alopecia with chlormethine, cyclophosphamide and ifosfamide.Mucositis with high-dose melphalan and cyclophosphamide.Rash and Stevens-Johnson syndrome with chlorambucil | |
| Carmustine and streptozocin | Facial flushing with carmustine. | |
| Dacarbazine and temozolomide | Photosensitivity phenomena | |
| Platinum drugs:Form covalent bonds with DNA; cause cell death by inhibiting DNA function | Cisplatin, carboplatin and oxaliplatin | Infusion reactions.Alopecia with cisplatin |
| Antimetabolites:Inhibit DNA synthesis and repair, mainly by interfering with the formation of nitrogenous bases | Antifolates: methotrexate and pemetrexed | Mucositis, dose-limiting with methotrexate.Skin rash, photosensitivity, hyperpigmentation, alopecia and recall reaction in irradiated areas with methotrexate |
| Fluoropyrimidines: fluorouracil and capecitabine | Mucositis, hand-foot syndrome, hyperpigmentation, photosensitivity, exfoliative dermatitis | |
| Nucleoside analogues: gemcitabine, cytarabine, azacitidine and hydroxycarbamide | Mucositis, maculopapular rash and recall phenomenon with gemcitabine.Mucositis, erythema, hidradenitis and alopecia with cytarabine. Rarely, hand-foot syndrome | |
| Purine analogs: mercaptopurine, tioguanine, fludarabine and cladribine | Mucositis, skin rash and photosensitivity | |
| Topoisomerase inhibitors:Break double-stranded DNA and cause cell death by inhibiting the enzymes DNA topoisomerase I (topo I) (camptothecins) and II (topo II) | Camptothecins: irinotecan and topotecan | Alopecia |
| Anthracyclines: doxorubicin, epirubicin, liposomal doxorubicin, daunorubicin, idarubicin | Mucositis, alopecia, nail hyperpigmentation, photosensitivity, recall phenomenon.With liposomal anthracyclines, less alopecia but higher incidence of mucositis, hand-foot syndrome and infusion reactions | |
| Mitoxantrone | Mucositis, alopecia, nail discoloration | |
| Dactinomycin | Mucositis, alopecia, hyperpigmentation, photosensitivity, recall reaction | |
| Etoposide and teniposide | Alopecia, mucositis, recall reaction.Infusion reactions. | |
| Anti-microtubule agents:Block microtubule formation, leading to inhibition of mitosis and cell division | Taxanes: paclitaxel, docetaxel, cabazitaxel and albumin-bound paclitaxel | Infusion reactions.Alopecia, onycholysis and mucositis with paclitaxel.Photosensitivity, maculopapular erythema, alopecia, nail hyperpigmentation, hand-foot syndrome and mucositis with docetaxel |
| Vinca alkaloids: vinorelbine, vincristine and vinblastine | Alopecia, mucositis and skin rash | |
Among type I hypersensitivity reactions, urticaria and angioedema are distinguished from anaphylactoid reactions. It is essential to differentiate between them because the therapeutic approach is completely different.10 Type I reactions include hypersensitivity to carboplatin and oxaliplatin, manifested as urticaria, angio-edema, bronchospasm and hypotension after 6 to 8 cycles of therapy, suggesting an IgE-mediated reaction. Due to cross-reaction between the various types of platinum drugs, their use is contraindicated following a previous hypersensitivity reaction.11 Treatment of the episode consists of administering adrenaline, corticosteroids and antihistamines, as well as stopping the drug.10 Due to the relevance of the schedules with carboplatin and oxaliplatin in the treatment of several tumors such as ovarian, lung or colon cancer, treatment strategies have been proposed to allow the maintenance of this class of drugs, including the reduction of the infusion rate, premedication with antihistaminic drugs or systemic corticosteroids or desensitization.12 Another example of type I hypersensitivity is the reaction caused by L-asparaginase, which also produces infusion reactions. With premedication and a slower infusion rate, patients who experience infusion reactions can continue therapy. However, those with L-asparaginase hypersensitivity should switch to Erwinia chrysanthemi asparaginase.13 One particular type I hypersensitivity reaction occurs with doxorubicin as an urticarial flare over the vein at the injection site. The linear wheal may be accompanied by a sensation of pain or stinging, which disappears after 45 minutes .14
Anaphylactoid reactions include those induced by taxanes, which are attributed to the vehicle used for administering them (Cremophor EL® and Tween 80). They manifest as flushing, breathing difficulties and rash, and can progress to severe hypotension, asystole and death.15 These reactions occur within a few minutes of the first infusion and are prevented by premedication with oral dexamethasone.10 Paclitaxel formulations containing no Cremophor® (nab-paclitaxel), with a lower incidence of this toxicity, have become commercially available .16
The classic example of type III hypersensitivity is vasculitis caused by immune complex formation, detected during high-dose methotrexate treatment or hydroxycarbamide therapy.17 A type IV hypersensitivity reaction of note is systemic contact dermatitis following intravesical administration of mitomycin. This is characterized by acute eczema on the palms of the hands, the soles of the feet, the ears, and the perineal region 24 hours post-treatment. Diagnosis is by epicutaneous testing .18
Patients on chemotherapy commonly experience photosensitivity phenomena, consisting of phototoxic reactions caused by exposure to ultraviolet rays (UVA). The sunburn-like lesions are confined to light-exposed areas and can result in hyperpigmentation. The substances most often involved are fluorouracil and its derivatives (capecitabine and tegafur), dacarbazine and vinblastine .19
Lesions predominantly involving the epidermisThe term “toxic erythema of chemotherapy” was introduced by Bolognia et al. to refer to a group of toxic reactions that share aspects of their pathogenesis, histology and clinical features.20 The pathogenic mechanism responsible for these reactions involves cytotoxicity against epidermal keratinocytes and, to a lesser extent, eccrine ductal cells in the epidermis.
The characteristic histological pattern is epidermal cytotoxicity with cell-poor interface dermatitis, basal hydropic degeneration, keratinocyte necrosis, keratinocyte atypia, and mitotic figures retained in upper layers of the epidermis. Clinically, these lesions manifest as areas of painful erythema, often associated with edema, and sometimes with blisters.
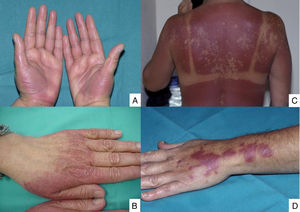
Lesions are mainly located on the hands and feet, in intertriginous areas (the axillae and groin, especially) and, less often, on the elbows, knees and ears. The lesions of toxic erythema of chemotherapy are self-limiting and tend to resolve with desquamation and post-inflammatory hyperpigmentation. The main risk with these lesions is misdiagnosis, and they are often described as hypersensitivity reactions, allergic drug eruptions and contact dermatitis, as well as graft versus host disease (GVHD), vasculitis or skin infections. This group of lesions includes chemotherapy-induced acral erythema, inflammatory lesions at the chemotherapy injection site, recall reactions, and chemotherapy-induced diffuse erythema (Fig. 1).
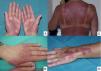
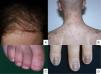
Acral erythema is also called palmar-plantar erythrodysaesthesia or hand-foot syndrome. It occurs from 48 hours post-treatment. It manifests with erythema, edema and palmar-plantar desquamation, associated with local discomfort experienced as paresthesia, pain and stinging. The most severe forms progress to fissured lesions and blisters. The lesions persist for 1 or 2 weeks and worsen with each chemotherapy cycle. The condition resolves after treatment withdrawal or dose reduction.21 The drugs most often associated with this type of toxicity are fluorouracil, capecitabine, cytarabine, liposomal doxorubicin, vinorelbine, docetaxel and paclitaxel .1,22 The eruption is dose-dependent, so regimens of high dose intensity are most often responsible for lesions of this type.22 Classical descriptions of acral erythema refer to localized lesions, typically on the palms and soles. However, acral erythema can sometimes occur in atypical places, such as the dorsum of the hands and feet, the elbows, or even in the ears .20,23 These atypical sites are more likely to be seen with taxanes and liposomal doxorubicin. The lesions consist of erythema, edema and desquamation on the dorsum of the hands and fingers, which requires differential diagnosis from phototoxic eruptions .24
Acral erythema is often associated with onycholysis, especially in patients on weekly docetaxel and paclitaxel regimens.25 It is a frequent cause of dose-limiting toxicity, particularly during capecitabine and liposomal doxorubicin therapy. In fact, it is the cutaneous injury most often responsible for dose-limiting toxicity. Other than reducing the dose, prolonging the dosing interval and, as a last resort, withdrawing the drug, there is no treatment for acral erythema. Patients may obtain symptomatic relief from local lesion care by applying cold compresses, emollients and topical corticosteroids, and from oral analgesics.21 Cooling the hands and feet during delivery of chemotherapy has been used with partial success in docetaxel-induced acral erythema .26 Systemic corticosteroid therapy may be helpful in some cases. This treatment has been used successfully to prevent acral erythema induced by fluorouracil and liposomal doxorubicin. Pyridoxine (vitamin B6), at doses of 300 and 1500 mg/day, has also been used when this injury occurs during treatment with fluorouracil, docetaxel, etoposide and doxorubicin ,27 although its efficacy has not been confirmed in controlled clinical trials.
Localized epidermal necrolysis at the injection siteEpidermal necrolysis is an erythematous and/or edematous, occasionally bullous eruption occurring 24 to 48 hours after injection of a cytostatic drug. It appears along the course of the vein injected. Histologically, it is characterized by a pattern of epidermal cytotoxicity. This injury always follows an uneventful chemotherapy session (no extravasation or chemical phlebitis). The eruption persists for 1 or 2 weeks and can leave residual hyperpigmentation when it heals.
Epidermal necrolysis has not been reported consistently in the literature, so the known clinical and histological data come from isolated case reports only. The various terms that have been used to define it are erythema multiforme-like injection site reaction, persistent supravenous eruption, fixed erythrodysesthesia plaques, and fixed drug eruption at the injection site.28–30 Cases of persistent supravenous eruption documented in the literature manifested with linear, macular, erythematous lesions along the vein injected with the chemotherapy drug.28 Cases of localized erythema multiforme consisted of edematous papules and vesicles clustered at the injection site, and following a linear trajectory along the vein into which the infusion was delivered.29 Cases reported as fixed erythema or erythrodysesthesia plaque manifested with a solitary erythematous plaque that progressed to a blister at the injection site.30 They all had clinical and histological features typical of toxic erythema of chemotherapy.
The drugs most often linked to this eruption are vinca alkaloids (especially vinorelbine), taxanes (especially docetaxel) and fluorouracil. These injuries arise from a cytotoxicity phenomenon directly affecting the epidermis, because of high local concentrations of the chemotherapy drug in the vein injected.
Recall reactions (recall phenomenon)Recall dermatitis is the result of an acute tissue toxicity in an area of the skin previously exposed to radiation or sunburn, when subsequently (days or years later) the patient receives chemotherapy, or much less frequently in the context of the administration of other medications”. The drugs most often related to lesions of this type are taxanes, methotrexate, dactinomycin and doxorubicin.31,32 The eruption is very well delimited and confined to the previously damaged area. It manifests clinically as erythema, edema and pain. The latent period between initial onset of the dermatosis and its reactivation by chemotherapy can range from 1 month to more than 5 years.32 The prevalence of radiation recall dermatitis among patients treated with radiotherapy and taxanes is calculated to be 6%, whereas in patients given radiotherapy and dactinomycin treatment it is 47% .33
Lesions predominantly involving the dermisTaxane-induced scleroderma-like reactionsThe occurrence of scleroderma-like changes has been associated with docetaxel and paclitaxel therapy. The sclerosis is induced by the taxanes themselves, not by excipients or solvent compounds.34 Elevated levels of TNF-α and IL-6 are implicated in the etiopathogenetic of these changes.35 Most of the cases described occurred 3 to 10 months after therapy started. The reaction begins as edema affecting both lower limbs, and progresses in a matter of weeks to skin induration and sclerosis.36 Cases involving the face and upper limbs have also been reported. This condition can usually be arrested by stopping treatment. It may even disappear but worsens when the drug is resumed. Although uncommon, there have been isolated cases involving systemic sclerosis (esophagus, muscles).35 Generally, however, other features typical of systemic scleroderma are absent, such as nailfold capillary changes, Raynaud's phenomenon, calcinosis, and scleroderma autoantibodies.37 Histological examination of the skin reveals changes indistinguishable from those seen in scleroderma, with a thickened dermis, composed of sclerotic collagen bundles, atrophy of the appendages, and interlobular septal thickening in the subcutaneous cellular tissue.
Bleomycin-induced Raynaud's phenomenonRaynaud's phenomenon is a cutaneous manifestation associated with bleomycin use. It generally occurs with a cumulative drug dose, so after 3-4 cycles of therapy 35%-45% of patients develop persistent Raynaud's phenomenon, and it is more likely to occur in those given bleomycin as intravenous boluses rather than by continuous infusion.38 However, Raynaud's phenomenon has reportedly occurred after the first infusion of the drug in patients with a history of idiopathic Raynaud's syndrome, and in smokers.38 The arterial spasm suffered by these patients produces intense pain and can even cause necrosis in the distal fingers or toes. The etiology of this phenomenon in the chemotherapy context has not been fully elucidated but two possible mechanisms have been suggested: direct drug damage to endothelial cells, or abnormal sympathetic stimulation due to chemotherapy-induced neuropathy, with resultant vasoconstriction.39 Vasodilator drugs ranging from nifedipine to sildenafil, iloprost or bosentan have been used to treat Raynaud's phenomenon.
Lesions predominantly involving the eccrine glandsEccrine squamous syringometaplasiaEccrine squamous syringometaplasia (ESS) is a histological term describing the replacement of cuboidal epithelium in the eccrine duct by 2-3 layers of squamous epithelium with keratinization inside the duct. This histological pattern has been reported as an incidental finding in the context of infectious, inflammatory and tumor-related dermatoses, phototoxic reactions, and even in ulcers of varied etiology. However, this adverse effect has been associated with the administration of a wide range of chemotherapy drugs. It is particularly common with pegylated liposomal doxorubicin, and more rarely occurs with cyclophosphamide, gemcitabine, taxanes and temsirolimus, as well as some of the new kinase inhibitor drugs, such as imatinib, sunitinib, vemurafenib and dabrafenib. It is speculated that ESS may occur due to the toxic effect of chemotherapy drugs on the eccrine ductal epithelium .40–44
Clinically, ESS tends to present within the first 30 days of chemotherapy treatment, as an eruption composed of macular lesions and erythematous plaques, some of which may be eroded or partially desquamated. They are usually located in skin folds (axillae, groin, under the breasts), and on the neck and eyelids. Applying moderately potent corticosteroids for 10 days is usually sufficient treatment. Oral corticosteroids should be reserved for more severe cases. If the patient needs further cycles of the same chemotherapy drugs, a 15%-20% dose reduction usually prevents recurrence of the lesions .40
Neutrophilic eccrine hidradenitisNeutrophilic eccrine hidradenitis (NEH) is currently regarded as part of the spectrum of neutrophilic dermatoses. It tends to present clinically as disseminated erythematous plaques, sometimes with an urticaria-like appearance, in the context of a febrile illness. Histologically, it is characterized by the presence of a neutrophilic infiltrate around the eccrine ducts together with necrosis of some ductal cells.
This adverse effect has often been linked to a wide range of malignancies and drugs but is most commonly associated with acute myeloid leukemia and cytarabine.45 As described for ESS above, NEH has been associated with the new targeted therapies used to treat melanoma, such as vemurafenib or dabrafenib.46 The pathogenesis of this condition is unknown, but a toxic effect of the drugs on the ductal epithelium has been suggested. Some authors regard NEH and ESS as part of a spectrum of cutaneous reactions to chemotherapy drugs that can even occur over time in the same patient. NEH would thus represent the early stage, with a dense neutrophilic infiltrate, and ESS would be the late stage, in which the ductal epithelium is undergoing squamous differentiation .47
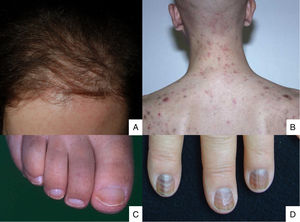
Lesions predominantly involving the hair follicle and nail apparatusAlopecia is common after chemotherapy because the follicular epithelium has a high mitotic rate. This condition mainly involves the proliferative phase of the follicle (anagen effluvium). How it presents is related to the chemotherapy drug used, the dose, the treatment duration, and the patient's response.48 Many drugs are capable of causing it (Table 2).49,50 It manifests within a few days or weeks of starting chemotherapy and is almost complete after 2-3 months (Fig. 2A). The hairline tends to be spared. Males are mainly affected in the occipital region (50% of cases) or frontal area (15%) or may have total alopecia (35%). In women it may be total (52%), or likewise involve the frontal region (40%) or occipital zone (8%).51 Hair loss is diffuse or patchy.
Histologically, necrotic and dyskeratotic keratinocytes are seen at early stages, with vacuolation of the follicular epithelium. Advanced stages display telogen hairs and few anagen follicles. There is very little inflammatory infiltrate.52 Hair regrowth occurs 1-3 months after chemotherapy stops. Some patients experience changes in texture, color and follicle thickness. If follicle regrowth is incomplete or absent beyond 6 months post-chemotherapy, it is termed permanent alopecia. This is more common in children than adults.53 It has been suggested that, at high doses, the action of some chemotherapy drugs on follicular stem cells or follicular signaling pathways may cause permanent alopecia.54,55 This condition is more commonly reported after the administration of taxanes and IFN-α-2a.56,57 Cooling the scalp might prevent the onset of alopecia.
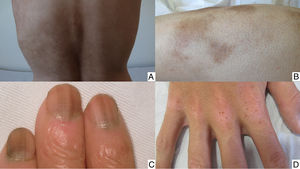
Chemotherapy-induced folliculitis has been described in isolated cases, despite occurring in up to 40% of patients on drugs such as dactinomycin (Fig. 2B).58,59 In the authors’ experience, folliculitis can also occur after the administration of paclitaxel, docetaxel, doxorubicin, cyclophosphamide and carboplatin. It manifests in a generalized manner on the face, trunk and buttocks, and sometimes progresses to comedones, which can be confused with acne in adolescent patients. On other occasions it occurs in a localized fashion on the face, scalp, forehead and nape of the neck. In these cases, it is associated with cyclophosphamide/ifosfamide therapy and platinum drugs. It is usually not accompanied by comedones but tends to be associated with febrile neutropenia. Cultures are negative and skin biopsy reveals suppurative folliculitis. Nevertheless, when pustular lesions appear in these patients, the need to perform a differential diagnosis with folliculitis secondary to the administration of oral corticosteroids (included in the medication administered prior to chemotherapy or prescribed by other reasons) should always be considered.Nail abnormalities are observed as brown discoloration, either transverse or longitudinal (melanonychia striata), or diffuse (total melanonychia).60 They are associated with cyclophosphamide, doxorubicin, hydroxycarbamide, busulfan, taxane, capecitabine, cisplatin or bleomycin therapy. These abnormalities may also manifest as transverse leukonychia or Mees’ lines, because nail keratinization is damaged by the effect of cytostatic drugs (doxorubicin, cyclophosphamide and vincristine). Leukonychia can also occur because of altered blood flow to the nails: half-and-half nails or Lindsay's nails (lower half opaque) (Fig. 2C), Muehrcke's lines (whitish bands running parallel to the lunula) (Fig. 2D), and Terry's nails (almost entirely opaque). Beau's lines are transverse lines on the nail plate caused by arrested matrix activity. This is termed onychomadesis when the groove is more severe and splits the nail plate in two. Changes in thickness can cause koilonychia (spoon nails), onychorrhexis (nails with longitudinal grooves), onychoschizia (distal fragmentation) or trachyonychia (rough nails). They can also produce onycholysis (distal detachment), which is sometimes hemorrhagic with taxanes. In these cases, it can be associated with erythema on the dorsum of the hands or around the ankles, and in the Achilles tendon area. This has been called periarticular thenar erythema and onycholysis (PATEO) syndrome .61 Lastly, paronychia and periungual pyogenic granulomas have been reported with capecitabine, methotrexate or doxorubicin. Bleomycin-associated abnormalities of the nail or nail region can be due to the induction of Raynaud's phenomenon or scleroderma-like changes .38
Lesions predominantly involving pigmentationChemotherapy-induced pigmentary changes are a common cutaneous adverse event that can affect the skin, mucosa or nail apparatus. Many chemotherapy agents can be involved. Although the pathogenic mechanism is not known precisely, it is thought that a direct toxic effect on melanocytes may be responsible .62
The pigmentary changes that may occur mainly take the form of hyperpigmentation, but vitiligo-like lesions have also been described.63 Hyperpigmentation is more common in patients with high phototypes, and it generally occurs after several cycles of therapy. Nevertheless, it can appear at the start of treatment, or at a late stage after it has stopped .64
Induced hyperpigmentation is not specific to a particular chemotherapy agent, and it can arise as a component of various clinical conditions:
- •
Diffuse hyperpigmentation (busulfan, fluorouracil, capecitabine, cyclophosphamide, doxorubicin, daunorubicin) (Fig. 3A); located in occluded areas, large skin folds, and on the elbows, knees and shins (cyclophosphamide, ifosfamide, busulfan, thiotepa, liposomal doxorubicin); in acral areas (fluorouracil, capecitabine, tegafur, cyclophosphamide, ifosfamide, doxorubicin65); light-exposed areas (fluorouracil, capecitabine, daunorubicin66); secondary to previous inflammatory dermatoses (anthracyclines, alkylating agents, antimetabolites) (Fig. 3B) .20
- •
Hyperpigmentation of the mucosa is uncommon, may be overlooked, and is not always easily distinguished from physiological pigmentation or coloration from other causes. It can be produced by antimetabolites, some alkylating agents, anthracyclines or bleomycin .67
- •
As mentioned above, nail pigmentation in the form of melanonychia can occur with various substances (Fig. 3C). It usually appears after several weeks or months of therapy and tends to disappear when treatment ends.
- •
Bleomycin-induced flagellate hyperpigmentation is highly characteristic of that drug, but not exclusively so. It has also been described with bendamustine, docetaxel and trastuzumab, in diseases such as dermatomyositis or Still's disease, and after eating shiitake mushrooms. It has a linear appearance and a flagellate distribution, predominantly on the trunk and at pressure points. It may be preceded by a pruritic inflammatory phase, and residual hyperpigmentation can last for months or years .68
- •
Serpentine supravenous hyperpigmentation is uncommon (fluorouracil, alkylating agents, anticancer antibiotics, taxanes, vinca alkaloids, proteasome inhibitors). It is usually located on the forearm (infusion site), and it follows the superficial venous network. The time of onset is variable, and it can get darker with each cycle .69,70
- •
Reticulate hyperpigmentation is also uncommon (bleomycin, cyclophosphamide, fluorouracil, cytarabine, idarubicin, paclitaxel). It affects the back particularly, and the abdomen, shoulders or buttocks to a lesser extent. The time of onset is variable, and it can sometimes be associated with pruritus or erythema. In most cases it disappears when therapy ends .71
- •
Eruptive naevi are most common in children and young adults following treatment for a hematological malignancy. Substances such as fluorouracil, capecitabine, methotrexate or doxorubicin can be involved (Fig. 3D). The palms and soles are especially prone. The naevi sometimes look dysplastic, and very occasionally a melanoma may develop .72
Chemotherapy-induced hyperpigmentation rarely requires treatment to be discontinued or stopped, and most cases resolve after therapy ends. Exposure to sunlight can encourage its onset, so adequate photoprotection is recommended.
Extravasation injuriesThe overall incidence of extravasation injuries is low and varies according to the series (0.01%-7%). They may become apparent immediately or several days post-infusion. According to the type of reaction, cases can be classified as irritant or vesicant.73 Irritant reactions are generally less serious (local irritation, pain, edema, erythema). They can be caused by alkylating agents, platinum drugs, antimetabolites, topoisomerase inhibitors or liposomal doxorubicin. Vesicant reactions are potentially more serious and can even cause extensive necrosis of surrounding tissues. They can occur with vinca alkaloids, taxanes, anthracyclines and some alkylating agents.
Prevention is crucial (avoid infusing into fragile or small-caliber veins), but if extravasation occurs treatment should be discontinued, and protocols followed according to the drug involved. Surgery is reserved for cases requiring surgical debridement or to treat sequelae .74
Description of the various toxicity scalesThe Common Terminology Criteria for Adverse Events (CTCAE), also known as “common toxicity criteria”, published by the National Cancer Institute (NCI) of the National Institutes of Health (NIH), is the most widely used classification scheme for adverse effects of chemotherapy.75 This scheme standardizes the definition of any treatment-induced toxicity. It is used in both healthcare practice and clinical trials. Toxicity ranges from grade 1 to grade 5 (mild, moderate, severe, life-threatening and fatal, respectively).
The NCI Patient Reported Outcomes-Common Terminology Criteria for Adverse Events (PRO-CTCAE) system is a new way to grade toxicities according to information reported by patients. It complements the CTCAE and is not yet in widespread use. It characterizes the frequency, severity and interference of 78 treatment-induced toxicities from the patient's point of view .76
Other complementary classification schemes exist, such as the World Health Organization Adverse Drug Reaction Terminology (WHO-ART),77 or the adverse effects reporting guidelines issued by the Radiation Therapy Oncology Group (RTOG). They both code clinical information about adverse reactions. In the first case, that information is drug-related; in the second case, it covers all aspects of treatment, including radiotherapy, surgery, and the devices and drugs used.
Adjusting and/or delaying doses or stopping therapy based on toxicityBecause of cutaneous toxicity, doses may be delayed or reduced, and/or therapy stopped, with consequent repercussions for treatment efficacy. No general dose adjustment criteria exist. It is recommended that steps be taken according to the prescribing information for each drug. In order to minimize dose reductions and delays, supportive dermatological treatment should be optimized.
How and when to refer a patient to the dermatology departmentBefore starting therapy, patients should be given detailed information about anticipated toxicities and what to do if they occur. Some sites hold medical consultations for assessing dermatological toxicity induced by chemotherapy and targeted therapies. Patients usually also have access to nursing staff, either face-to-face or by telephone, for unscheduled consultations.
When cutaneous toxicity occurs, it should be assessed for whether it needs to be referred to the dermatology department, and if so, how urgently. If it requires urgent attention, emergency dermatology consultations are very helpful. If none are available, hospital admission or referral to an emergency department should be considered.
It is advisable for patients whose therapy has a high rate of cutaneous toxicity to be referred to the dermatology department for a baseline assessment before treatment begins. A set of recommendations should be drawn up, and the patient should be monitored during therapy.
ConclusionsIn the management of cancer patients, diagnosing and treating chemotherapy-induced cutaneous toxicity is of proven importance. Cutaneous adverse events need to be understood and managed correctly. They require specific treatments, to enable early diagnosis and to prevent disorders that might have repercussions for the patient's cancer therapy. These include delayed or reduced drug doses, switching chemotherapy drugs, or poor treatment adherence, with resultant implications for the patient's prognosis.
Close cooperation between oncologists and dermatologists is critical, because complex pathology can lead to misdiagnosis and mismanagement of the adverse effect. The approach to all chemotherapy-related complications is improving all the time. In the specific case of cutaneous lesions, this is being achieved by multidisciplinary management aimed at preventing, diagnosing and treating these toxicities more effectively, thereby improving the prognosis and quality of life of patients on chemotherapy.
As well as serious toxicities requiring urgent treatment, dermatological complications can also cause cutaneous lesions that have a major impact on one's own body image. Patients may be constantly reminded of their illness, with consequent psychological repercussions. Those who manage to retain a good body image have better quality of life and tolerate their disease and therapies better.
Ethical statementThe study has been performed in accordance with the ethical standards of the Declaration of Helsinki and its later amendments. This article does not contain any studies with human participants or animals performed by any of the authors. All patients gave inform consent for the publication of their images that illustrate this article.
Declaration of AuthorshipOnofre Sanmartín, Carmen Beato, Hae Jin Suh Oh, Isabel Aragón, Agustín España, Margarita Majem, Sonia Segura, Alfonso Gúrpide, Rafael Botella, Cristina Grávalos participated in the conception and design of the study, gathering and analysis of data, writing, critically reviewing and approval of the final version of the manuscript.
Conflict of interestThe authors declare that, when writing and revising the text, they did not know the names of the companies that provided financial support for this project, so this support has not influenced the content of this article.
The authors are grateful for the editorial assistance of Fernando Sánchez-Barbero of HealthCo (Madrid, Spain) in the production of this manuscript. SEOM and AEVD are grateful for financial support for this project in the form of unrestricted grants from AstraZeneca, Avène, Boehringer Ingelheim, Pierre Fabre, Roche Farma, La Roche Posay.
Please cite this article as: Sanmartín O, Beato C, Suh-Oh HJ, Aragón I, España A, Majem M, et al. Manejo clínico de los eventos adversos cutáneos en pacientes tratados con quimioterapia: consenso nacional de la Academia Española de Dermatología y Venereología y de la Sociedad Española de Oncología Médica. Actas Dermosifiliogr. 2019;110:448–459.