Corticosteroids can cause hypersensitivity reactions, particularly delayed-type allergic reactions. A new classification system for testing hypersensitivity to corticosteroids distributes the drugs into 3 groups according to molecular structure; patients are classified according to whether they are allergic to agents in 1 or more of the groups. We aimed to describe the clinical characteristics of corticosteroid-allergic patients treated at our clinic and apply the new classification system to them; we also compared these patients’ characteristics to those of others treated at our clinic.
Material and methodsRetrospective study of cases of delayed-type corticosteroid hypersensitivity treated in the skin allergy clinic of a tertiary level hospital over an 11-year period.
ResultsWe reviewed the records of 2857 patients, finding 33 with at least one positive patch test result showing corticosteroid hypersensitivity. Atopic dermatitis and hand involvement were less common in our corticosteroid-allergic patients. All were allergic to a group 1 corticosteroid (most often, budesonide, the culprit in 87.9%). Testing with a specific corticosteroid series revealed that 14 (42.4%) were also allergic to corticosteroids in group 2 and/or group 3. None were allergic exclusively to group 2 or group 3 agents. Twenty-one patients were exposed to a corticosteroid cream from a group their patch test results indicated allergy to; 13 of them (61.9%) did not develop a hypersensitivity reaction.
ConclusionsThe Spanish standard series only contains group 1 corticosteroids. In the interest of improving allergy management, we recommend testing with a specific corticosteroid series and a patient's own creams whenever patch testing with a standard series reveals a hypersensitivity reaction to corticosteroids.
Los corticoides pueden producir reacciones de hipersensibilidad, sobre todo retardadas. Se ha propuesto una nueva clasificación para el estudio de la alergia a corticoides que los divide en 3 grupos según su estructura molecular y establece 2 perfiles de pacientes según estén sensibilizados a uno o varios grupos. Los objetivos de este estudio son describir las características clínicas de nuestros pacientes alérgicos a corticoides, compararlas con las del resto de la población estudiada y analizar su distribución según la nueva clasificación.
Material y métodosEstudio retrospectivo de 11 años que incluye los casos de pacientes con reacciones de hipersensibilidad retardada a corticoides en la Unidad de Alergia Cutánea del Servicio de Dermatología de un hospital terciario.
ResultadosEstudiamos a 2.857 pacientes, de los cuales 33 presentaron uno o más parches positivos a los corticoides. Estos pacientes presentaron menos dermatitis atópica y menor afectación de las manos. Todos fueron alérgicos a algún corticoide del grupo 1 y la budesonida fue el más frecuente (87,9%). Con la batería específica de corticoides observamos que 14 (42,4%) eran, además, alérgicos a corticoides del grupo 2 o 3. Ninguno fue alérgico solo a corticoides del grupo 2 o 3. El 61,9% (13/21) de los pacientes que fueron testados con cremas con un corticoide del grupo al cual era alérgicos no presentó reacción a aquellas.
ConclusionesLa batería estándar española tiene solo marcadores para la alergia a corticoides del grupo 1. Recomendamos aplicar una batería específica de corticoides y los fármacos propios si los marcadores son positivos para poder clasificarlos mejor y adecuar su manejo terapéutico.
Corticosteroids are widely used in dermatology. They were introduced as topical agents in 1952,1 and, although they have proven very efficacious, they are not free from adverse effects. Despite their anti-inflammatory and immunomodulatory properties, corticosteroids can also behave—albeit paradoxically—as allergens and cause mainly delayed-type hypersensitivity reactions.2–6
Corticosteroid-induced allergic contact dermatitis is increasingly common, and prevalence ranges from 0.2% to 5%. Attempts have been made to classify corticosteroids into groups in order to better define sensitization to them. In 1989, corticosteroids were classified into 4 groups, namely, A, B, C, and D,7 which in 2000 was subdivided into D1 and D28 depending on chemical structure and cross-reactivity patterns. The drugs with the greatest sensitizing capacity were in groups A and D2. Subsequently, it was observed that cross-reactions were occasionally not as predicted or expected according to the classification, which was then modified. In 2009, Baeck et al.9 studied molecular models of corticosteroids and patch test results from 315 corticosteroid-sensitized patients. In 2011, they proposed a new, simpler classification that divided corticosteroids into 3 groups (Table 1).10,11 Group 1 comprises those drugs that most commonly produce allergic reactions, and group 3 those that have the lowest sensitizing capacity and produce the fewest cross-reactions.12 The authors also classified patients into 2 profiles depending on whether they were sensitized to 1 or more groups; therefore, patients with profile 1 only react to a single group and those with profile 2 react to several groups.
Classification of Corticosteroids According to Baeck et al10
| Group 1 | Group 2 | Group 3 |
|---|---|---|
| Nonmethylated nonhalogenated molecules | Halogenated molecules (except*) with a cis-ketal or diol structure in C16/C17 | Halogenated methylated molecules in C16 |
| Correspond to budesonide and group A and group D2 of the classification of Coopman et al.7 | Correspond to group B of the classification of Coopman et al. | Correspond to group C and group D1 of the classification of Coopman et al. |
| BudesonideCloprednolCortisone acetateDichlorisone acetateDifluprednateFludrocortisone acetateFluorometholoneFluprednisolone acetateHydrocortisoneHydrocortisone aceponateHydrocortisone acetateHydrocortisone 17-butyrateHydrocortisone 21-butyrateHydrocortisone hemisuccinateIsofluprednone acetateMazipredoneMedrysoneMethylprednisolone acetateMethylprednisolone aceponateMethylprednisolone hemisuccinatePrednicarbatePrednisolonePrednisolone caproatePrednisolone pivalatePrednisolone sodium metasulfobenzoatePrednisolone succinatePrednisoneTixocortol pivalate | TriamcinoloneAmcinonideDesonide*FluchloronideFlumoxonideFlunisolideFluocinolone acetonideFluocinonideHalcinonide*Triamcinolone acetonideTriamcinolone benetonideTriamcinolone diacetateTriamcinolone hexacetonide | Alclometasone dipropionateBeclomethasone dipropionateBetamethasoneBetamethasone 17-valerateBetamethasone dipropionateBetamethasone sodium phosphateClobetasol propionateClobetasone butyrateDesoximetasoneDexamethasoneDexamethasone acetateDexamethasone sodium phosphateDiflucortolone valerateDiflorasone diacetateFlumethasone pivalateFluocortin butylFluocortoloneFluocortolone caprylateFluocortolone pivalateFluocortolone acetateFluprednidene acetateHalometasoneMeprednisoneFluticasone propionateMometasone furoate |
The main objective of the present study was to analyze the clinical and demographic characteristics of corticosteroid-sensitized patients followed at the skin allergy unit of our dermatology service during the last 11 years. The secondary objectives were to compare these characteristics with those of the other study patients and to group patients according to the new classification.
Material and MethodsStudy DesignThe study was performed in the Skin Allergy Unit of the Dermatology Department of Hospital General Universitario de Alicante, Alicante, Spain and took the form of a retrospective review of all patients diagnosed with delayed-type sensitivity reactions to corticosteroids between January 2004 and December 2014.
Study PopulationWe included all study patients who underwent patch testing and were sensitized to a corticosteroid. The clinical data recorded for each patient were age and sex, profession, history of atopy, location of skin lesions, and subsequent use of corticosteroids. The results recorded from the patch tests were positivity to corticosteroids, positivity to the patient's own commercially available creams, and cosensitization to other allergens. We used the MOAHLFA index to compare clinical data from these patients with those of the other patients on whom we performed skin tests during the study period. The data were retrieved from the electronic database at the clinic.
Method and Patch Test ReadingsAll patients underwent patch testing with the standard series of the Spanish Contact Dermatitis and Skin Allergy Research Group (GEIDAC), which includes budesonide and tixocortol 21-pivalate as markers of allergy to corticosteroids. Furthermore, patients assessed from October 2008 onward (20 patients in total) also underwent patch testing with hydrocortisone 17-butyrate. Budesonide was applied at 0.01% in petrolatum (pet), tixocortol 21-pivalate at 0.1% pet, and hydrocortisone 17-butyrate in 1% alcohol (all supplied by Chemotechnique Diagnostics AB). Patients with positive results to one of these markers were assessed using a specific corticosteroid series (Chemotechnique Diagnostics AB), which, in addition to the corticosteroids from our standard series, included triamcinolone acetonide (1.0% pet), dexamethasone 21-phosphate (1.0% pet), clobetasol 17-proprionate (1.0% pet), betamethasone 17-valerate (1.0% pet), and alclometasone 17,21-diproprionate (1.0% pet). Many patients underwent testing with their own commercially available corticosteroid-containing cream or a cream that was available at the clinic. The patches were prepared using adhesive Finn chamber strips (SmartPractice), which were stuck to the patient's back for 48hours. Test results were read at 48 and 96hours according to the criteria of the International Contact Dermatitis Research Group (+, ++, and +++).
Statistical AnalysisData were processed using SPSS. Qualitative variables were analyzed using the chi-square test. When the conditions for application of the chi-square test were not met, we used the Fisher exact test. Statistical significance was set at P≤.05. The results were analyzed, interpreted, and compared with those obtained from similar studies.
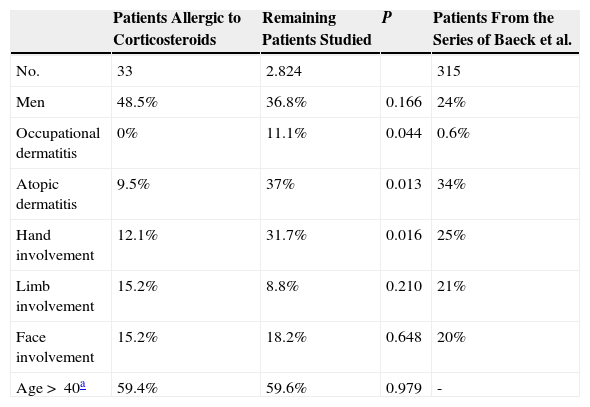
ResultsClinical Characteristics of Corticosteroid-Sensitized Patients and Comparison With Data From the Total Population Studied and With Data From the Series of Baeck et al.9Patch testing was performed on 2857 patients, of whom 33 had a positive result to 1 or more corticosteroids (sensitization rate of 1.16%). The clinical and demographic characteristics of these patients, those of the rest of the study population, and those of patients from the series of Baeck et al.9 are shown in Table 2. It is noteworthy that the hands were less commonly involved in corticosteroid-allergic patients than in the rest of the study population and that as many as 7 of the 33 patients (21.2%) had generalized eczema. No cases of occupational allergy were detected among the corticosteroid-allergic patients. It is striking that only 9.5% of the corticosteroid-allergic patients had underlying atopic dermatitis, in contrast with 37% in the rest of the study population.
Clinical and Demographic Characteristics of Patients Sensitized to Corticosteroids in our Series, of the Other Patients Studied Using Patch Testing in our Department, and of Patients From the Series of Baeck et al.9 Using the MOAHLFA Index.
| Patients Allergic to Corticosteroids | Remaining Patients Studied | P | Patients From the Series of Baeck et al. | |
|---|---|---|---|---|
| No. | 33 | 2.824 | 315 | |
| Men | 48.5% | 36.8% | 0.166 | 24% |
| Occupational dermatitis | 0% | 11.1% | 0.044 | 0.6% |
| Atopic dermatitis | 9.5% | 37% | 0.013 | 34% |
| Hand involvement | 12.1% | 31.7% | 0.016 | 25% |
| Limb involvement | 15.2% | 8.8% | 0.210 | 21% |
| Face involvement | 15.2% | 18.2% | 0.648 | 20% |
| Age>40a | 59.4% | 59.6% | 0.979 | - |
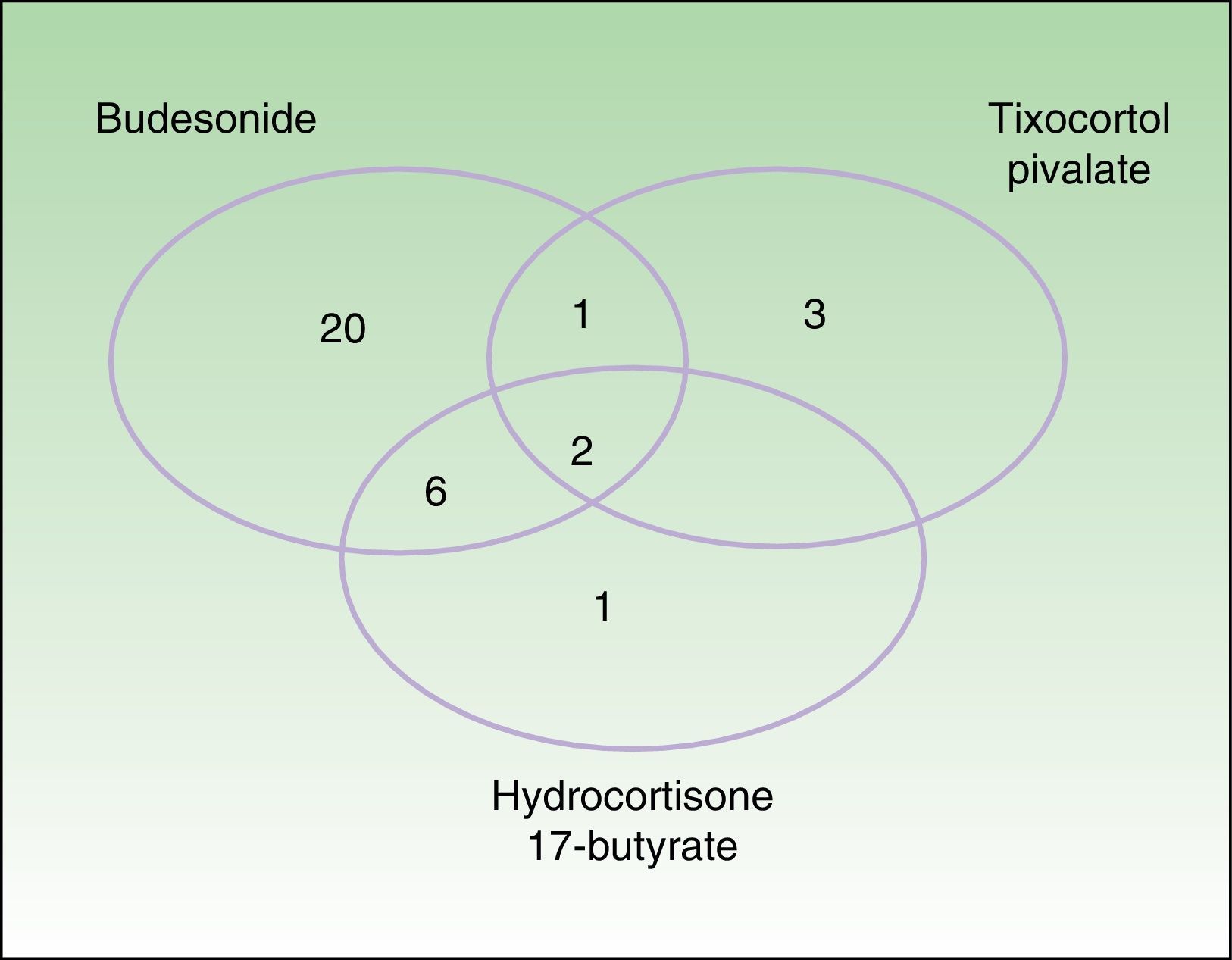
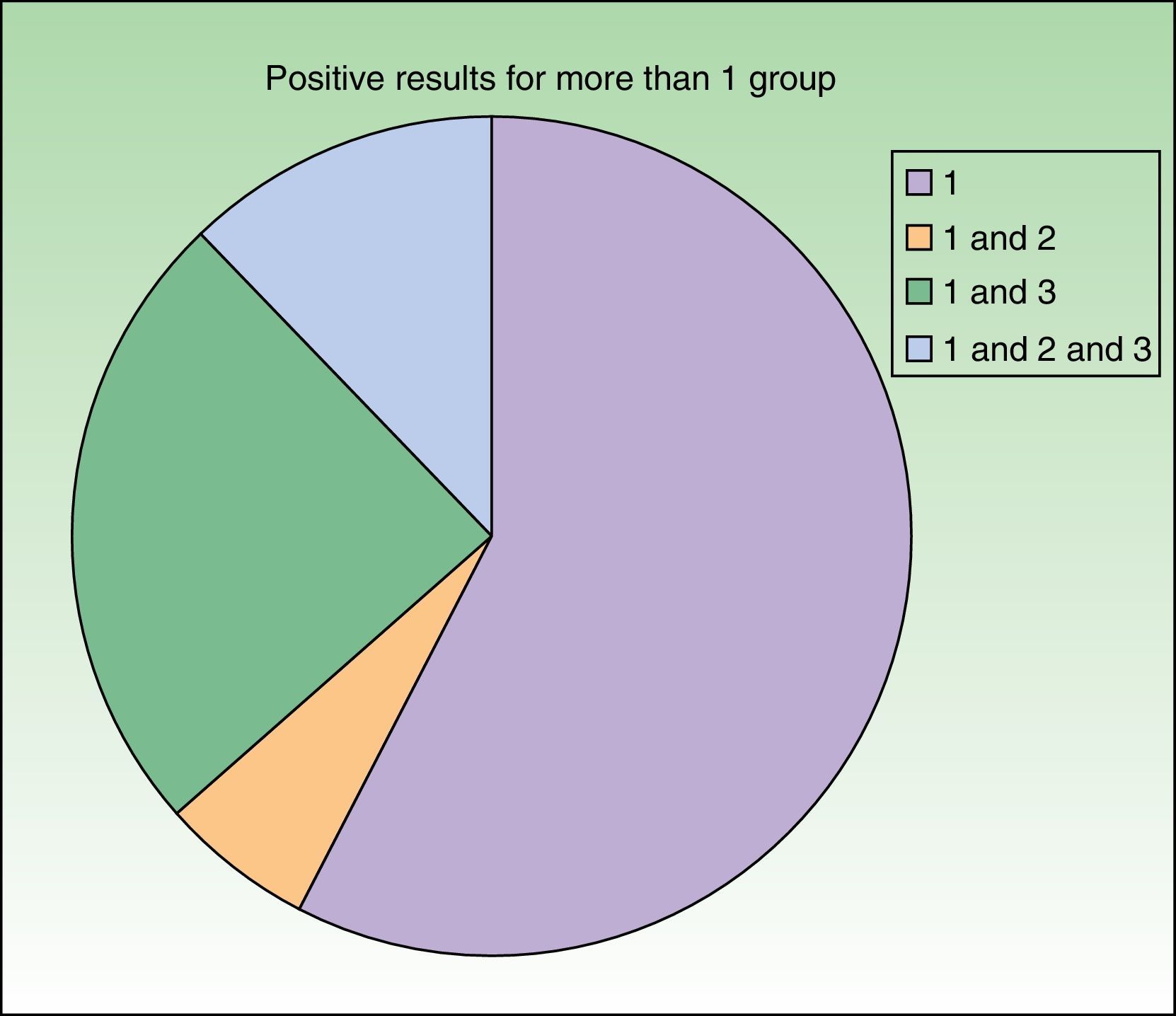
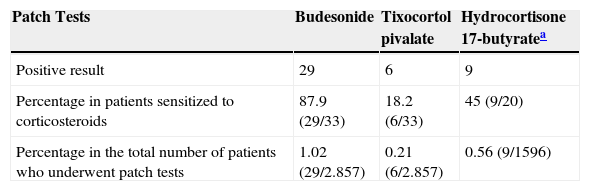
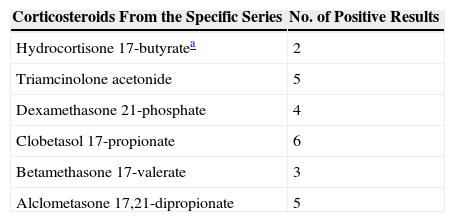
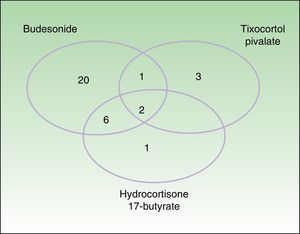
Testing with markers of corticosteroid allergy in our standard series yielded positive results in 33 patients. The prevalence of allergy to budesonide was 87.9% (29/33), that of tixocortol pivalate was 18.2% (6/33), and that of hydrocortisone 17-butyrate was 45% (9/20) (Table 3). Figure 1 shows the distribution of positive results with the corticosteroids from the standard series. Of note, only 4 patients had a negative result for budesonide; 3 were detected with tixocortol pivalate and 1 with hydrocortisone 17-butyrate. The results for the specific corticosteroid series are shown in Table 4. The specific series made it possible to detect 14 patients who, in addition to being allergic to group 1 corticosteroids, were also allergic to those from group 2 or 3. Figure 2 shows the distribution of patients according to the new classification of Baeck et al.10
Frequency of Positive Results With Markers of Allergy to Corticosteroids From the Standard Series.
| Patch Tests | Budesonide | Tixocortol pivalate | Hydrocortisone 17-butyratea |
|---|---|---|---|
| Positive result | 29 | 6 | 9 |
| Percentage in patients sensitized to corticosteroids | 87.9 (29/33) | 18.2 (6/33) | 45 (9/20) |
| Percentage in the total number of patients who underwent patch tests | 1.02 (29/2.857) | 0.21 (6/2.857) | 0.56 (9/1596) |
Frequency of Positive Results With the Corticosteroids Studied in the Specific Series in Patients With Allergic Contact Dermatitis to the Corticosteroids in Our Series.
| Corticosteroids From the Specific Series | No. of Positive Results |
|---|---|
| Hydrocortisone 17-butyratea | 2 |
| Triamcinolone acetonide | 5 |
| Dexamethasone 21-phosphate | 4 |
| Clobetasol 17-propionate | 6 |
| Betamethasone 17-valerate | 3 |
| Alclometasone 17,21-dipropionate | 5 |
Distribution of patients according to the classification of Baeck et al.10 All patients were sensitized to group 1 corticosteroids. Sensitization was to group 1 only in 19 cases (57.6%) and to more than 1 group in 14 cases (42.4%), as follows: 2 patients (6.1%) were sensitized to groups 1 and 2; 8 patients (24.2%) were sensitized to groups 1 and 3; and 4 patients (12.1%) were sensitized to groups 1, 2, and 3.
We performed patch testing with commercially available corticosteroid creams in 21/33 patients (63.6%). However, we observed no reactions in 13 of these patients (61.9%) after testing with a cream that contained a corticosteroid from the group to which the patient was allergic. The clinical history revealed that after the diagnosis of corticosteroid allergy was made, 5 patients had been prescribed a topical corticosteroid from the same group to which they were sensitized, without ever having presented a reaction. It also revealed that no reactions were recorded in a further 5 patients who had taken oral prednisone. Only 1 patient (allergic to corticosteroids from all 3 groups) presented a generalized reaction after application of intralesional triamcinolone acetonide to treat a nasal polyp, although no reaction was observed after inhalation of fluocinolone acetonide.
Thirty patients (90.9%) were cosensitized to other allergens. Of note, 8 patients (24.2%) were allergic to fragrances. This frequency was higher than that of the rest of the study population, among whom 8.3% were sensitized to fragrances. It is also noteworthy that 6 patients (18.2%) were cosensitized to ethylenediamine dihydrochloride and 3 (9.1%) to neomycin.
DiscussionThe prevalence of sensitization to corticosteroids in the present study (1.16%) reflects that reported in other Spanish series,13–15 including a similar study performed at our hospital between 2004 and 2007. However, these values are lower than those reported in studies from Europe (2.6%),16 the United States (4.6%),17 and Asia (3.29%),18 probably because of the lower corticosteroid concentrations used in patch tests in Europe. In addition, compared with the rest of Europe, it is possible that the corticosteroids used in Spain are less potent, treatment is administered earlier, or treatment schedules are shorter.
Corticosteroid-allergic patients have specific demographic characteristics. Thus, in our series, we did not find as high a percentage of women as in the rest of the population that underwent patch testing, although the differences are not statistically significant. Similarly, we did not find as high a percentage of women as in other series (51.8% compared with 76%).9 We were surprised by the lower percentage of atopic dermatitis, given that patients with this condition more frequently use aerosols with corticosteroids and apply topical corticosteroids to their skin. These findings are not consistent with those of Baeck et al9—much larger than ours—in which 34% of patients were atopic; therefore, it remains unclear whether atopy is a risk factor for developing corticosteroid allergy. The distribution by anatomical site affected is also interesting, since the allergy is usually generalized or diffuse, mainly on the trunk, and its presentation as hand eczema is much less common than in the rest of the study population. We are unable to provide an explanation for this finding, since hand eczema tends to be chronic, with subsequent long-term use of corticosteroids. It may be because the corticosteroids used (clobetasol 17-propionate, mometasone furoate) are highly potent group 2 and 3 agents. As expected, we found no occupational cases, since reporting of this condition is exceptional in the literature.19
The GEIDAC standard series only contains budesonide and tixocortol pivalate but not hydrocortisone 17-butyrate. In our series, it is striking that most corticosteroid-allergic patients were detected with budesonide, consistent with previous data from Spanish series.13–15 In the United States, however, most patients were detected with tixocortol20; in Europe, sensitization rates for budesonide, tixocortol, and hydrocortisone 17-butyrate seem to be similar.21 We believe these discrepancies are due to factors such as national prescription habits and to the diagnostic tests used (budesonide and tixocortol are applied in higher concentrations [0.1% and 1%, respectively] in the American standard series). As for the usefulness of these substances as markers of corticosteroid allergy, it is thought that 90% of corticosteroid-allergic patients are detected with budesonide and tixocortol.22 We do not think it is necessary to add hydrocortisone 17-butyrate to the GEIDAC standard series. We did not detect allergy to group 2 or 3 corticosteroids separately, since all the markers in the standard series are from group 1. We believe that group 2 and 3 corticosteroids should be temporarily added to the standard series in order to determine the prevalence of allergy to the drugs they contain.
The study of the specific corticosteroid series and commercially available creams was extremely useful, because it enabled us to detect 14 patients who were sensitized to drugs from groups 2 and 3 (Fig. 3). We believe that the best approach would be to have a specific series that reflected the use of corticosteroids in a particular country. We recommend patch testing with the patient's own commercially available corticosteroid-containing cream, since we can then choose the corticosteroids the patient will be able to use. It is not unusual for a patient to tolerate corticosteroids belonging to a group to which he/she is, in theory, allergic. Therefore, even though the recent classification by Baeck et al.10 has made it possible to better reclassify corticosteroids according to their chemical structure and patients according to whether they are allergic to corticosteroids from 1 or more groups, it is not ideal, since many of the patients subsequently do not present reactions after using topical corticosteroids from the group they are sensitized to. Similarly, it does not seem useful to recommend inhaled or systemic corticosteroids.
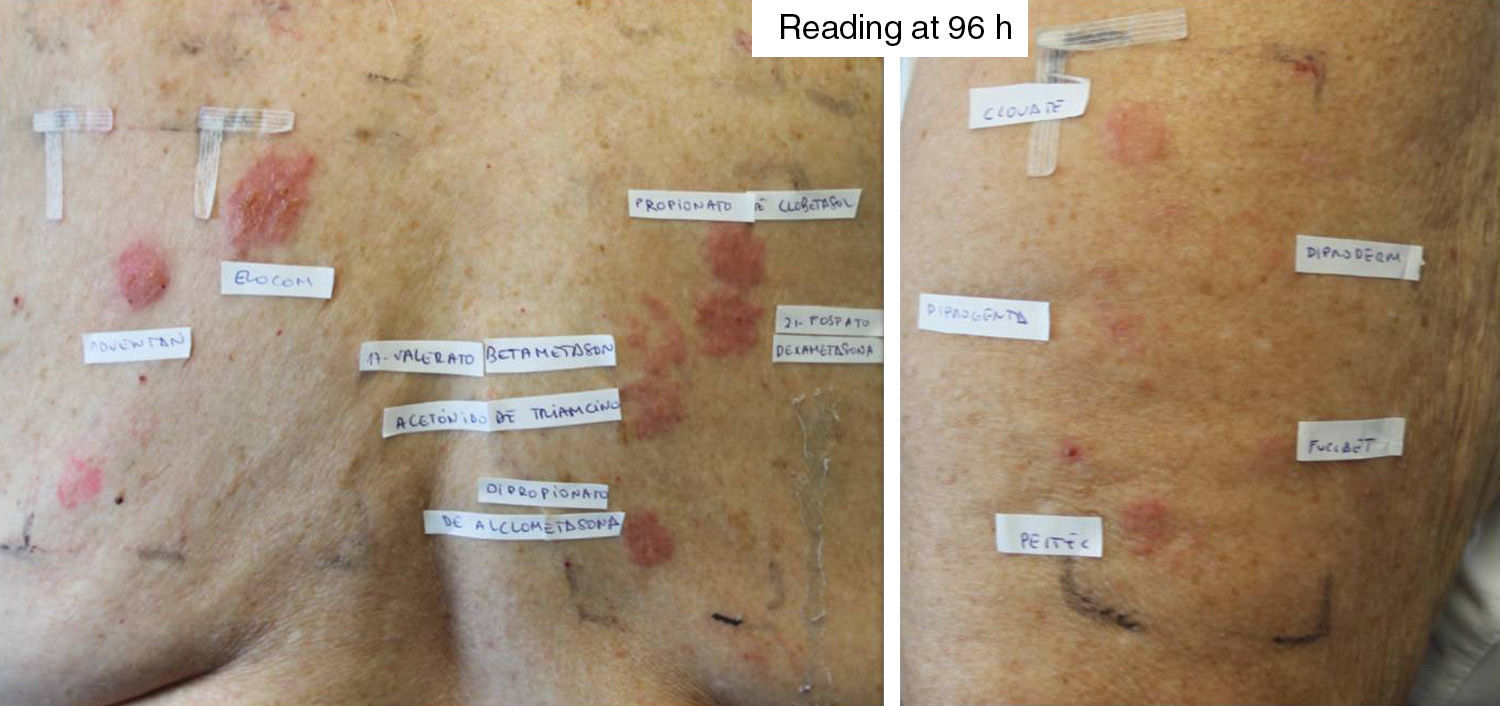
Patient diagnosed with Hailey-Hailey disease sensitized to all 3 groups of corticosteroids. The standard series revealed positive results for budesonide, tixocortol pivalate, and hydrocortisone 17-butyrate. A reading at 96hours with the patients’ own corticosteroids revealed the following results: Adventan (methylprednisolone) +++, Elocom (mometasone furoate) +++, Clovate (clobetasol propionate) ++, Diproderm (betamethasone 17,21-dipropionate)+Diprogenta (betamethasone)+Fucibet (fusidic acid and betamethasone valerate)+Peitel (prednicarbate) ++. With the corticosteroid series the results were as follows: clobetasol propionate +++, dexamethasone 21-phosphate +++, betamethasone 17-valerate ++, triamcinolone acetonide ++, and alclometasone dipropionate ++.
Corticosteroid-allergic patients are cosensitized to multiple allergens, the most commonly involved being neomycin sulfate and ethylenediamine dihydrochloride. Cosensitization has been attributed to the presence of these allergens in a single pharmaceutical cream. In our series, the high percentage of cosensitization with fragrances is noteworthy. We do not know if this cosensitization is due to the presence of fragrances in topical treatments or to the use of perfumed products by patients with chronic eczema.
ConclusionsCorticosteroid-induced allergic contact dermatitis represents a major restriction for the patient and a diagnostic and therapeutic challenge for the dermatologist. At present, the GEIDAC standard series has 2 good markers that serve to detect allergy to group 1 corticosteroids. However, these do not seem likely to be useful for detecting allergy to corticosteroids from other groups; therefore, our standard series should include, at least temporarily, representatives of groups 2 and 3. Testing the specific corticosteroid series and the products of patients with positive results to any of the markers from the standard series is highly recommended, since it provides information on the patient's sensitivity profile. Nevertheless, the many inconsistencies between real-life results for patch testing and patient tolerance to the various commercially available corticosteroid-containing creams complicate therapy and hamper recommendations. In the meantime, it seems prudent to advise corticosteroid-allergic patients to avoid corticosteroids from the group they are allergic to. Furthermore, it would be appropriate to perform patch testing or test with the drugs the patient is likely to use. An alternative would be to use calcineurin inhibitors and reserve corticosteroids as the therapeutic option of last resort. The use of oral corticosteroids should also be restricted, especially in patients sensitized to corticosteroids from several groups, although most of the patients in the present series did not experience problems after use.
Ethical DisclosuresProtection of Persons and AnimalsThe authors declare that this research did not involve experiments performed on humans or animals.
Confidentiality of DataThe authors declare that no private patient data are disclosed in this article.
Right to Privacy and Informed ConsentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We are grateful to Dr. Sánchez (Preventive Medicine Department, Hospital Universitario de Alicante) for help with the statistical analysis.
Please cite this article as: Berbegal L, DeLeon FJ, Silvestre JF. Estudio de sensibilización a corticoides en una consulta de alergia cutánea. Actas Dermosifiliogr. 2015;106:816–822.