Surgical excision with margins of 0.5cm is the standard treatment for lentigo maligna (LM). Excision, however, is often incomplete as many of these tumors have indistinct borders.
ObjectiveTo identify clinical predictors of subclinical extension in primary and recurrent LM of the head and thereby determine which lesions might require wider surgical margins.
Material and methodsWe reviewed the clinical records of patients with LM of the head treated definitively with conventional surgical excision or slow micrographic Mohs surgery (MMS) at the dermatology department of Instituto Valenciano de Oncología between January 1993 and April 2011.
ResultsSurgical margins larger than 0.5cm were required in 69.2% of recurrent LM and 26.5% of primary LM. Factors associated with the need for wider margins were prior treatment that might have interfered with the clinical delineation of the border, lesions in the center of the face, and skin phototypes III to V.
ConclusionsSurgical margins of 0.5cm are inadequate for the treatment of a considerable number of LM lesions located on the head, particularly if these are recurrent. Slow MMS using paraffin-embedded sections appears to be the treatment of choice in such cases, particularly for recurrent lesions or lesions with poorly defined borders or possible subclinical extension.
El tratamiento estándar del lentigo maligno (LM) es la escisión quirúrgica con márgenes de 0,5cm. Sin embargo, dada la mala delimitación de muchos tumores, es frecuente que esta exéresis sea incompleta.
Objetivoidentificar parámetros clínicos que puedan predecir qué LM localizados en la cabeza, extirpados de forma primaria o tras recidivar, se extienden más allá de los límites visibles y por tanto, puedan requerir márgenes quirúrgicos más amplios.
Material y métodosse revisó retrospectivamente la información clínica de los pacientes con LM localizado en la cabeza cuyo tratamiento quirúrgico definitivo, mediante cirugía convencional o cirugía de Mohs diferida, fue realizado en el Servicio de Dermatología del Instituto Valenciano de Oncología (IVO) entre enero de 1993 y abril de 2011.
Resultadosun 69,2% de los LM recidivados y un 26,5% de los tumores primarios requirieron márgenes de más de 0,5cm. La administración previa de tratamientos que puedan interferir en la delimitación clínica, la localización centrofacial y las lesiones que se presentan en pacientes con fototipos altos (III-V) fueron los factores asociados a la necesidad de márgenes quirúrgicos más amplios.
Conclusionesla utilización de márgenes de 0,5cm para el tratamiento del LM es insuficiente para un número importante de casos localizados en la cabeza, especialmente los recidivados. La cirugía de Mohs diferida, con el estudio de todos los márgenes en parafina, parece el tratamiento de elección en particular para los casos recidivados o en los que la delimitación clínica pueda verse dificultada.
Lentigo maligna (LM) is a subtype of melanoma in situ that develops in older patients, typically appearing in sun-exposed skin that has suffered chronic actinic damage.1 The standard treatment for LM is surgical excision of the tumor with margins of 0.5cm. Excision is often incomplete, however, as many of these tumors have indistinct borders.2–5 Between 6% and 20% of LM melanomas recur, probably because tumor cells have spread beyond the clinically apparent border and because conventional histology evaluates only 5% of the margin removed.6–8 The subclinical extension of the LM seems to be related to tumor size and some authors have therefore suggested that when larger tumors are removed, larger margins should be taken to ensure complete excision.9 In fact, the latest recommendations of the National Comprehensive Cancer Network state that margins wider than 0.5cm may be necessary for complete removal and that it is advisable to perform complete circumferential peripheral and deep margin assessment.
Various surgical techniques, such as Mohs surgery in its different forms, facilitate the evaluation of 100% of the tumor margin while minimizing the removal of healthy surrounding tissue.10 Several retrospective studies have shown that these methods can reduce LM recurrence to between 0.5% and 3%.9,11–15 Most authors prefer some version of slow Mohs surgery in which paraffin-embedded tissue is processed because it is difficult to identify melanocytes in frozen sections even with the aid of immunohistochemical staining.6,16–19 We have also observed fewer recurrences since we implemented the Mohs technique in our practice. In most cases the tumor can be removed in a single stage, but more than 2 stages may be required in some patients. The size of the LM melanoma, and perhaps other clinical features, potentially affect the margin width that would be adequate for curative excision. We hypothesized that there may be a correlation between certain clinical LM features and the likelihood of subclinical spread. If so, we think that identifying such features would allow us to plan the most appropriate approach, whether using conventional surgery with preestablished margins or slow Mohs surgery.
The main aim of this study was to identify the clinical variables that can predict which primary or recurrent LM melanomas located on the head are likely to have spread beyond the visible borders and therefore require us to remove wider margins. The second aim was to determine the ideal surgical margin required for a cure, according to the selected clinical variables.
MethodsThe patients included had LM melanomas on the head that were definitively treated with conventional or slow Mohs surgery by the dermatology department of the Instituto Valenciano de Oncología between January 1993 and April 2011. All patient and lesion data as well as images related to these cases were extracted from the institute's melanoma database20 for retrospective analysis.
Conventional surgery consisted of excision of the visible tumor plus a margin extending 0.5cm from the visible border followed by conventional histology, in which lateral and deep margins were sliced thinly and evaluated separately. In the slow Mohs procedure the paraffin-embedded tissue is sectioned and processed according to the usual Mohs procedure. All portions of the margins are examined and the excised tissue is mapped with the aid of printed images provided by the pathologist. Immunohistochemical stains (Melan-A and HMB-45) are used when required. A margin of approximately 0.5cm is removed in each stage of Mohs surgery too, though from the second stage onward, tissue is excised only from the area adjacent to the point where cells were found in the margin of the previous stage.
We first included histologically confirmed primary LM melanomas removed by incisional or excisional biopsy. Primary tumors were defined as those that had not previously received a curative treatment. Three approaches to treatment were considered curative. The first was conventional surgery with tumor-free margins of 0.5cm or with follow-up removal of wider margins if the initial margins were found to contain tumor cells. The second was Mohs surgery, and the third was topical imiquimod therapy leading to clinical disappearance of the tumor. Among the treatments considered noncurative were all procedures done before histologic diagnosis of malignancy and/or clinical suspicion of malignancy; such treatments would have been indicated for a benign lesion and would not have been recommended once a diagnosis of LM melanoma had been reached. A history of shave excision or conventional surgery with margins less than 0.5cm and other procedures performed mainly for cosmetic purposes would imply that the pigmented lesion had been judged clinically benign. Cryotherapy, electrocoagulation, various types of laser therapy, use of depigmenting or “skin-lightening” creams fall into this category and as a class will be referred to as treatments used to manage skin artifacts.
We also included the LM recurrences we treated. These were defined as lesions that reappeared after a treatment that had been considered curative, whether the previous attempt had been made in our facility or elsewhere.
We decided that it would be reasonable to consider each conventional LM excision and each slow Mohs stage to be equivalent for purposes of analysis. The margins recommended for conventional surgery are 0.5cm wide and we evaluate 100% of such margins in our hospital; thus we can assume that we removed the same amount of skin as at a slow Mohs stage. When LM tumors required additional interventions to achieve tumor-free margins, we added the number of stages for cumulative analysis. In this way we established that the principle variable to analyze for each tumor was the number of surgical stages (equivalent to the removal of a 0.5-cm margin) required to achieve a tumor-free margin. This variable was analyzed categorically, as requiring 1 stage or more stages. Conventional excision with a margin of less than 0.5cm and shave excisions were not classified as treatments with curative intent and thus neither was counted as a surgical stage.
Patient characteristics analyzed were age at the time of surgery, sex, and skin phototype. Tumor characteristics were age of the lesion (time elapsed between the patient's first observation of a lesion and diagnosis), location, approximate size in centimeters, predominant color, and definition of borders (categorized as well defined or poorly defined). Subjective perception of color was categorized as mainly light (light brown and erythematous) or dark (dark brown or black). We recorded previous treatments in the group with recurrent LM melanomas, including therapies used to manage whatever skin artifacts had been noted.
We excluded patients with invasive melanoma identified on biopsy or excision.
Only cases with full tumor information available were included. All quantitative variables were expressed categorically. Descriptive statistics were compiled for patient characteristics and clinical characteristics of the tumor. Differences in these variables were analyzed between patients requiring only 1 surgical stage and those requiring more than 1 stage to achieve complete excision. We used the Pearson χ2 test to compare the groups when the expected frequency of a variable was more than 5; when the frequency was less than 5 we used the Fisher exact test. Statistical significance was set at a value of P less than .05.
ResultsOf 62 LM melanomas from 57 patients (30 men, 27 women), 49 were primary tumors and 13 were recurrent ones. One man had 2 LM melanomas at different locations and 4 patients were first treated by our department for a primary tumor and later for a recurrence. The median age was 67.5 years (interquartile range, 60-76 years). Twenty out of 55 patients (36.4%) had a fair skin phototype (Fitzpatrick I-II) and 35/55 were darker (Fitzpatrick III-V).
Nearly half (48.4%) of the tumors were on the cheek. In order of decreasing frequency, the remaining locations were the nose (22.6%), scalp (14.5%), forehead or temple (4.8% each), the ear (3.2%), and the perioral area (1.6%).
The tumors had been developing for periods ranging from a few months to years. The age of the lesion exceeded 10 years in 11 cases, but in a majority of cases (59.6%), the patient had noticed it for the first time in the preceding 5 years. For recurrent tumors the time elapsed between treatment with curative intent of the primary tumor until our treatment of the recurrence ranged from a few months to 6 years.
In 38 of 54 patients (70.4%), the tumor measured 2cm or less just before curative treatment. In 16 (29.6%) the tumors were larger.
In 30 of 48 cases (62.5%) the color of the tumor was described as dark and in 18 as light (37.5%); information about color was not on record for 14 of the LM melanomas.
Most tumors (47/54, 87%) had irregular, poorly defined borders. Only 7 (13%)—all of them primary tumors—had well-defined borders.
For 14 of the 62 LM melanomas (22.6%), some form of treatment had previously been given to manage apparently benign skin artifacts observed during the course of disease.
We used conventional surgery with margins of 0.5cm in 1 or more stages, depending on histologic findings, in about a quarter of the procedures performed by our department to treat primary tumors (13/49, 26.5%) and in about a third of the treatments for recurrent tumors (4/13, 30.7%). In the LM melanomas treated this way, only 4 (3 recurrent, 1 primary) required a second stage to take another 0.5cm of tissue because tumor cells were found in the margins after the first attempt at excision. In 33 of the 49 primary LM melanomas (67.3%) and in 8 of the 13 recurrent ones (61.5%), slow Mohs surgery was used. From 1 to 4 stages were required to obtain tumor-free margins: 29 were fully excised in 1 stage, 10 in 2 stages, 1 in 3 stages, and 1 in 4 stages. Finally, Mohs surgery (in 1 or more stages) was used after a conventional excision that had left affected margins in 3 of the 49 primary LM melanomas and 13 of the recurrent ones. A single additional stage was sufficient in 3 of these cases. When conventional surgical excisions had been performed with 0.5-cm margins in other hospitals, these were also counted as a surgical stage. The choice of one technique or the other was individualized, based on the distinctness of the tumor border, size, location, or patient characteristics.
Nine of the 13 tumors we treated after recurrence had at first been managed in other centers and 4 had been treated in our department. Two of the treatments done with curative intent in these 13 cases of recurrence had used imiquimod, which led to a clinical cure. In 10 of the 13 recurrences, the primary tumor had been treated surgically and the margins had been found to be tumor-free. One of the LM melanomas initially treated with imiquimod recurred twice more; the tumor was then treated surgically, with 2 years passing between the first and second recurrences. Of the 11 recurrent tumors that had previously been treated surgically, conventional surgery had been used in 9 cases and Mohs surgery in 2 cases. One of these last 2 cases involved a lesion with a long history of ablative treatments with laser and several imiquimod cycles. In fact, various treatments had been undertaken to manage artifacts observed on 1 or more occasions during the course of disease in 7 of the 13 recurrent LM melanomas. Three of the 4 tumors that recurred more than once also had a history of such treatments. Regarding the 3 tumors treated with imiquimod, all required several cycles before a clinical cure was achieved. In these cases treatments for apparently benign artifacts had also been used at some point in the clinical course.
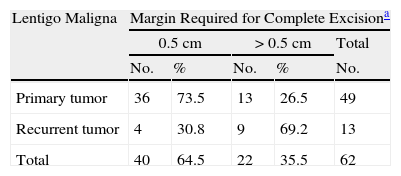
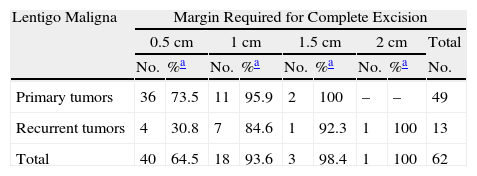
The analysis of variables considered candidate predictors of the number of stages that would be needed for complete excision showed that primary LM melanomas required a mean of 1.31 stages while recurrent ones required a mean of 1.92 stages. We were able to remove all of the primary tumor with a single 0.5-cm margin in 73.5% of the cases; 95.5% of these tumors were completely removed by the second stage (margins of 0.5cm or 1cm (Tables 1 and 2). Only 38.8% of the recurrent tumors were fully excised with removal of a 0.5-cm margin); 84.6% were excised with removal of up to 1cm and 92.3% were fully excised with removal of up to 1.5cm around the tumor (P<.008) (Tables 1 and 2).
Complete Removal of Primary and Recurrent Lentigo Maligna Melanomas With Margins of 0.5 cm or>0.5 cm.
| Lentigo Maligna | Margin Required for Complete Excisiona | ||||
| 0.5cm | >0.5cm | Total | |||
| No. | % | No. | % | No. | |
| Primary tumor | 36 | 73.5 | 13 | 26.5 | 49 |
| Recurrent tumor | 4 | 30.8 | 9 | 69.2 | 13 |
| Total | 40 | 64.5 | 22 | 35.5 | 62 |
Complete Excision of the Primary and Recurrent Lentigo Maligna Melanomas According to Total Surgical Margin Required, Recorded as Increments of 0.5cm.
Tumors that had previously been treated for benign artifacts generally required 2 or more surgical stages, with only 35.7% being fully excised with margins of 0.5cm. In contrast, in tumors that had not received earlier treatment for presumably benign artifacts, a surgical cure was achieved with a margin of 0.5cm in 72.9% of the cases (P=.023) (Table 3).
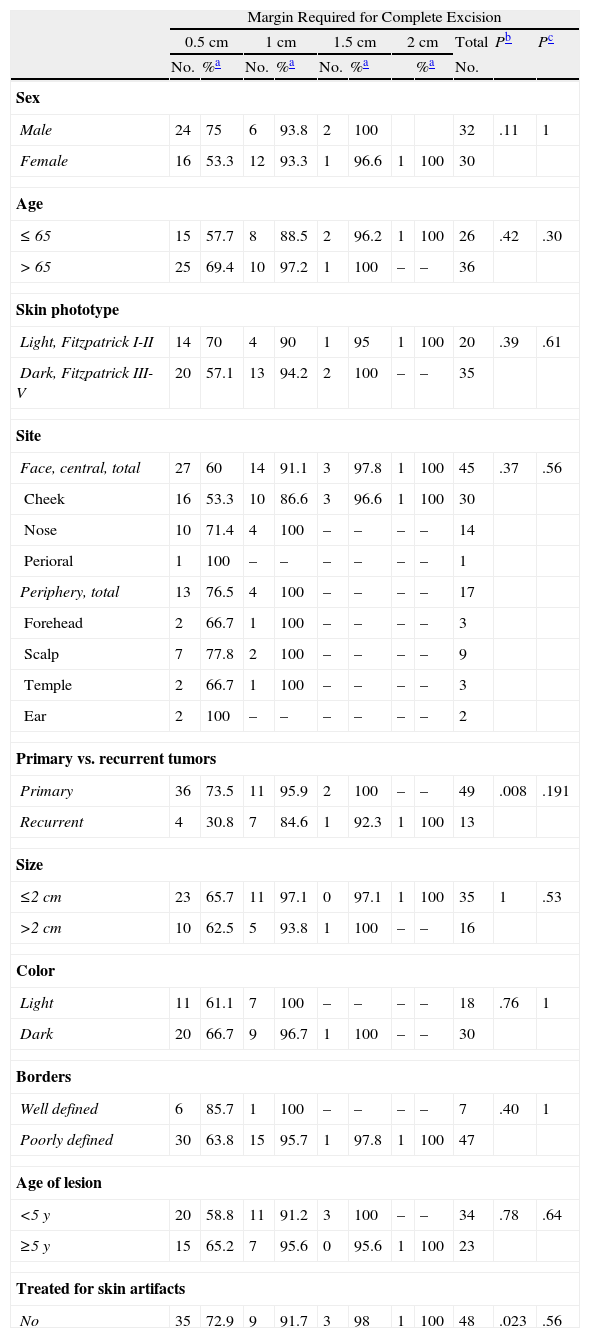
Total Surgical Margin Required to Completely Excise Primary and Recurrent Lentigo Maligna Melanomas, Analyzed According to Clinical and Patient Variables.
| Margin Required for Complete Excision | |||||||||||
| 0.5cm | 1cm | 1.5cm | 2cm | Total | Pb | Pc | |||||
| No. | %a | No. | %a | No. | %a | %a | No. | ||||
| Sex | |||||||||||
| Male | 24 | 75 | 6 | 93.8 | 2 | 100 | 32 | .11 | 1 | ||
| Female | 16 | 53.3 | 12 | 93.3 | 1 | 96.6 | 1 | 100 | 30 | ||
| Age | |||||||||||
| ≤65 | 15 | 57.7 | 8 | 88.5 | 2 | 96.2 | 1 | 100 | 26 | .42 | .30 |
| >65 | 25 | 69.4 | 10 | 97.2 | 1 | 100 | – | – | 36 | ||
| Skin phototype | |||||||||||
| Light, Fitzpatrick I-II | 14 | 70 | 4 | 90 | 1 | 95 | 1 | 100 | 20 | .39 | .61 |
| Dark, Fitzpatrick III-V | 20 | 57.1 | 13 | 94.2 | 2 | 100 | – | – | 35 | ||
| Site | |||||||||||
| Face, central, total | 27 | 60 | 14 | 91.1 | 3 | 97.8 | 1 | 100 | 45 | .37 | .56 |
| Cheek | 16 | 53.3 | 10 | 86.6 | 3 | 96.6 | 1 | 100 | 30 | ||
| Nose | 10 | 71.4 | 4 | 100 | – | – | – | – | 14 | ||
| Perioral | 1 | 100 | – | – | – | – | – | – | 1 | ||
| Periphery, total | 13 | 76.5 | 4 | 100 | – | – | – | – | 17 | ||
| Forehead | 2 | 66.7 | 1 | 100 | – | – | – | – | 3 | ||
| Scalp | 7 | 77.8 | 2 | 100 | – | – | – | – | 9 | ||
| Temple | 2 | 66.7 | 1 | 100 | – | – | – | – | 3 | ||
| Ear | 2 | 100 | – | – | – | – | – | – | 2 | ||
| Primary vs. recurrent tumors | |||||||||||
| Primary | 36 | 73.5 | 11 | 95.9 | 2 | 100 | – | – | 49 | .008 | .191 |
| Recurrent | 4 | 30.8 | 7 | 84.6 | 1 | 92.3 | 1 | 100 | 13 | ||
| Size | |||||||||||
| ≤2cm | 23 | 65.7 | 11 | 97.1 | 0 | 97.1 | 1 | 100 | 35 | 1 | .53 |
| >2cm | 10 | 62.5 | 5 | 93.8 | 1 | 100 | – | – | 16 | ||
| Color | |||||||||||
| Light | 11 | 61.1 | 7 | 100 | – | – | – | – | 18 | .76 | 1 |
| Dark | 20 | 66.7 | 9 | 96.7 | 1 | 100 | – | – | 30 | ||
| Borders | |||||||||||
| Well defined | 6 | 85.7 | 1 | 100 | – | – | – | – | 7 | .40 | 1 |
| Poorly defined | 30 | 63.8 | 15 | 95.7 | 1 | 97.8 | 1 | 100 | 47 | ||
| Age of lesion | |||||||||||
| <5y | 20 | 58.8 | 11 | 91.2 | 3 | 100 | – | – | 34 | .78 | .64 |
| ≥5y | 15 | 65.2 | 7 | 95.6 | 0 | 95.6 | 1 | 100 | 23 | ||
| Treated for skin artifacts | |||||||||||
| No | 35 | 72.9 | 9 | 91.7 | 3 | 98 | 1 | 100 | 48 | .023 | .56 |
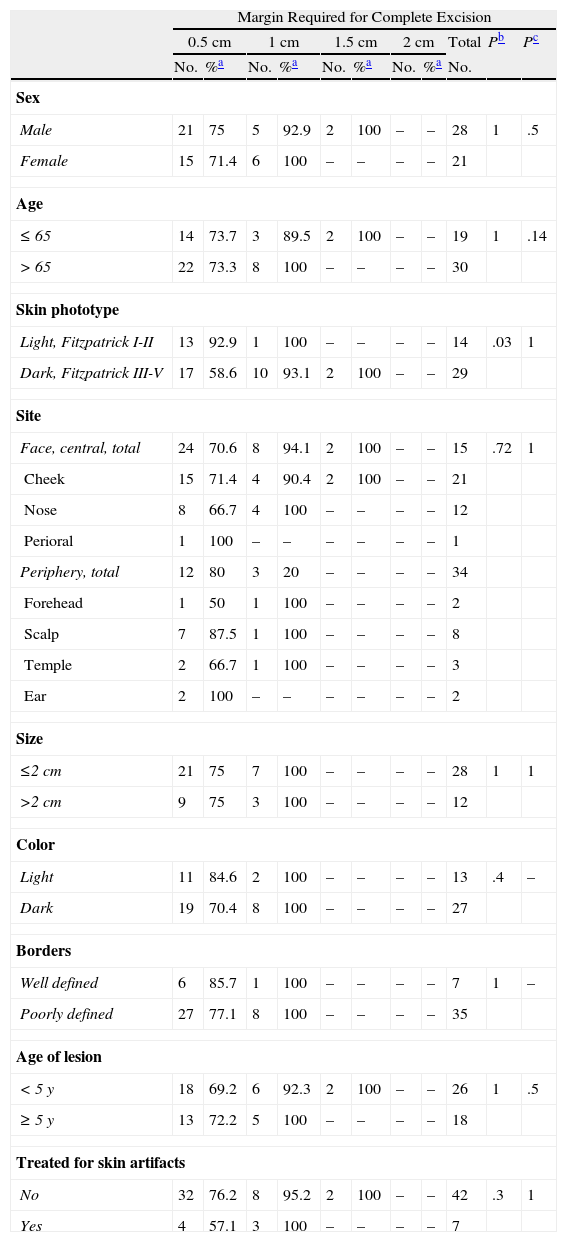
In light-skinned patients with primary LM melanomas, 92.9% of the tumors were removed in a single stage, whereas 93.1% could be completely excised when margins up to 1cm were taken (P=.03) (Table 4). The opposite pattern was seen in recurrent tumors, for which tumors in patients with light-skin phototypes required more surgical stages (Table 5).
Removal of Primary Lentigo Maligna Melanomas: Surgical Margin Required for Complete Excision Analyzed According to Clinical and Patient Variables.
| Margin Required for Complete Excision | |||||||||||
| 0.5cm | 1cm | 1.5cm | 2cm | Total | Pb | Pc | |||||
| No. | %a | No. | %a | No. | %a | No. | %a | No. | |||
| Sex | |||||||||||
| Male | 21 | 75 | 5 | 92.9 | 2 | 100 | – | – | 28 | 1 | .5 |
| Female | 15 | 71.4 | 6 | 100 | – | – | – | – | 21 | ||
| Age | |||||||||||
| ≤65 | 14 | 73.7 | 3 | 89.5 | 2 | 100 | – | – | 19 | 1 | .14 |
| >65 | 22 | 73.3 | 8 | 100 | – | – | – | – | 30 | ||
| Skin phototype | |||||||||||
| Light, Fitzpatrick I-II | 13 | 92.9 | 1 | 100 | – | – | – | – | 14 | .03 | 1 |
| Dark, Fitzpatrick III-V | 17 | 58.6 | 10 | 93.1 | 2 | 100 | – | – | 29 | ||
| Site | |||||||||||
| Face, central, total | 24 | 70.6 | 8 | 94.1 | 2 | 100 | – | – | 15 | .72 | 1 |
| Cheek | 15 | 71.4 | 4 | 90.4 | 2 | 100 | – | – | 21 | ||
| Nose | 8 | 66.7 | 4 | 100 | – | – | – | – | 12 | ||
| Perioral | 1 | 100 | – | – | – | – | – | – | 1 | ||
| Periphery, total | 12 | 80 | 3 | 20 | – | – | – | – | 34 | ||
| Forehead | 1 | 50 | 1 | 100 | – | – | – | – | 2 | ||
| Scalp | 7 | 87.5 | 1 | 100 | – | – | – | – | 8 | ||
| Temple | 2 | 66.7 | 1 | 100 | – | – | – | – | 3 | ||
| Ear | 2 | 100 | – | – | – | – | – | – | 2 | ||
| Size | |||||||||||
| ≤2cm | 21 | 75 | 7 | 100 | – | – | – | – | 28 | 1 | 1 |
| >2cm | 9 | 75 | 3 | 100 | – | – | – | – | 12 | ||
| Color | |||||||||||
| Light | 11 | 84.6 | 2 | 100 | – | – | – | – | 13 | .4 | – |
| Dark | 19 | 70.4 | 8 | 100 | – | – | – | – | 27 | ||
| Borders | |||||||||||
| Well defined | 6 | 85.7 | 1 | 100 | – | – | – | – | 7 | 1 | – |
| Poorly defined | 27 | 77.1 | 8 | 100 | – | – | – | – | 35 | ||
| Age of lesion | |||||||||||
| <5 y | 18 | 69.2 | 6 | 92.3 | 2 | 100 | – | – | 26 | 1 | .5 |
| ≥5 y | 13 | 72.2 | 5 | 100 | – | – | – | – | 18 | ||
| Treated for skin artifacts | |||||||||||
| No | 32 | 76.2 | 8 | 95.2 | 2 | 100 | – | – | 42 | .3 | 1 |
| Yes | 4 | 57.1 | 3 | 100 | – | – | – | – | 7 | ||
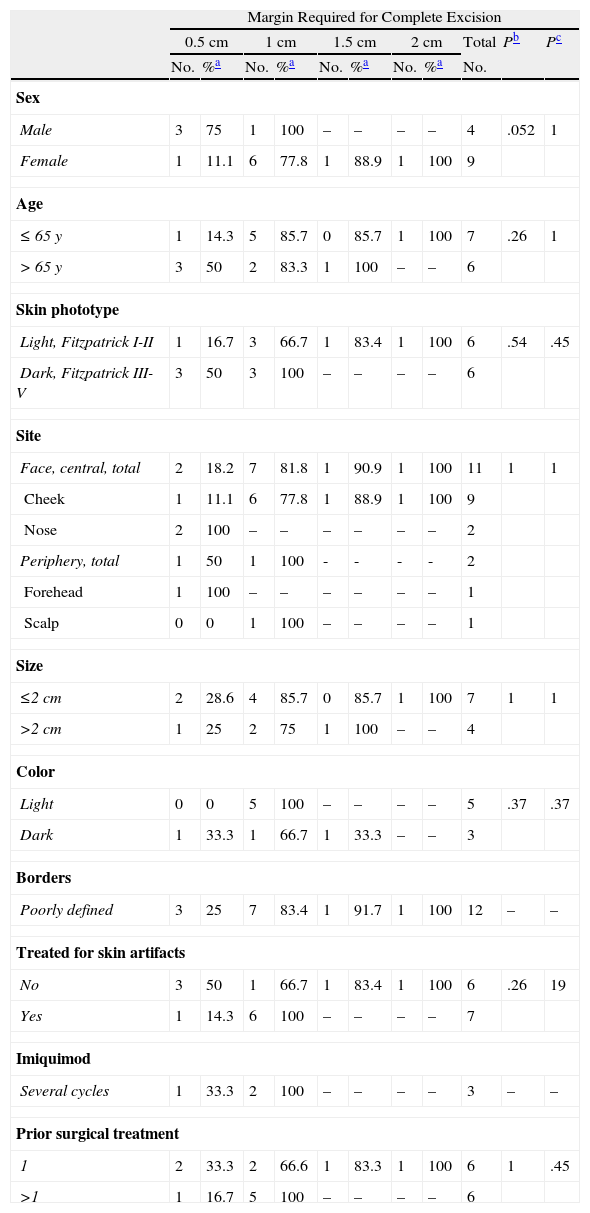
Removal of Recurrent Lentigo Maligna Melanomas: Surgical Margin Required for Complete Excision Analyzed According to Clinical and Patient Variables.
| Margin Required for Complete Excision | |||||||||||
| 0.5cm | 1cm | 1.5cm | 2cm | Total | Pb | Pc | |||||
| No. | %a | No. | %a | No. | %a | No. | %a | No. | |||
| Sex | |||||||||||
| Male | 3 | 75 | 1 | 100 | – | – | – | – | 4 | .052 | 1 |
| Female | 1 | 11.1 | 6 | 77.8 | 1 | 88.9 | 1 | 100 | 9 | ||
| Age | |||||||||||
| ≤65 y | 1 | 14.3 | 5 | 85.7 | 0 | 85.7 | 1 | 100 | 7 | .26 | 1 |
| >65 y | 3 | 50 | 2 | 83.3 | 1 | 100 | – | – | 6 | ||
| Skin phototype | |||||||||||
| Light, Fitzpatrick I-II | 1 | 16.7 | 3 | 66.7 | 1 | 83.4 | 1 | 100 | 6 | .54 | .45 |
| Dark, Fitzpatrick III-V | 3 | 50 | 3 | 100 | – | – | – | – | 6 | ||
| Site | |||||||||||
| Face, central, total | 2 | 18.2 | 7 | 81.8 | 1 | 90.9 | 1 | 100 | 11 | 1 | 1 |
| Cheek | 1 | 11.1 | 6 | 77.8 | 1 | 88.9 | 1 | 100 | 9 | ||
| Nose | 2 | 100 | – | – | – | – | – | – | 2 | ||
| Periphery, total | 1 | 50 | 1 | 100 | - | - | - | - | 2 | ||
| Forehead | 1 | 100 | – | – | – | – | – | – | 1 | ||
| Scalp | 0 | 0 | 1 | 100 | – | – | – | – | 1 | ||
| Size | |||||||||||
| ≤2cm | 2 | 28.6 | 4 | 85.7 | 0 | 85.7 | 1 | 100 | 7 | 1 | 1 |
| >2cm | 1 | 25 | 2 | 75 | 1 | 100 | – | – | 4 | ||
| Color | |||||||||||
| Light | 0 | 0 | 5 | 100 | – | – | – | – | 5 | .37 | .37 |
| Dark | 1 | 33.3 | 1 | 66.7 | 1 | 33.3 | – | – | 3 | ||
| Borders | |||||||||||
| Poorly defined | 3 | 25 | 7 | 83.4 | 1 | 91.7 | 1 | 100 | 12 | – | – |
| Treated for skin artifacts | |||||||||||
| No | 3 | 50 | 1 | 66.7 | 1 | 83.4 | 1 | 100 | 6 | .26 | 19 |
| Yes | 1 | 14.3 | 6 | 100 | – | – | – | – | 7 | ||
| Imiquimod | |||||||||||
| Several cycles | 1 | 33.3 | 2 | 100 | – | – | – | – | 3 | – | – |
| Prior surgical treatment | |||||||||||
| 1 | 2 | 33.3 | 2 | 66.6 | 1 | 83.3 | 1 | 100 | 6 | 1 | .45 |
| >1 | 1 | 16.7 | 5 | 100 | – | – | – | – | 6 | ||
Sex also accounted for significant differences in the number of surgical stages required to remove recurrent tumors: more stages were needed to achieve a cure in women than in men. With margins of 0.5cm, we completely removed 11.1% of the LM melanomas from women and 75% of the melanomas from men. The cure rates rose to 77.8% and 100%, respectively, with removal of 1-cm margins (P=.052) (Table 5). The trend was similar when all tumors (primary and recurrent) were analyzed together (Table 3).
Other noteworthy but statistically nonsignificant trends were observed (Tables 3–5). More steps tended to be required to completely remove tumors from patients over the age of 65 years and from the center of the face (cheek, nose, perioral region) in comparison with the periphery (forehead, temple, ear) or the scalp. Lightly pigmented tumors and those described as well defined tended to be removed with smaller margins than those required to excise dark or poorly defined ones.
No differences were found in relation to size or age of the lesions.
Neither the number of prior curative treatments nor the type (conventional surgery, Mohs surgery, or imiquimod) predicted the number of stages required to fully remove recurrent tumors (Table 5).
DiscussionLM management is still a subject of debate: although a variety of options are available, surgery continues to offer certain advantages. The first and most important advantage is that it allows the surgeon to make certain that the melanoma has not invaded adjacent tissue or, if it has, to measure Breslow depth, which is the key to both management and prognosis. The second is that surgery deals with the periadnexal involvement that is often found when there is subclinical tumor spread.7,21 Finally, unlike other ablative techniques, surgery does not cause changes in pigmentation that could complicate or prevent the recognition of possible LM recurrence.
Imiquimod has been linked to some striking reports of LM cures, but reviews of studies in which this drug has been used suggest that the follow-up times have been inadequate to demonstrate its efficacy against cancer.22,23 Also supportive of surgical treatment is research that demonstrates a 22% probability of finding histologic evidence that the tumor has invaded surrounding tissue when material removed by incisional biopsy is assessed24; incisional biopsy is not currently recommended for the management of LM, therefore.22 The results of our small series also discourage the use of this approach. We think that such biopsy procedures could produce artifacts that confound the features that can be observed in an LM melanoma and should be reserved for cases in which surgery is contraindicated and alternative techniques, such as radiotherapy, have also been ruled out. Conventional surgery with margins of 0.5cm and Mohs surgery are the most widely applied techniques. Our study of the surgical margin required for complete excision of an LM is the first to look at the relevance of clinical and demographic variables in patients treated with both conventional surgery and slow Mohs surgery.
Ours is also the first study to record procedures undertaken to treat skin artifacts in presumably benign lesions before malignancy is suspected or diagnosed. Although such treatments have met with success in some LM case series, we believe it is important to look at the reason for undertaking them. When cryotherapy with curative intent has been applied, the high recurrence rates have been attributed to failure to reach the proper temperature or depth.25–29
In the interpretation of a retrospective observational study, it is essential to consider certain limitations. One is that some variables were not systematically recorded as they would have been in a prospective study starting at the time of diagnosis. Another is that that some information (color, size, skin phototype, etc.) might not be found in the records. It should also be noted that some clinical features, such as color or definition of borders, are based on subjective assessment. Furthermore, we used neither dermatoscopy nor Wood's light30 to optimally identify borders before surgery, and this was also a limitation. Finally, particularly important in interpreting our findings is the possibility of selection bias: our series is not representative of the population of patients with LM, given that ours is a referral center for Mohs surgery.
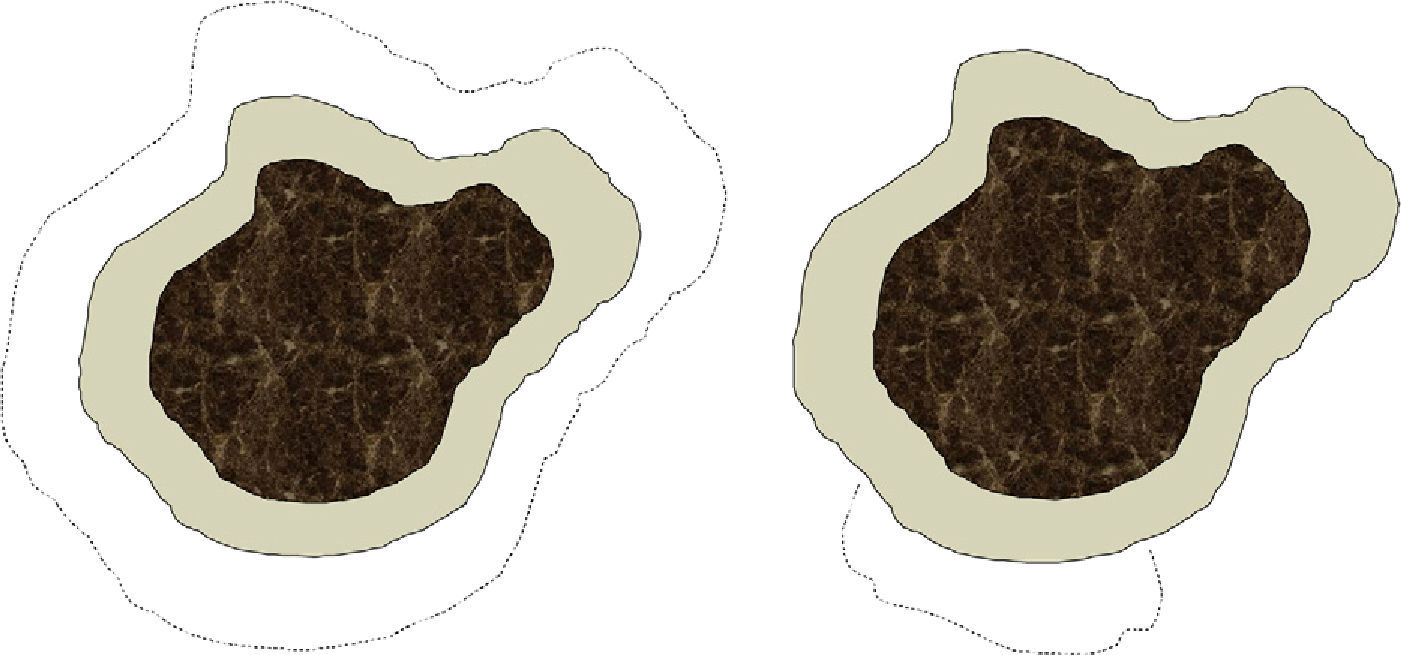
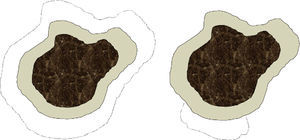
We are aware that conventional surgery and Mohs surgery differ in significant ways that affect excision technique (angle, depth, precise measurement of the margin, etc.), tissue processing, and skin defects left after the procedure (Fig. 1). For practical purposes, however, we think that each conventional surgical procedure or each Mohs stage can be analyzed together under the concept of surgical step, which would be defined as the removal of a specified surgical margin beyond the visible border of the tumor. Lateral margins removed by conventional surgery are sliced thinly and examined in their entirety in our hospital, so the great difference between the 2 techniques would lie in the mapping of the lesion as performed during slow Mohs surgery. Such mapping identifies exactly where a surgical margin has been inadequate. Thus, we arbitrarily chose to consider a conventional surgical procedure and a Mohs surgery stage (each corresponding to the removal of a margin of 0.5cm) to be equivalent for purposes of analysis, even though there might be potentially significant variability, especially when a surgeon is working in an area where it is important to attempt to spare as much healthy tissue as possible (such as around the eye). Our assumption of equivalence should be considered a limitation of the study.
Depiction of differences in the defect left after conventional surgery (left) and after Mohs surgery (right) The dark central area denotes the lentigo maligna melanoma. The more lightly tinted area shows the standard margin (e.g., 0.5cm) of healthy skin removed from around the tumor. The dotted lines indicate the margin that would be taken in a second stage if tumor cells had invaded the first margin. In conventional surgery the defect left by the procedure would be greater than in slow Mohs surgery, which only excises tissue adjacent to the area where the first margin was found to have been invaded.
We also emphasize as a relevant feature of our study design that all the patients were treated at the same facility, which specializes in skin cancer. Probably many of the patients were referred to our hospital because they had complicated tumors with multiple recurrences or because previous treatments had been unsuccessful.
We found that recurrent LM melanomas required wider surgical margins than primary tumors. In addition, LM melanomas that had previously undergone treatments for presumably benign artifacts, whether they were primary or recurrent LM tumors, also required more surgical steps to achieve a cure. Our observations seem to suggest that recurrent LM melanomas had poorly defined borders from the start or had undergone extensive subclinical spread. They might have been incorrectly excised or scarring might have interfered with the early diagnosis of recurrence, allowing the tumor to grow further. Cosmetic treatments that were erroneously given while LM developed also seem to have interfered significantly with the clinical appearance of the lesions, which presented poorly defined borders on recurrence. Previous studies have mentioned the high recurrence rates after cryotherapy, argon laser therapy, or electrocautery, emphasizing the difficulty of recognizing recurrences clinically.13,30
The surgical cure rates described in the literature are similar to ours. Huilgol and coworkers13 reported that 20% of the primary tumors in their series recurred and that 56% of the recurrent tumors required more than a single 0.5-cm margin stage. The comparable rates in our series were 26.5% and 69.2%, respectively. Huilgol and coworkers used a serial surgical technique, however, in which margin assessment and histologic processing was that of conventional surgery, unlike the examination of 100% of the margin in Mohs surgery.13 Aagarwal-Antal and coworkers3 reported a cure rate of only 42% for the excision of LM melanomas from sun-exposed skin with margins of 0.5cm. In another study of 46 LM melanomas treated with standard Mohs procedures and immunohistochemical stains, Zalla and coworkers31 reported a cure rate of 50% with margins of 0.6cm.
Our finding that more stages were required to achieve complete excision of primary LM melanomas from patients with darker phototypes can be explained by the difficulty of distinguishing borders on pigmented skin. Furthermore, the assessment of borders during histology can also be affected by changes in the density and distribution of melanocytes in sun-exposed skin, a common occurrence and one which may be accompanied by slight to moderate atypia even in the absence of a tumor.32,33
The trend toward more surgical stages required to achieve a cure when LM melanomas are located in the center of the face may be the result of the surgeon's tendency to act more conservatively in perioral skin in order to preserve facial structures for aesthetic purposes. The same explanation may be relevant to the finding that more stages were needed in women and patients under the age of 65 years.
A mean of 1.31 stages (of 0.5-cm margin removal) was required to excise primary LM melanomas in our study; the mean was 1.91 stages for recurrent tumors. Clayton and coworkers34 reported a mean of 1.65 stages required for excising a total of 77 LM melanomas by slow Mohs surgery. In a prospective study of 116 patients treated with conventional Mohs surgery, a mean of 1.97 surgical stages was required; the authors demonstrated the need for slow processing in that study by finding that a new slice taken from the paraffin-embedded tissue after processing frozen tissues revealed tumor cells in the margins of 8 out of 167 cases of melanoma in situ.11 Siew-Yin and coworkers35 observed that a mean of 1.64 stages (taking margins of 0.2-0.5cm) had been needed in a retrospective study of slow Mohs surgical treatment of 14 periocular melanomas, 8 of which were diagnosed as LM.
Of the 45 LM melanomas we treated with slow Mohs surgery, only 2 were recurrences, an observation that confirms the high cure rates reported in the literature.9,11–15
We believe that the choice of one surgical technique over another should be tailored to fit the case, according to the previously described variables. Conventional surgical excision is an excellent choice for well-defined primary LM melanomas that are small and located on peripheral areas of the face in patients with light skin. In other cases wider margins (0.5-1cm) or techniques involving complete assessment of margins (such as Mohs surgery) should be considered. Such techniques also offer better cure rates and minimize the amount of healthy skin removed from aesthetically sensitive areas.
In conclusion, our observations in this series are consistent with the literature that finds margins of 0.5cm to be insufficient for removing some LM melanomas. Recurrent tumors, those that have undergone noncurative treatments that might interfere with clinical observation of borders, tumors in persons with dark skin, and those located in areas at the center of the face may initially require wider margins for complete removal. LM melanoma features (size, color, or age of the lesion) did not help to predict how wide a margin beyond the visible border would be needed in our series, even though other authors have found size to be relevant.30 Larger, prospective, randomized studies are required to obtain a higher evidence level. The use of confocal microscopy to clearly identify tumor borders, monitor noninvasive therapies applied, or detect recurrences early might substantially improve outcomes in the management of LM; adjuvant treatments, such as applying 0.1% imiquimod cream around the scar to reduce recurrences might also help.36–38
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank Dr. Vicente García Patos for his critical reading of our manuscript.
Please cite this article as: Hilari H, Llorca D, Traves V, Villanueva A, Serra-Guillén C, Requena C et al. Tratamiento quirúrgico del lentigo malign: cirugía convencional vs. Mohs diferida. Estudio retrospectivo de 62 casos. Actas Dermosifiliogr.2012;103:614-623.