Atopy is almost always associated with an elevated immunoglobulin (Ig) E production. Omalizumab is a monoclonal anti-IgE antibody that is currently indicated for the treatment of cases of asthma that satisfy certain criteria. A number of studies have been published on the usefulness of omalizumab in the treatment of atopic dermatitis, and the results have been variable. We present our experience in the treatment of 9 patients with severe atopic dermatitis refractory to at least 2 systemic drugs. All patients reported a decrease in pruritus and an improvement in quality of life. Good control of the skin disease was achieved with omalizumab in monotherapy in 2 patients, and there was a slight improvement in the eczematous lesions in 4 patients. Those patients who also had asthma achieved good control of their respiratory symptoms and did not require additional therapy. Omalizumab is a well-tolerated and safe drug that can be useful for the treatment of severe atopic dermatitis refractory to other systemic therapies. This monoclonal anti-IgE antibody is a major therapeutic advance as it opens the door to the management of atopic dermatitis using systemic immunomodulating therapies.
La atopia se acompaña de forma casi constante de una producción elevada de inmunoglobulina E (IgE). Omalizumab es un anticuerpo monoclonal anti-IgE, que actualmente está indicado en pacientes con asma que cumplen determinados criterios. Se han publicado algunos estudios sobre la utilidad del omalizumab en el tratamiento de la dermatitis atópica (DA), con resultados variables.
Presentamos nuestra experiencia en 9 pacientes con dermatitis atópica grave, refractaria al menos a dos fármacos sistémicos. Todos los pacientes tratados referían una disminución del prurito y una mejoría en su calidad de vida. Los que presentaban asma consiguieron un buen control desde el punto de vista respiratorio, sin precisar otros tratamientos adicionales. En dos casos se consiguió un buen control en monoterapia, apreciándose una discreta mejoría de las lesiones de eccema en 4 de ellos.
El omalizumab es un fármaco bien tolerado y seguro. Puede ser útil en el tratamiento de pacientes con DA grave, refractaria a otros tratamientos sistémicos. Este anticuerpo monoclonal anti-IgE abre la puerta a los tratamientos inmunomoduladores sistémicos para el manejo de la DA, lo que supone un gran avance terapéutico.
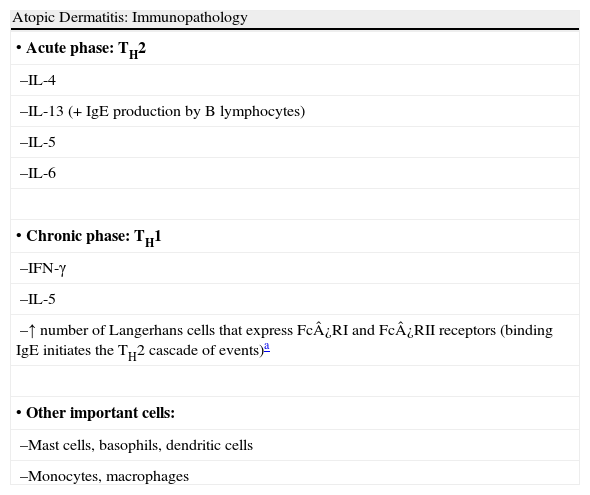
Atopic diseases, including asthma, rhinoconjunctivitis, and eczema, are defined as a group of chronic conditions with a strong hereditary component. They are caused by hypersensitivity of the skin and/or mucosas to certain environmental substances. Atopy is associated with elevated immunoglobulin (Ig) E production and/or altered nonspecific reactivity (Table 1).1
Summary of the Immunopathology of Atopic Dermatitis.
| Atopic Dermatitis: Immunopathology |
| • Acute phase: TH2 |
| –IL-4 |
| –IL-13 (+IgE production by B lymphocytes) |
| –IL-5 |
| –IL-6 |
| • Chronic phase: TH1 |
| –IFN-γ |
| –IL-5 |
| –↑ number of Langerhans cells that express Fc¿RI and Fc¿RII receptors (binding IgE initiates the TH2 cascade of events)a |
| • Other important cells: |
| –Mast cells, basophils, dendritic cells |
| –Monocytes, macrophages |
Abbreviations: IFN, interferon; Ig, immunoglobulin; IL, interleukin; TH, T helper.
Omalizumab is a chimeric humanized monoclonal antibody that is 95% human IgG1 and 5% a specific epitope of murine IgE. It binds to the high-affinity IgE receptor (Fc¿RI) and thus prevents IgE from binding to the surface of mast cells or basophils, blocking mast-cell degranulation and inhibiting the release of inflammatory mediators. Furthermore, it has been shown that the response to treatment with omalizumab occurs due to a downregulation phenomenon and a quantitative reduction in the cell-surface expression of Fc¿RI receptors on basophils and dendritic cells.2,3 Treatment with omalizumab is currently indicated in patients over 12 years of age with moderate to severe persistent allergic asthma satisfying certain criteria (forced expiratory volume in the first second < 80%, positive skin tests or in vitro reactivity, and frequent daytime symptoms or night-time awakenings), not symptomatically controlled with conventional management (inhaled corticosteroids combined with inhaled β2-agonists), and associated with a serum IgE between 30 and 700 IU/mL. Higher IgE levels are not a contraindication. It is administered as a subcutaneous injection, adjusting the dose according to the weight of the patient and the pretreatment IgE levels. It is marketed in vials of 150mg, and the dose typically administered is a multiple of this quantity, with a dose interval of 2 to 3 weeks. The most commonly reported adverse effects are mild and include local reactions at the site of injection (45% of cases), infections (viral infections in 23%, upper respiratory tract infection in 20%, sinusitis in 16%, and pharyngitis in 11%), and headache (15% of cases). Anaphylaxis is very rare, although postmarketing studies indicate that it can occur in up to 0.1% of patients receiving the drug. There is no demonstrated increase in the risk of malignancy or in the onset of other treatment-related immunological syndromes.4
Since omalizumab came onto the market, there have been reports about its possible use in other disorders, including allergic rhinoconjunctivitis, food allergies, chronic urticaria, hyperimmunoglobulin E syndrome (Job syndrome), and atopic dermatitis.5
Case DescriptionsWe present 9 patients with severe atopic dermatitis (AD) treated with omalizumab in our hospital between January 2007 and October 2009. All patients were over 18 years of age and presented severe AD that was refractory to at least 2 systemic drugs. Omalizumab was prescribed as off-label treatment. Patients were informed in detail about the available therapeutic options and signed the corresponding informed consent form.
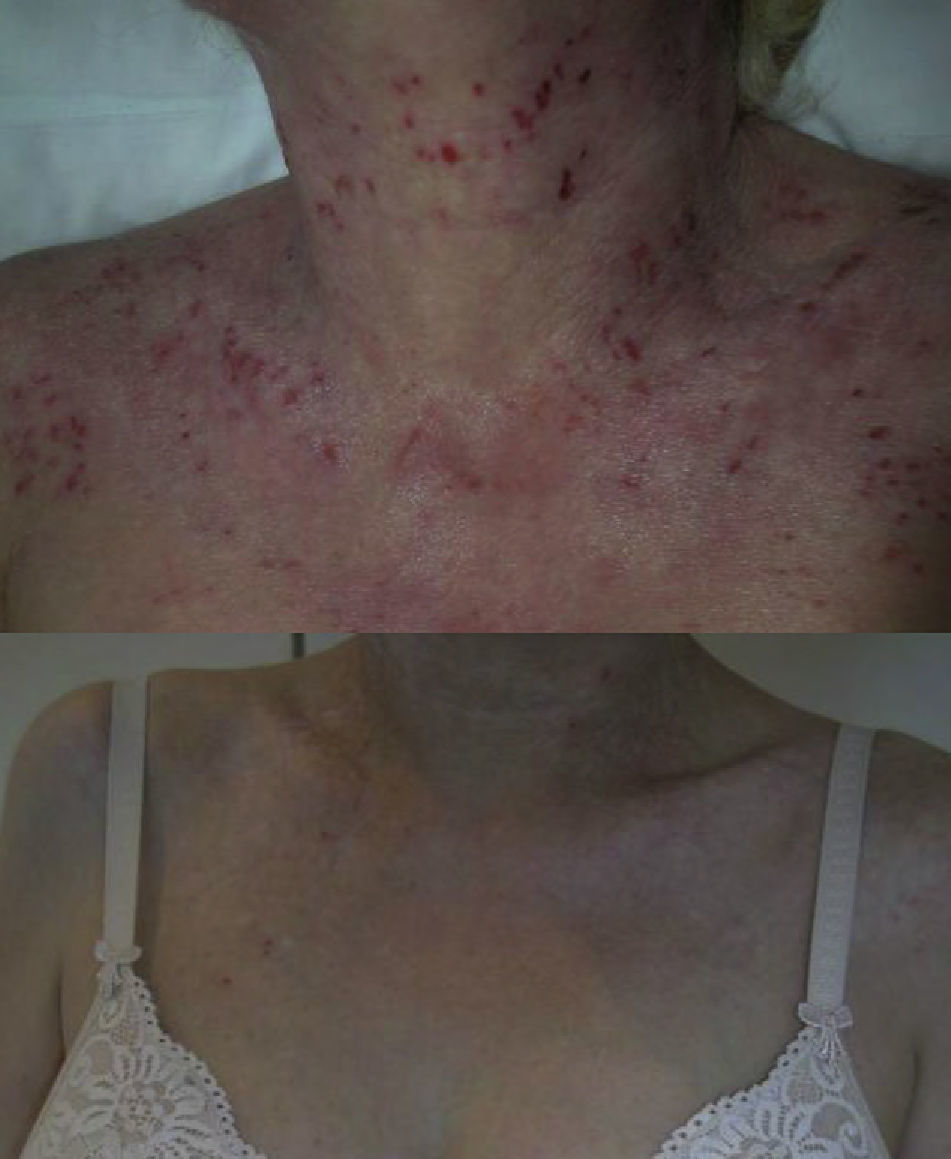
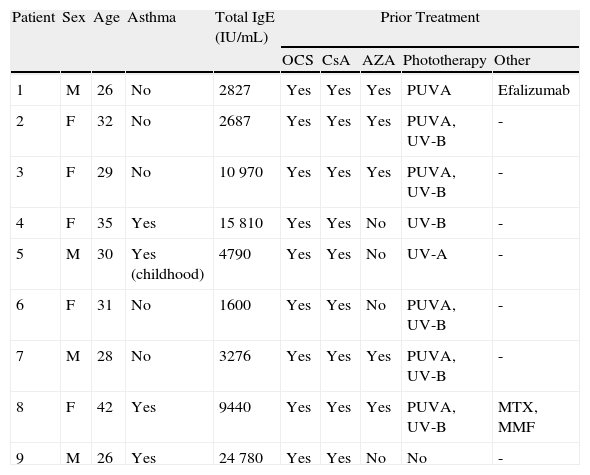
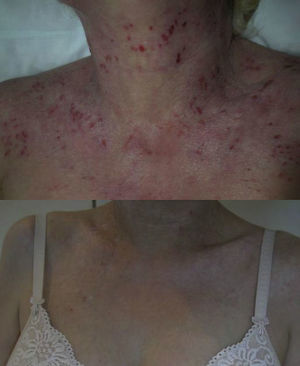
The group comprised 5 women and 4 men aged between 26 and 42 years. Three of the 9 patients also had asthma. Elevated IgE levels were detected in all patients. All had received treatment with oral corticosteroids and ciclosporin, 8 had had phototherapy, and 5 had been treated with azathioprine. The omalizumab regimen was 450mg every 3 weeks in 7 patients, and a weight-adjusted dose was administered in the 2 remaining patients. The total number of injections administered ranged from 2 to 24. After treatment with omalizumab, all patients reported a decrease in pruritus and an improvement in their quality of life. The patients with asthma presented good control of their condition and required no additional treatments. Good control of the AD was achieved with omalizumab in monotherapy in 2 cases (Fig. 1), and there was a slight improvement in the eczema lesions in 4 patients (Table 2). A progressive loss of efficacy was observed in 1 patient after a good initial clinical response. At the time of writing, 1 patient was still receiving treatment and had almost no lesions (Fig. 2). The reasons for the discontinuation of treatment are shown in Table 2. One patient developed ductal breast cancer, and it was therefore decided to interrupt the treatment with omalizumab despite the good clinical response.
Epidemiological Characteristics, Laboratory Findings, and Treatment of Our Series of Patients.
| Patient | Sex | Age | Asthma | Total IgE (IU/mL) | Prior Treatment | ||||
| OCS | CsA | AZA | Phototherapy | Other | |||||
| 1 | M | 26 | No | 2827 | Yes | Yes | Yes | PUVA | Efalizumab |
| 2 | F | 32 | No | 2687 | Yes | Yes | Yes | PUVA, UV-B | - |
| 3 | F | 29 | No | 10 970 | Yes | Yes | Yes | PUVA, UV-B | - |
| 4 | F | 35 | Yes | 15 810 | Yes | Yes | No | UV-B | - |
| 5 | M | 30 | Yes (childhood) | 4790 | Yes | Yes | No | UV-A | - |
| 6 | F | 31 | No | 1600 | Yes | Yes | No | PUVA, UV-B | - |
| 7 | M | 28 | No | 3276 | Yes | Yes | Yes | PUVA, UV-B | - |
| 8 | F | 42 | Yes | 9440 | Yes | Yes | Yes | PUVA, UV-B | MTX, MMF |
| 9 | M | 26 | Yes | 24 780 | Yes | Yes | No | No | - |
| Dose of Omalizumab | No. of Injections | Reason for Interruption | Pruritus | Quality of Life | Eczema | Asthma |
| 450mg/3 wk | 2 | Injection site reaction | Not evaluablea | Not evaluablea | Not evaluablea | Not evaluablea |
| 450mg/3 wk | 4 | Vasovagal syncope | Improvement | Improvement | No improvement | - |
| 450mg/3 wk | 9 | Lack of efficacy | Improvement | Improvement | Slight improvement | - |
| 450mg/3 wk | 7 | Lack of efficacy | Improvement | Improvement | Slight improvement | Improvement |
| 450mg/3 wk | 4 | Lack of efficacy | Improvement | Improvement | Slight improvement | - |
| 450mg/3 wk | 8 | Lack of efficacy | Improvement | Improvement | Slight improvement | - |
| 600mg/3 wk | 24 | Recurrence of lesions | Improvement | Improvement | Slight improvement | - |
| 450mg/3 wk | 20 | Concomitant breast cancer | Improvement | Improvement | Controlled on monotherapy | Improvement |
| 300mg/2 wk | 22 | Continues on treatment | Improvement | Improvement | Controlled on monotherapy | Improvement |
Abbreviations: AZA, azathioprine; CsA, ciclosporin A; F, female; M, male; MMF, mycophenolate mofetil; MTX, methotrexate; OCS, oral corticosteroids; PUVA, psoralen-UV-A.
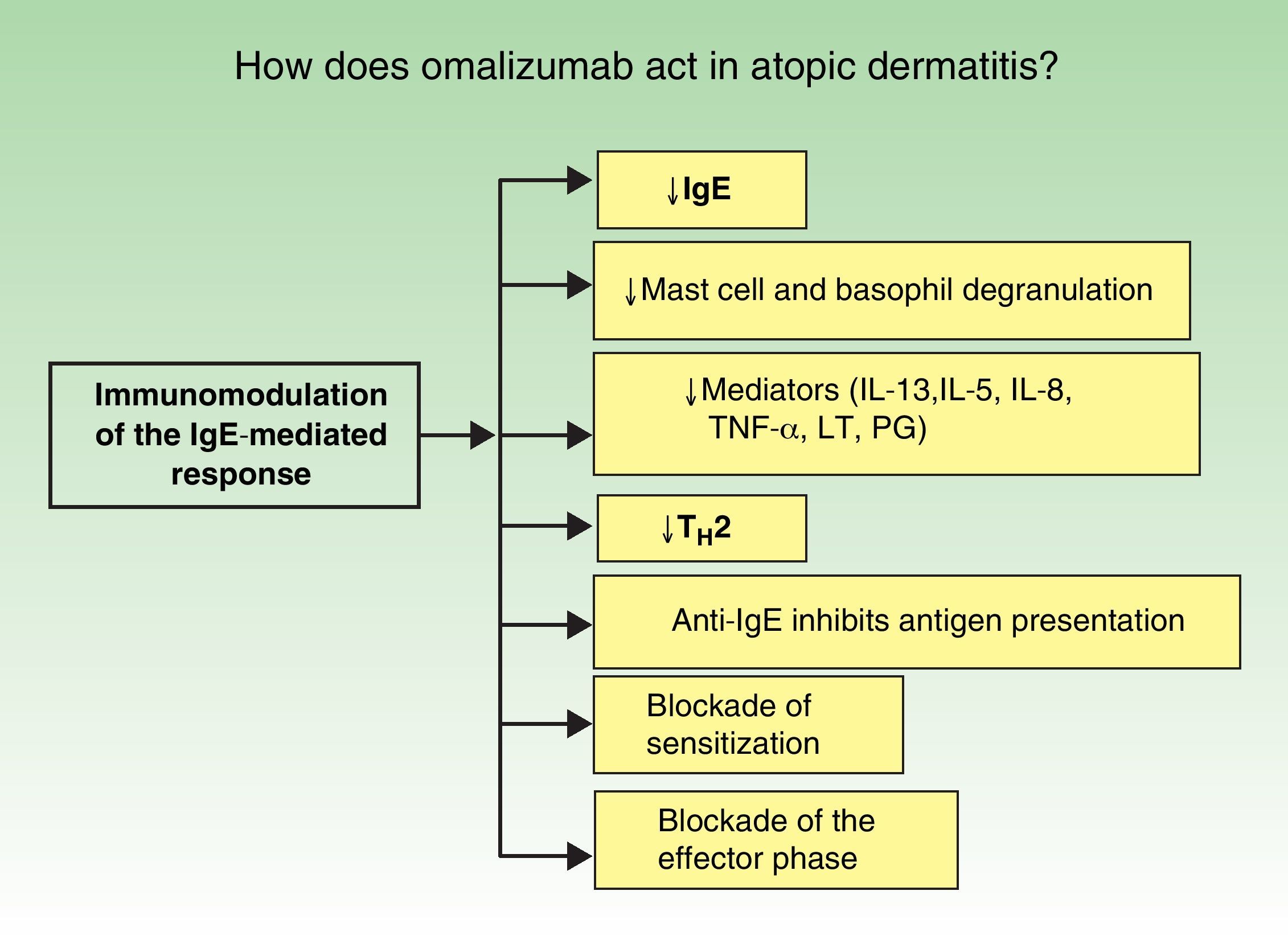
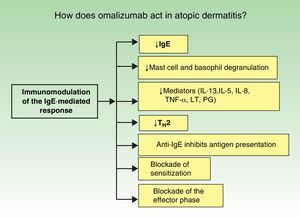
The immunopathology of AD varies according to the stage of the disease (Fig. 1). In atopy, omalizumab acts via more than 1 mechanism of action and it produces global immunomodulation. Its initial action is to decrease IgE levels, inhibit mast cell and macrophage degranulation, and reduce the levels of many of the inflammatory mediators implicated in AD (interleukin [IL] 13, IL-5, IL-8, tumor necrosis factor α, leukotrienes, and prostaglandins). It also blocks the type 2 helper T cell (TH2) cascade of events, a key factor in the pathophysiology of atopy. Finally, but no less important, the anti-IgE antibodies significantly inhibit antigen presentation. All the above actions lead to a blockade both of sensitization and of the effector phase in a novel therapeutic approach (Fig. 2).6
The use of omalizumab in AD is associated with certain problems. First, IgE is not the only etiological and pathogenic factor in this disease. Second, the majority of atopic patients have IgE levels much higher than the recommended limit for the treatment of asthma (700 IU/mL), and the need for a higher dose of omalizumab in these patients must be considered. Some authors have suggested that a higher dose should be used, but the main problem would be a potentially higher risk of anaphylaxis.7 The possible relationship between pre- and post-omalizumab IgE levels and the response to treatment is also an interesting aspect.8 In our study, we did not routinely monitor IgE levels during treatment with omalizumab because of interference by omalizumab in IgE measurement (the nephelometry technique used in our hospital does not distinguish between IgE and anti-IgE). Another controversial aspect that must be mentioned is the very high cost of treatment; the cost-benefit relationship must be taken into account.9
The results currently available on the response of AD to treatment with omalizumab are very variable. Krathen and Shu10 described their experience in 3 adult patients with severe AD treated with omalizumab in whom a dose of 450mg every 2 weeks for 4 months achieved no clinical response whatsoever. The patients in that study had very high pretreatment IgE levels, ranging from 5440 to 24 000 IU/mL, and the authors suggested that such high levels of IgE would probably require a higher dose of omalizumab to achieve a response. Lane11 reported an improvement in the severity of AD refractory to conventional treatments in 3 nonasthmatic patients aged between 10 and 13 years with pretreatment IgE levels of between 1990 and 6120 IU/mL. Treatment with omalizumab was maintained for 22 weeks at doses of up to 450mg every 2 weeks. Vigo et al.12 studied 7 patients aged 7 to 58 years with asthma and AD treated with omalizumab. The maximum IgE level in those patients was 2020 IU/mL and the maximum dose of omalizumab used was 375mg every 2 weeks. After 3 months of treatment the authors detected a clinical improvement, which proved to be statistically significant when month 0 was compared with month 7. Lee et al.13 conducted a pilot study with 21 patients, all of whom had moderate to severe persistent asthma and AD of variable severity. The pretreatment IgE levels in their patients were between 18.2 and 8396 IU/mL, and all patients showed a statistically significant clinical improvement. Amrol14 presented 3 patients with severe, treatment-resistant AD whose skin symptoms improved significantly after treatment with omalizumab. Recently, Park et al.,15 in Korea, described a case of recalcitrant AD treated with omalizumab. The patient was a 34-year-old man with severe AD refractory to a number of topical and oral agents who had very high IgE levels. After 8 months of treatment with omalizumab his symptoms had improved considerably and the AD severity index—SCORing Atopic Dermatitis (SCORAD)—had decreased.
From the results of our series we can conclude that omalizumab is a well-tolerated and safe drug that may be useful for the treatment of patients with severe AD refractory to other systemic treatments. This anti-IgE monoclonal antibody opens the door to the use of immune modulators in the management of AD and represents a major therapeutic advance.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Fernández-Antón Martínez MC, Leis-Dosil V, Alfageme-Roldán F, Paravisini A, Sánchez-Ramón S, Suárez Fernández R. Omalizumab en el tratamiento de la dermatitis atópica. Actas Dermosifiliogr.2012;103:624-628.