Cutaneous squamous cell carcinoma is sometimes characterized by an increased risk of locoregional recurrence and occasionally distant metastasis. Several clinical and pathological factors, including perineural invasion, have been shown to have prognostic value in this setting. Perineural invasion, that is, the spread of tumor cells into the space surrounding a nerve, is usually an incidental finding. In the presence of symptoms or radiographic evidence of perineural spread, the diagnosis is clinical perineural invasion, which is associated with an increased risk of local recurrence and mortality.
En ocasiones el carcinoma epidermoide cutáneo se caracteriza por tener un mayor riesgo de desarrollar recurrencia locorregional y ocasionalmente metástasis a distancia. Existen diversos factores clínico-patológicos de reconocido valor pronóstico, entre ellos la presencia de infiltración perineural. Esta consiste en la diseminación de células tumorales a través de los nervios, siendo en la mayoría de los casos un hallazgo incidental. Cuando aparecen síntomas y/o evidencia radiológica de la extensión de la infiltración perineural, se clasifica como infiltración perineural clínica y se asocia a un mayor riesgo de recurrencia local y de mortalidad.
Perineural invasion (PNI) is defined as the spread of tumor cells into the space surrounding a nerve,1 with little tendency to invade inwards. It was first described by Cruveilhier2 in 1835 in a case of breast cancer invading the facial nerve. Therefore, PNI is not a variety of lymphatic or hematic spread, but rather a direct extension of the primary tumor.3,4
EpidemiologyPNI is underestimated in cutaneous tumors and often goes unnoticed in terms of presence of symptoms and on conventional radiography. The incidence of PNI in cutaneous squamous cell carcinoma (CSCC) is 2.4% to 14%,5–7 increasing to 24% in recurrent CSCC8 and to 29% in CSCC of the mucosa and head and neck.9
The incidence of PNI also varies with the histologic subtype—it is more common in poorly differentiated CSCC9—and with the size of the tumor: PNI can be observed in 64% of tumors larger than 2.5cm and in 11% of those smaller than 2.5cm.10
The mean age of patients is 64 years, with a clear predominance in men (77%).11
PathogenesisPNI was initially thought to involve lymphatic spread of the tumor to the nerves. Subsequently, the nerve sheath was thought to act as a low resistance pathway for the spread of tumor cells.10 This theory was refuted when electron microscopy revealed the high selectivity of the blood-nerve barrier.4,12,13
PNI is currently thought to occur by invasion, as the result of a reciprocal and dynamic association between the tumor and the nerve endings.4,14 Several neurotrophic agents have been shown to be involved, including nerve growth factor,15 the nerve cell adhesion molecule,16,17 brain-derived neurotrophic factor, and neurotrophin 3.4
Plexin-B1 is a subtype of the proteins that intervene in nerve cell adhesion and axon migration during development.18 Binmadi et al.19 showed that plexin-B1 was overexpressed in tissues and in cell lines from neurotropic malignancies and is attracted to nerves that express its ligand, semaphorin 4D, in a Rho/Rho kinase-dependent manner. Nerves are attracted to tumors via this same protein system, thus suggesting that both plexin B1 and semaphorin 4D play a major role in the development of PNI.
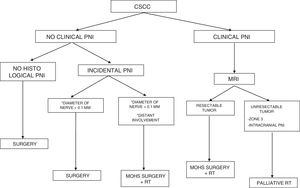
DiagnosisClassification of Perineural InfiltrationPNI is an incidental finding in 60%-70% of patients with CSCC and PNI, that is, it is identified in the histopathology examination of the tumor after surgery. The patient is asymptomatic, and there is no radiological evidence. This form of PNI is known as incidental or microscopic PNI.20,21
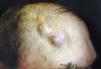
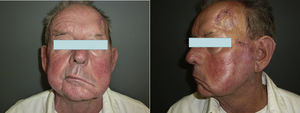
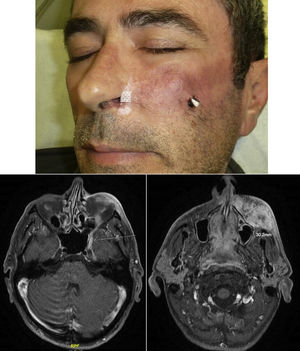
When there are symptoms such as pain, paresthesia, anesthesia, or paralysis and/or radiological evidence of spread of PNI, it is classed as clinical PNI22,23 (Figure 1). The initial symptoms are usually very subtle, with a tingling or numb sensation; these could go unnoticed if the physician does not have a high suspicion of PNI.24 The absence of abnormalities in imaging tests performed on patients with clinical PNI further hampers management.25,26 Pain and motor deficit may be seen in more advanced cases (Figure 2).
These findings could lead to an erroneous diagnosis of Bell palsy, trigeminal neuralgia, or cerebrovascular accident, thus delaying diagnosis and worsening prognosis.27
Anatomy of the Facial NervesThe facial nerve and trigeminal nerve are the most frequently affected, especially the maxillary branch,28,29 with isolated cases of CSCC reported outside the head and neck in patients with clinical PNI.30,31
Knowledge of the anatomical distribution of these nerves is essential for an understanding of PNI and its treatment.32–34
Trigeminal NerveThe trigeminal nerve is the cranial nerve that is most vulnerable to PNI in cutaneous cancer, owing to the abundant innervation of the skin in most of the areas of the head and neck exposed to UV rays. It emerges from the pons, with a sensory root and a motor root. Its sensory (semilunar) ganglion lies in the trigeminal cave on the floor of the middle cranial fossa. It divides into 3 branches from the distal part of the ganglion, as follows:
-V1: The ophthalmic branch, which enters the orbit via the superior orbital fissure. Before doing so, it divides into its 3 main branches (lachrymal, frontal, and nasociliary). The supraorbital nerve is the terminal branch.
-V2: The maxillary branch is a sensory nerve that exits the foramen rotundum and crosses the pterygomaxillary fossa and floor of the orbit. On reaching the infraorbital foramen, it enters the malar area, where it is then known as the infraorbital nerve.
-V3: The mandibular branch arises from the ganglion of Gasser as a sensory nerve. It then courses towards the foramen ovale, through which it enters the zygomatic fossa. As it passes through this foramen, it merges with the motor root of the trigeminal nerve, thus making it a mixed nerve.
Its sensory root is divided into 3 branches: the auriculotemporal nerve, which courses to the parotid gland, where it anastomoses with branches of the facial nerve; the lingual nerve; and the inferior alveolar nerve, which exits the mental foramen as the mental nerve.
The motor root divides into the masticator nerve and the mylohyoid nerve.
Facial NerveTogether with the trigeminal nerve, the facial nerve is the most commonly involved nerve, especially in cases of CSCC that metastasize in the parotid gland.
It emerges from the pontomedullary sulcus, reaches the cerebellopontine angle cistern, and inserts into the internal auditory canal, where it divides into the labyrinthine, tympanic, and mastoid segments. The facial nerve then exits the cranium via the stylomastoid foramen before passing into the parotid gland, where it divides into 2 major trunks, the cervicofacial trunk, which in turn divides into the maxillary, mandibular, and cervical nerves, and the temporofacial trunk, which divides into the temporal branch and the zygomatic branch.
HistologyThe peripheral nerves are formed by bundles of nerve fibers covered by 3 layers known as the epineurium, perineurium, and endoneurium. The endoneurium is a lax connective tissue that surrounds each individual nerve fiber. The perineurium is a specialized connective tissue that surrounds each fascicle of nerve fibers. The epineurium is a dense irregular connective tissue that surrounds a whole peripheral nerve and fills the spaces between the nerve fascicles. The perineurium is the main component of the blood-nerve barrier and is characterized by highly selective permeability.
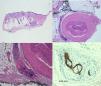
At present, there is no agreed definition of PNI, since establishing histological criteria is controversial. Dunn et al.1 propose the following diagnostic findings. In the presence of a malignant neoplasm, PNI can be diagnosed by observing cytologically malignant cells in the space surrounding the nerve. In equivocal cases, total or near total circumferential involvement is useful, as is the presence of perineural tracking in tangential sections and intraneural involvement.
In contrast, Liebig et al.4 state that the finding of tumor cells within any of the 3 layers of the nerve sheath or tumor foci outside the nerve with involvement of 33% of the circumference is sufficiently characteristic to define PNI.
It is important to remember that other conditions resemble PNI.42–44 These include peritumoral fibrosis, perineural inflammation, squamous epithelium in areas of previous excision, and epithelial sheath neuroma. Immunohistochemistry techniques can prove very useful for establishing the definitive diagnosis.
RadiologyMagnetic resonance is the most sensitive imaging technique for diagnosis of PNI.32,45–47 The study of the whole nerve tract makes it possible to evaluate the presence and extension of PNI in patients who present with signs or symptoms of neurological involvement and thus to plan therapy. The primary findings48 are uptake of the whole circumference of the nerve in gadolinium-enhanced T1-weighted sequences, increase in the normal diameter of the nerve, and obliteration of juxta-foraminal fat pads (Fig. 3).
Atrophy caused by denervation with involvement of a motor branch has been reported to be an indirect sign of PNI.49,50 This is seen in magnetic resonance as a hyperintense signal in acute phases in enhanced T2 images (edema due to denervation) and a lower muscle volume and fatty infiltration in later phases.
These radiological signs may persist indefinitely despite the improvement in symptoms; therefore, recurrence is to be suspected when the lesion grows or symptoms progress.
Computed tomography45,47 is less sensitive, although it is very useful for evaluating bone changes: abnormalities of the cortex, shape, and size of nerve foramina, as well as dilatation of the foramina and erosion of bone in the most advanced cases.
Positron emission tomography51 is very useful in cancer of the head and neck. It detects invasion of the lymphatic system, metastases, and residual or recurrent tumors. However, to date, there have been no analyses of its sensitivity for identifying PNI. Its sensitivity may be very low, since it only detects recurrence and metastasis in tumors over a certain size (>1.5-2.0cm in most cases).
If imaging tests reveal intracranial PNI or perineural spread, the lesion may be considered unresectable.
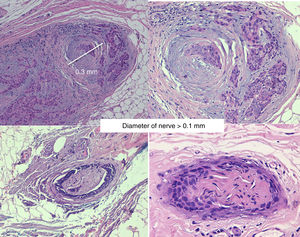
PrognosisIn the absence of other risk factors, the presence of PNI does not indicate a poorer prognosis if the diameter of the affected nerve is lower than 0.1mm and it is not near the primary tumor. Therefore, adjuvant therapy is not considered necessary in these cases.35,36
Campoli et al9 revealed that while PNI is an uncommon finding in CSCC, it is associated with previous markers of poor prognosis, namely, the size and thickness of the tumor, clinical risk factors, and significant subclinical extension. The authors suggest that incidental PNI may serve as a marker to improve the accuracy of prognostic evaluation in these patients.
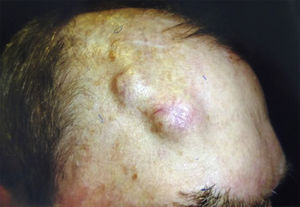
Invasion of thicker nerves or nerves at some distance from the main focus of the tumor,35 irrespective of the diameter (Figs. 4 and 5), implies a poorer prognosis. Clayman et al.,40 found that disease-specific survival at 3 years was 64% in patients with CSCC and PNI, compared with 91% in patients with CSCC without PNI.
A recent systematic review based on 12 studies covering 640 cases of CSCC with PNI found no differences in the risk of lymph node metastasis or distant metastasis between patients with microscopic PNI and patients with clinical PNI. However, the latter have a greater risk of local recurrence and a risk of death of 30%.37–39,41
Zonal ClassificationWilliams et al.47 classified PNI by zone of involvement in order to determine its spread along the facial and trigeminal nerves in magnetic resonance imaging and thus identify which cases warranted surgical resection. Three zones were established, as follows: peripheral (zone 1), central/skull base (zone 2), and cisternal (zone 3) (Table 1).
Classification of Perineural Infiltration by Zone in Head and Neck Tumors.
| Zone 1 |
| V1: to the superior orbital fissure |
| V2: to the external aperture of the foramen rotundum |
| V3: to the external aperture of the foramen ovale |
| VII: to the external aperture of the stylomastoid foramen |
| Zone 2 |
| V1, V2, and V3: from zone 1 to the Gasserian ganglion |
| VII: from zone 1 to the lateral end of the internal auditory canal |
| Zone 3 |
| The whole nerve from the Gasserian ganglion or internal auditory canal into the cistern and brain stem |
This classification has also been shown to be a key predictor of adverse outcomes,52 with a 5-year survival rate of 85.7% for zone 1, 64.4% for zone 2, and 20% for zone 3.
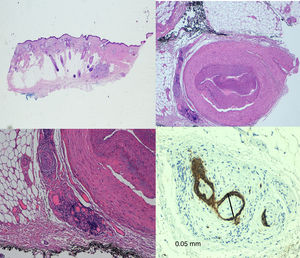
TreatmentMohs surgery53,54 is considered the technique of choice for the treatment of CSCC. It achieves lower recurrence rates and greater sensitivity for detection of PNI than standard surgery, since it enables full examination of the lateral and deep margins and comes as close as possible to complete removal of the tumor. The most widely used technique may be the slow version with formalin-fixed tissue (Fig. 6).
Rowe et al.55 reported a local recurrence rate of 47% for standard surgery and 0% for Mohs surgery. Based on 17 cases of CSCC with PNI treated with Mohs surgery and followed for 42 months, Cottel5 reported no cases of local recurrence and 1 case of metastasis (5.9%). In their retrospective study of 70 patients treated with Mohs surgery, Leibovitch et al.11 reported a recurrence rate of 8% in 5 years.
The presence of an inflammatory infiltrate around a nerve, which is considered a marker of PNI, indicates the need to continue monitoring the nerve until tumor cells are identified or until the inflammation resolves. In fact, even without the inflammatory infiltrate, distant lesions have been observed further along the nerve at up to 14cm beyond the initial tumor site. Such lesions are known as skip lesions.56 These could explain the relatively higher rate of postsurgical recurrence of CSCC with PNI than of lesions without PNI.
Therefore, some researchers consider that an additional safety margin should be used with Mohs surgery, even after ensuring a negative histological margin in cases of PNI.11 Other researchers, however, do not recommend this approach because they believe that the presence and location of skip lesions are unpredictable and, therefore, treat patients with adjuvant radiotherapy, preserving the healthy tissue as much as possible.54
PNI is generally an incidental histological finding after complete removal of the primary tumor. In patients with negative surgical margins and PNI affecting small cutaneous nerves (diameter <0.1mm), management should be limited to clinical follow-up. In the case of significant PNI (diameter>0.1mm), even when the surgical margins are free after removal, adjuvant radiotherapy is inidicated.57
Patients with clinical PNI must undergo an exhaustive assessment of the signs and symptoms of the condition during the pretreatment clinical examination. In addition, therapy must be aggressive in order to minimize the risk of tumor recurrence and metastasis.
If imaging tests indicate that the primary tumor is resectable, then the approach should be aggressive surgical resection followed by radiotherapy. The 2017 update of the National Comprehensive Cancer Network recommends adjuvant radiotherapy in CSCC with extensive PNI.
When this approach is not possible—disease affecting zone 3, PNI near or within the cranial cavity, and tumors that spread along a cranial nerve to the skull base—palliative radiotherapy is indicated, thus considerably reducing the possibility of cure.
No prospective studies have been performed to confirm the efficacy of adding chemotherapy in patients with CSCC and PNI.29 Clinical trials are ongoing with an anti-PD1 monoclonal antibody in patients with metastatic or locally advanced CSCC, and it would be interesting to evaluate its usefulness as adjuvant therapy in patients with PNI in order to minimize the risk of local recurrence and metastasis.58–61
Follow-upGiven the considerable invasiveness and high recurrence rates of PNI, affected patients should be followed very closely when the condition is significant. The first 24 months are the period when the risk of local recurrence is highest. Follow-up should be based on routine detailed examination of the trigeminal and facial nerves, as well as assessment of ocular motility.62 Furthermore, twice-yearly magnetic resonance imaging should be performed. While the usefulness of radiological monitoring has not been quantified, early detection and subsequent retreatment can increase survival.
ConclusionsGiven the role of PNI in the prognosis of CSCC, early detection is essential. Optimal therapeutic management depends on a detailed clinical examination, an exhaustive radiological and histological assessment, and implementation of a follow-up protocol.
Funding(2017-172-001) Universidad Católica de Valencia (UCV), internal funding for research.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Pérez García MP, Mateu Puchades A, Sanmartín Jiménez O. Invasión perineural en el carcinoma epidermoide cutáneo. Actas Dermosifiliogr. 2019;110:426–433.