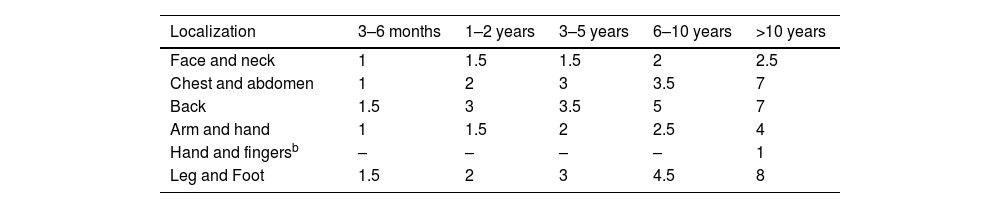The Spanish Academy of Dermatology and Venereology (AEDV) Psoriasis and Pediatric Working Groups (PSW and PWG) have developed a set of recommendations for the management of pediatric psoriasis based on the best available evidence and experts’ opinion.
MethodologyThe methodology of nominal groups was followed, with help from a scoping review. A coordinator was designated, and a group of experts was selected based on their experience and knowledge on the management of psoriasis. The coordinator defined both the objectives and the key points of the document. Then, with help from a documentalist, a systematic literature review was conducted across Medline, Embase and Cochrane Library until May 2023. Systematic literature reviews, meta-analyses, and observational studies were included. National and international clinical practice guidelines and consensus documents were reviewed. With this information, the coordinator proposed preliminary recommendations that were discussed and modified in a nominal group meeting with all experts. After several review processes, which included an external review, the final document was generated.
ResultsPractical recommendations on the evaluation and management of patients with pediatric psoriasis are presented in association with other AEDV documents. The evaluation of the pediatric patient, the definition of the therapeutic objectives, the criteria for indication and selection of treatment are addressed. Practical issues such as therapeutic failure, response maintenance, comorbidity and risk management are also included.
Los Grupos de Psoriasis y Dermatología Pediátrica (GPS y GEDP) de la Academia Española de Dermatología y Venereología (AEDV) han generado recomendaciones para el tratamiento de la psoriasis pediátrica, basadas en la mejor evidencia disponible y la experiencia de expertos.
MetodologíaSe siguió la metodología de grupos nominales, con la ayuda de una revisión sistemática de la literatura (RSL). Tras designar una coordinadora, se seleccionó un grupo de integrantes en función de su experiencia y conocimiento en la psoriasis pediátrica. La coordinadora definió los objetivos y los puntos clave del documento, y con la ayuda de una documentalista, se realizó una RSL en Medline, Embase y Cochrane Library (hasta mayo de 2023). Se seleccionaron revisiones sistemáticas, metaanálisis, ensayos clínicos, así como estudios observacionales. Se revisaron otras guías de práctica clínica y documentos de consenso nacionales e internacionales. Con esta información, la coordinadora generó una serie de recomendaciones preliminares que fueron evaluadas y modificadas en una reunión de grupo nominal. Tras varios procesos de revisión y una evaluación externa se redactó el documento definitivo.
ResultadosSe presentan en el documento recomendaciones prácticas, en línea con otros documentos de la AEDV, sobre la evaluación y el manejo de los pacientes con psoriasis pediátrica. Se aborda la valoración del paciente pediátrico, la definición de los objetivos terapéuticos en estos pacientes, así como los criterios de indicación y selección del tratamiento. Se incluyen, asimismo, cuestiones prácticas como el fracaso terapéutico, el mantenimiento de la respuesta, la comorbilidad o la gestión del riesgo.
Psoriasis is one of the most common dermatological conditions among the pediatric population, significantly impacting children's quality of life and development—often to a greater extent than other chronic conditions such as epilepsy or diabetes.1–4 European studies have reported a psoriasis prevalence of 0.18% up to 0.55% in children aged 0–9 years and 0.83% up to 1.37% in those aged 10–19 years.1,5,6 Onset most commonly occurs during adolescence and tends to follow a relapsing course.1,7 Pediatric psoriasis, like its adult counterpart, is a multisystemic disease often associated with comorbidities such as psoriatic arthritis (PsA), obesity, depression, and metabolic syndrome.8
Although pediatric psoriasis shares the same clinical subtypes as adult psoriasis, the lesions can differ in distribution and morphology. Similarly, the clinical symptoms and impact of pediatric psoriasis may vary depending on the patient's age.
Currently, alongside phototherapy, a wide range of pharmacological treatments is available for pediatric psoriasis, including topical and systemic therapies as well as biologic therapies.
The Psoriasis and Pediatric Dermatology Working Groups (GPS and GEPD) of the Spanish Academy of Dermatology and Venereology (AEDV), together with other published clinical practice guidelines on the management of adult psoriasis, developed recommendations for the specific pharmacological management of pediatric psoriasis (ages 0–18 years). Non-pharmacological treatments (e.g., exercise, heliotherapy, psychological support, multidisciplinary care), though equally important in the comprehensive management of children with psoriasis, are outside the scope of this document.
The main goal of this consensus document is to provide dermatologists with a reference tool to support therapeutic decision-making and facilitate the selection of the best available treatment for pediatric psoriatic patients, including special locations. The document also aims to standardize and consolidate proposals implemented in clinical practice by pediatric psoriasis experts in Spain, based on the best available evidence and expert opinion.
This document is particularly relevant given the lack of specific guidelines for this age group in our setting and studies highlighting that many pediatric psoriatic patients are undertreated due to a lack of knowledge or apprehension.9,10 Of note, patient age should not restrict access to any drugs.
The recommendations address the assessment of psoriasis severity in pediatric patients and its practical implications, the indications for available treatments, therapeutic goals, and response to treatment, always considering the unique characteristics (physiology, pharmacokinetics, family structure, etc.) of this population vs adults.
Furthermore, these recommendations can aid other health care professionals involved in managing these patients, such as primary care physicians, rheumatologists, nurses, pediatricians, and health care administrators.
MethodologyStudy designThis consensus document was initiated by AEDV GPS and GEPD. The consensus was developed using a nominal group methodology supported by a systematic literature review (SLR). The project fully complied with the principles established in the Declaration of Helsinki on medical research involving human subjects and complied with applicable Good Clinical Practice regulations.
Participant selection and document developmentFirst, a coordinator was appointed, and a group of dermatologists was selected based on their expertise in pediatric psoriasis. During the initial nominal group meeting, experts, with methodological support, defined the objectives, scope, audience, and sections of the document. They also decided to conduct a SLR to analyze the safety and efficacy profile of pharmacological treatments and phototherapy for pediatric psoriasis (Tables 1–3 of the annex in the supplementary data). The SLR was conducted with the help of a professional documentalist who developed search strategies using Medical Subject Headings (MeSH) and free-text terms across major bibliographic databases (Medline, Embase, and the Cochrane Library) up until May 2023. Quality SLRs, meta-analyses, randomized clinical trials (RCTs), and observational studies were included. Two independent reviewers selected the studies and extracted the data.
The results of the SLR and other national and international consensus documents8,11–15 formed the basis for drafting preliminary recommendations and text.
These were discussed during a second nominal group meeting, where the final recommendations were generated. After several review processes, including external review by GPS members, the final document was drafted.
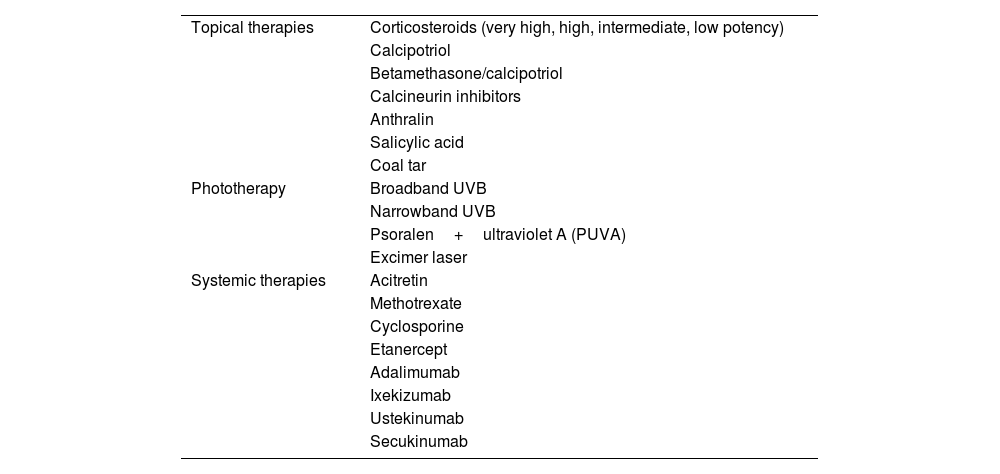
ResultsAlongside phototherapy, pharmacological treatments for pediatric psoriasis include topical and systemic therapies (Table 1). However, currently, phototherapy and biologic therapies are the only treatments approved for pediatric psoriasis.
Available treatments for pediatric psoriasis.
| Topical therapies | Corticosteroids (very high, high, intermediate, low potency) |
| Calcipotriol | |
| Betamethasone/calcipotriol | |
| Calcineurin inhibitors | |
| Anthralin | |
| Salicylic acid | |
| Coal tar | |
| Phototherapy | Broadband UVB |
| Narrowband UVB | |
| Psoralen+ultraviolet A (PUVA) | |
| Excimer laser | |
| Systemic therapies | Acitretin |
| Methotrexate | |
| Cyclosporine | |
| Etanercept | |
| Adalimumab | |
| Ixekizumab | |
| Ustekinumab | |
| Secukinumab |
Table 1 lists the treatments available for pediatric psoriasis.
Characteristics of pediatric psoriasisAs with adult psoriasis, the selection/prioritization of treatments must consider various drug-related factors, patient, environment, psoriasis per se, the health care system and its organization.16,17
Pediatric psoriasis also has several characteristics different from adult psoriasis regarding:
- •
Clinical features of psoriasis (distribution, morphology, symptoms, impact).
- •
Pediatric-specific factors (anatomophysiological, psychological, familial, educational, and social context).
- •
Disease assessment by the dermatologist and indications for drug use.
- •
Parental or caregiver involvement in disease assessment and therapeutic decision-making.
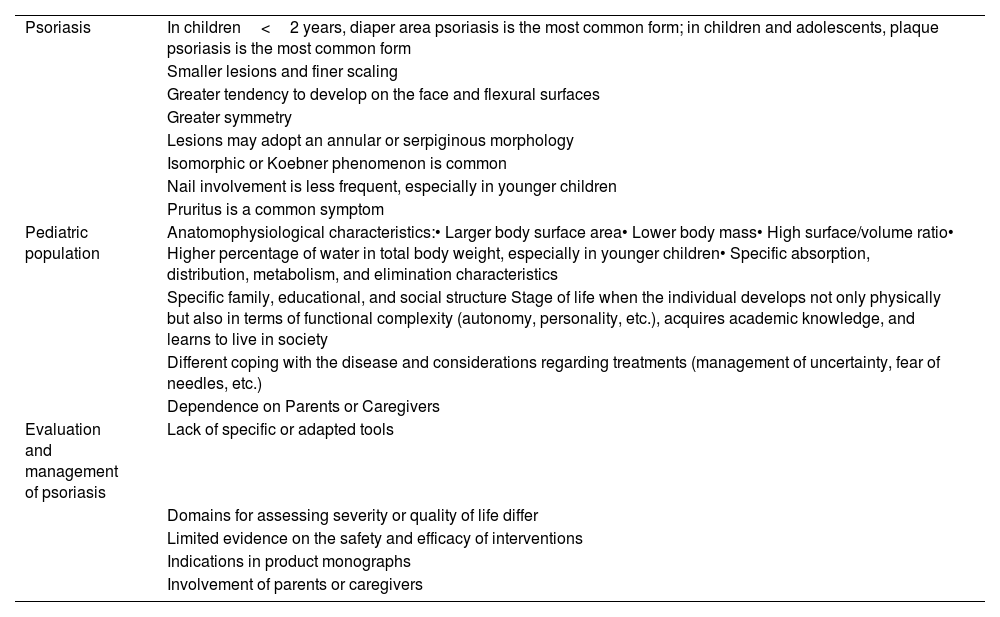
Table 2 illustrates the main differences between pediatric and adult psoriasis.
Characteristics and differences in the management of pediatric psoriasis vs adult psoriasis.
| Psoriasis | In children<2 years, diaper area psoriasis is the most common form; in children and adolescents, plaque psoriasis is the most common form |
| Smaller lesions and finer scaling | |
| Greater tendency to develop on the face and flexural surfaces | |
| Greater symmetry | |
| Lesions may adopt an annular or serpiginous morphology | |
| Isomorphic or Koebner phenomenon is common | |
| Nail involvement is less frequent, especially in younger children | |
| Pruritus is a common symptom | |
| Pediatric population | Anatomophysiological characteristics:• Larger body surface area• Lower body mass• High surface/volume ratio• Higher percentage of water in total body weight, especially in younger children• Specific absorption, distribution, metabolism, and elimination characteristics |
| Specific family, educational, and social structure Stage of life when the individual develops not only physically but also in terms of functional complexity (autonomy, personality, etc.), acquires academic knowledge, and learns to live in society | |
| Different coping with the disease and considerations regarding treatments (management of uncertainty, fear of needles, etc.) | |
| Dependence on Parents or Caregivers | |
| Evaluation and management of psoriasis | Lack of specific or adapted tools |
| Domains for assessing severity or quality of life differ | |
| Limited evidence on the safety and efficacy of interventions | |
| Indications in product monographs | |
| Involvement of parents or caregivers |
From a disease perspective, while the types and locations of pediatric psoriasis are similar to those in adults, there are notable differences. For instance, in children younger than 2 years, diaper-area psoriasis is the most common on,18 whereas plaque psoriasis is more prevalent in children and adolescents.19,20 Guttate psoriasis is the second most common type.21 Nail involvement occurs in up to 40% of cases but is less common vs adults.22 Additionally, pediatric psoriasis lesions tend to be smaller, exhibit finer scaling vs adults,20 and are more symmetrical.23 They also more frequently develop on the face and flexor surfaces.20 Pruritus is a common symptom that significantly disrupts daily life and sleep.7,24–26As with adults, the impact of the disease on children can be profound, extending to their environment. Children with psoriasis may experience disruptions in educational, physical, psychological, emotional, familial, and social well-being.2–4
Managing pediatric patients requires a holistic approach, considering skin involvement, comorbidities, and other individual factors.27,28 The final decision on which drug should be prescribed rests with the dermatologist's clinical judgment. After evaluating the above-mentioned variables and applying them to the individual patient, the dermatologist will make a justified decision in collaboration with the patient's parents or guardians. Patient age should not limit access to therapeutic options.
Assessment of severity in pediatric psoriasisAssessing disease severity is of paramount importance for setting therapeutic goals, making treatment decisions, and monitoring response. In the pediatric population, severity is evaluated similarly to adults,16,17 while accounting for specific characteristics of pediatric psoriasis. Moderate-to-severe psoriasis is defined as follows:
- •
Psoriasis Area and Severity Index (PASI)≥10, Body Surface Area (BSA)≥10, Physician's Global Assessment (PGA)≥3, or Children's Dermatology Life Quality Index (CDLQI)≥10
- •
Special locations
- •
Severe forms (erythrodermic and pustular psoriasis)
- •
Presence of PsA
- •
Failure of topical treatments
In addition to these criteria, other factors influencing disease severity, such as its impact on academic performance, should also be considered. Collaboration with parents or guardians is often necessary, particularly for younger children, to accurately assess disease severity.
However, in the pediatric population, it is necessary to address several issues related to the severity indices used in adults (Table 3). The PASI and BSA and their cutoff points are not formally validated and/or adapted for children. Additionally, they may present certain limitations due to differences in body composition between children and adults. For this reason, a standard method of measuring BSA in children has been proposed using the “rule of nines,” adjusted for regional and age-related body proportions29. The CDLQI, on the other hand, is a validated 10-question survey to assess quality of life in patients aged 4 up to 16 years, derived from the adult Dermatology Life Quality Index (DLQI)30, which includes variables such as itching, sleep, hygiene, impact on relationships and activities, and treatment efficacy. It is available both as text and in animated vignette format30,31. While it measures quality of life, it can serve as a surrogate index of disease severity. For patients aged 16 and older, the adult DLQI can be used. The Simplified Psoriasis Index (PSI) is a validated index for children that not only evaluates the severity of skin lesions but also includes the psychosocial burden and previous treatments used32. However, it is not widely used.
Main characteristics of tools for assessing the severity of psoriasis in children and adolescents.
| Tool | Limitations of use in children and adolescents | Validation in pediatric population | Reference |
|---|---|---|---|
| PASI | Different body proportions compared to adults | NO | – |
| BSA | Different body proportions compared to adults | NO | – |
| CDLQI | Difficulty for the child to understand/express/complete the items asked | 4–16 years | 30 |
| PGA | – | NO | – |
| PSI | – | 4–17 years | 32 |
PASI, Psoriasis Area and Severity Index; BSA, Body Surface Area; CDLQI, Children's Dermatology Life Quality Index; PGA, Physician's Global Assessment; PSI, Simplified Psoriasis Index.
These indices are difficult to apply in children under 4 years of age, in whom the PGA may serve as a valid alternative to assess disease severity. In very complex cases, even an individual visual analog scale (VAS) could greatly aid in evaluating severity.
Despite the limitations noted with severity indices, based on expert experience, these indices are considered perfectly generalizable to the pediatric population, using the same cutoff points established for adults.
As outlined in other GPS documents, in addition to disease severity, associated symptoms, quality-of-life impact, psychosocial and educational development of the child, as well as the opinions of parents or caregivers, should be considered in therapeutic decision-making.16,17
Therapeutic goalsRegarding the establishment of therapeutic goals (Table 4), as in adults,33 the aim should always be excellence (complete skin clearance and absence of symptoms and disease impact on the patient). This goal is particularly critical in children because they are in a vital stage of development and are likely to live with psoriasis for many years. Thus, tight disease control from the moment of diagnosis is crucial.
Considerations on establishing therapeutic goals in pediatric psoriasis.
| Consideration | Details |
|---|---|
| 1. The therapeutic goal must: | • Be individualized• Be adapted to the characteristics of the disease• Be adapted to the characteristics of the pediatric patient• Be established independently of the type of medication• Weigh the impact on safety, quality of life, and efficiency that attempting to achieve complete disease clearance may have• Consider the peculiarities of severity assessment in pediatric patients |
| 2. When establishing the therapeutic goal, it is recommended to differentiate between: | • Optimal goal• Clinically adequate goal |
| 3. The optimal goals should include: | • Achieving a PASI100 response, absolute PASI 0, or complete clearance• Absence of psoriasis-related clinical signs• Absence of psoriasis impact on the physical, psychological, emotional, educational, familial, and social spheres |
| 4. The clinically adequate goals should include: | • Achieving a PASI90 response, absolute PASI≤3, BSA<3, and PGA 0–1 (in special locations, PGA≤1)• Minimizing the impact on quality of life and child development• Achieving minimal disease activity (MDA) |
| 5. In specific patients or situations (multiple previous failures, toxicity issues, etc.), other less demanding therapeutic goals may be considered clinically adequate, such as PASI75 or absolute PASI≤5 | |
PASI, Psoriasis Area and Severity Index; BSA, Body Surface Area; CDLQI, Children's Dermatology Life Quality Index; PGA, Physician's Global Assessment.
However, several pediatric patients, despite responding, do not achieve this therapeutic goal.33 Consequently, individualized less demanding therapeutic goals may be acceptable (e.g., in patients refractory to multiple therapies, due to toxicity issues, etc.).
On the other hand, as previously mentioned, everything must be customized to the pediatric patient context. Collaboration with parents or caregivers is fundamental.
In the pediatric context, it is particularly important to explain the characteristics, as well as disease progression, therapeutic options, and expectations in detail, considering the need for informed consent for certain drugs.
Criteria for selecting topical, systemic, and phototherapy treatmentsOnce psoriasis severity has been determined, treatment selection and prioritization should consider factors related to (1) type of psoriasis, (2) the patient (age and comorbidities), (3) the drug (available evidence, administration route, onset speed, convenience, etc.), (4) preferences of the patient and family, (5) health care system organization.16,17
The child's idiosyncrasies and environment should also be considered. It is essential to evaluate whether a specific treatment is the most suitable or whether it could negatively impact various aspects of the child's daily life.34 Negative impacts include missing school for treatment or laboratory tests, stigma, cosmetic unacceptability of topical treatments, or phobia of needles causing stress and anxiety.
Regulatory aspects described in drug fact sheets must also be considered (Tables 5 and 6).
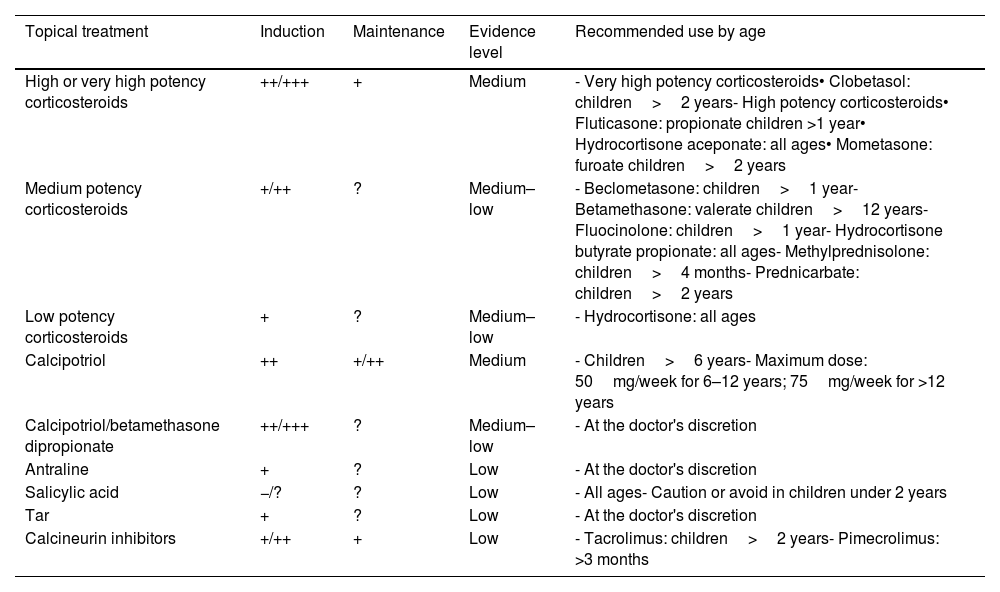
Main characteristics of safety, efficacy, and use of topical therapies.
| Topical treatment | Induction | Maintenance | Evidence level | Recommended use by age |
|---|---|---|---|---|
| High or very high potency corticosteroids | ++/+++ | + | Medium | - Very high potency corticosteroids• Clobetasol: children>2 years- High potency corticosteroids• Fluticasone: propionate children >1 year• Hydrocortisone aceponate: all ages• Mometasone: furoate children>2 years |
| Medium potency corticosteroids | +/++ | ? | Medium–low | - Beclometasone: children>1 year- Betamethasone: valerate children>12 years- Fluocinolone: children>1 year- Hydrocortisone butyrate propionate: all ages- Methylprednisolone: children>4 months- Prednicarbate: children>2 years |
| Low potency corticosteroids | + | ? | Medium–low | - Hydrocortisone: all ages |
| Calcipotriol | ++ | +/++ | Medium | - Children>6 years- Maximum dose: 50mg/week for 6–12 years; 75mg/week for >12 years |
| Calcipotriol/betamethasone dipropionate | ++/+++ | ? | Medium–low | - At the doctor's discretion |
| Antraline | + | ? | Low | - At the doctor's discretion |
| Salicylic acid | −/? | ? | Low | - All ages- Caution or avoid in children under 2 years |
| Tar | + | ? | Low | - At the doctor's discretion |
| Calcineurin inhibitors | +/++ | + | Low | - Tacrolimus: children>2 years- Pimecrolimus: >3 months |
+++: Very (effective, safe, adherent, preferred by the patient); ++: Quite (effective, safe, adherent, preferred by the patient); +: Somewhat (effective, safe, adherent, preferred by the patient); −/+: Doubtful (effective, safe, adherent, preferred by the patient); −: Not (effective, safe, adherent, preferred by the patient); ?: Unknown.
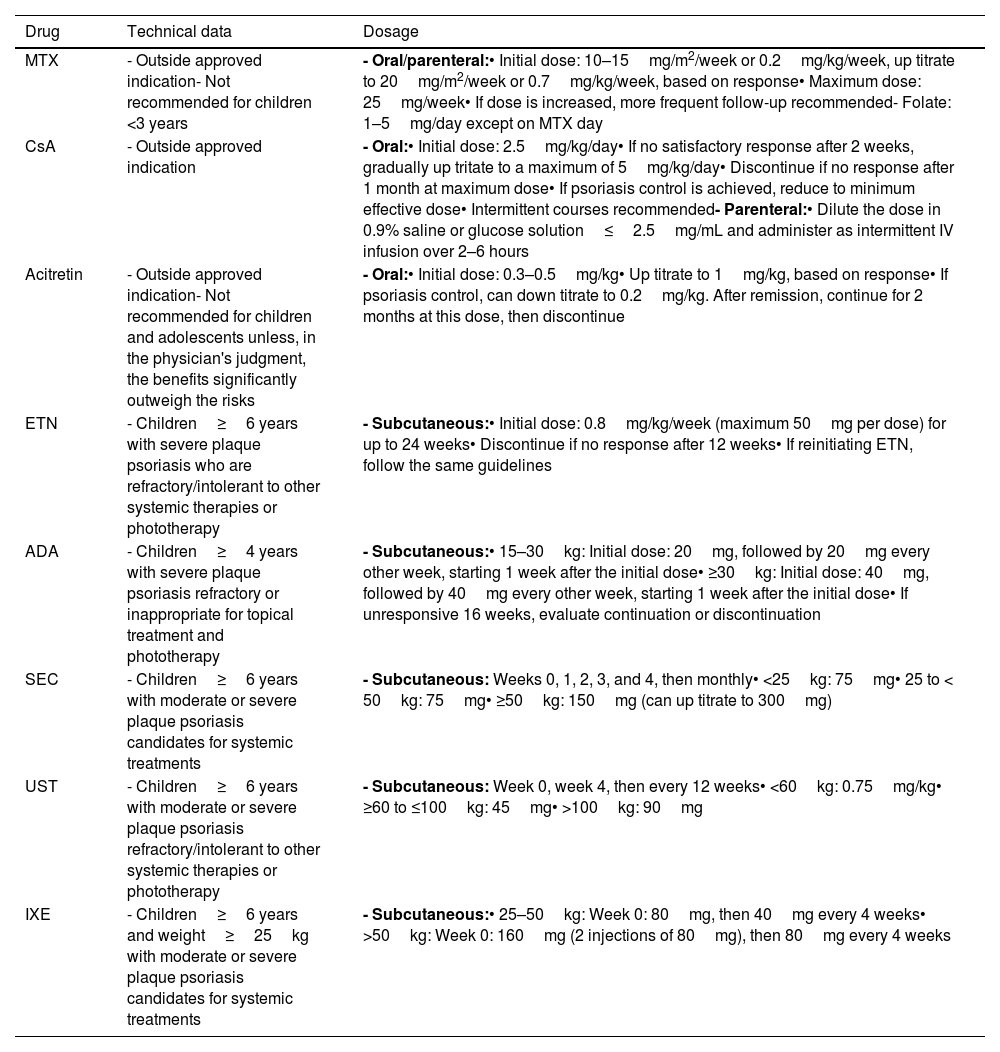
Guidelines on the use of systemic drugs in pediatric psoriasis.
| Drug | Technical data | Dosage |
|---|---|---|
| MTX | - Outside approved indication- Not recommended for children <3 years | - Oral/parenteral:• Initial dose: 10–15mg/m2/week or 0.2mg/kg/week, up titrate to 20mg/m2/week or 0.7mg/kg/week, based on response• Maximum dose: 25mg/week• If dose is increased, more frequent follow-up recommended- Folate: 1–5mg/day except on MTX day |
| CsA | - Outside approved indication | - Oral:• Initial dose: 2.5mg/kg/day• If no satisfactory response after 2 weeks, gradually up tritate to a maximum of 5mg/kg/day• Discontinue if no response after 1 month at maximum dose• If psoriasis control is achieved, reduce to minimum effective dose• Intermittent courses recommended- Parenteral:• Dilute the dose in 0.9% saline or glucose solution≤2.5mg/mL and administer as intermittent IV infusion over 2–6 hours |
| Acitretin | - Outside approved indication- Not recommended for children and adolescents unless, in the physician's judgment, the benefits significantly outweigh the risks | - Oral:• Initial dose: 0.3–0.5mg/kg• Up titrate to 1mg/kg, based on response• If psoriasis control, can down titrate to 0.2mg/kg. After remission, continue for 2 months at this dose, then discontinue |
| ETN | - Children≥6 years with severe plaque psoriasis who are refractory/intolerant to other systemic therapies or phototherapy | - Subcutaneous:• Initial dose: 0.8mg/kg/week (maximum 50mg per dose) for up to 24 weeks• Discontinue if no response after 12 weeks• If reinitiating ETN, follow the same guidelines |
| ADA | - Children≥4 years with severe plaque psoriasis refractory or inappropriate for topical treatment and phototherapy | - Subcutaneous:• 15–30kg: Initial dose: 20mg, followed by 20mg every other week, starting 1 week after the initial dose• ≥30kg: Initial dose: 40mg, followed by 40mg every other week, starting 1 week after the initial dose• If unresponsive 16 weeks, evaluate continuation or discontinuation |
| SEC | - Children≥6 years with moderate or severe plaque psoriasis candidates for systemic treatments | - Subcutaneous: Weeks 0, 1, 2, 3, and 4, then monthly• <25kg: 75mg• 25 to < 50kg: 75mg• ≥50kg: 150mg (can up titrate to 300mg) |
| UST | - Children≥6 years with moderate or severe plaque psoriasis refractory/intolerant to other systemic therapies or phototherapy | - Subcutaneous: Week 0, week 4, then every 12 weeks• <60kg: 0.75mg/kg• ≥60 to ≤100kg: 45mg• >100kg: 90mg |
| IXE | - Children≥6 years and weight≥25kg with moderate or severe plaque psoriasis candidates for systemic treatments | - Subcutaneous:• 25–50kg: Week 0: 80mg, then 40mg every 4 weeks• >50kg: Week 0: 160mg (2 injections of 80mg), then 80mg every 4 weeks |
MTX: Methotrexate; CsA: Cyclosporine; ETN: Etanercept; mg: milligrams; m2: square meter; week: week; kg: kilogram; mL: milliliter.
To define therapeutic failure, the same considerations as in adults16,17 are used, adapted to the particularities of the pediatric population. Therapeutic failure is defined as:
- •
Failure to achieve the proposed therapeutic goal: primary therapeutic failure.
- •
Loss of the proposed therapeutic goal: secondary therapeutic failure.
- •
Achievement of the therapeutic goal at the expense of significant toxicity, requiring treatment discontinuation: safety failure.
- •
The treatment negatively impacts the patient's quality of life.
The timeframe for defining therapeutic failure depends on the type of treatment used.
Finally, it is crucial to evaluate the patient's adherence to treatment before determining the presence of definitive therapeutic failure.
Evidence and use of available treatments in pediatric psoriasisTopical treatmentsTopical treatments, except for topical corticosteroids, are not indicated in the drug fact sheet for pediatric psoriasis. However, they are widely used in the routine clinical practice for both mild and severe psoriasis as coadjuvant therapy (Table 5). Although specific RCTs are lacking, their safety and efficacy profile and have been analyzed in multiple observational studies, both in the short- and long-term.35–48
Although there are no head-to-head comparative studies, indirect data suggest that corticosteroids, vitamin D analogs, and the combination of calcipotriol/betamethasone dipropionate11 seem to be more effective than other topical treatments for pediatric plaque psoriasis (both mild and severe forms).
Topical corticosteroidsEvidence on the safety and efficacy profile comes from moderate-to-low quality observational studies (Table 5). Plaque improvement/resolution after 8–12 weeks of use is estimated at 72.7%, with greater efficacy at higher corticosteroid potency.35–37
The safety profile is acceptable and similar to that of adults, with irritation and itching at the application site being the most common adverse events.36 Systemic adverse events are very rare.
High/very high-potency topical corticosteroids should be used with caution and for the shortest periods of time possible. Dermatologists should monitor patients closely, tailoring this monitoring to the characteristics of each child.
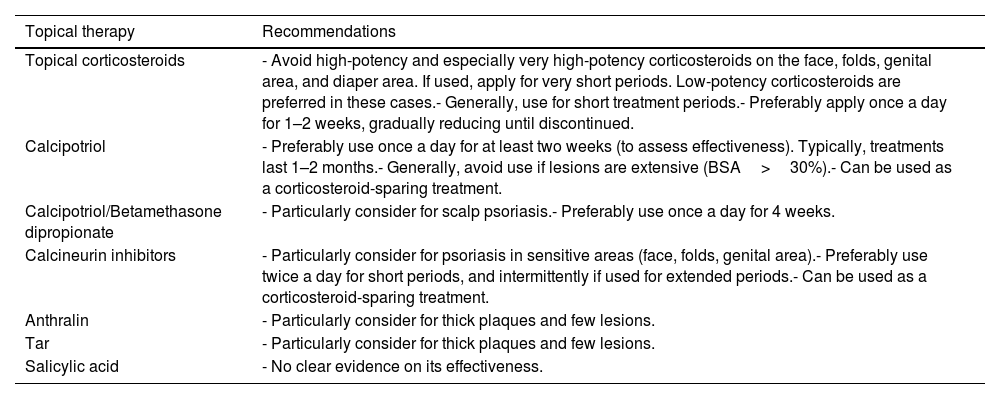
Experts recommend explaining to parents/caregivers and older children the amount and method of application and the possibility of rebound effects if discontinued abruptly (especially for more potent corticosteroids).49,50Tables 5 and 7 provide indications and recommendations, and Table 8 outlines a usage guide based on the lesion location and patient age.
Practical recommendations on the use of topical therapies in pediatric psoriasis.
| Topical therapy | Recommendations |
|---|---|
| Topical corticosteroids | - Avoid high-potency and especially very high-potency corticosteroids on the face, folds, genital area, and diaper area. If used, apply for very short periods. Low-potency corticosteroids are preferred in these cases.- Generally, use for short treatment periods.- Preferably apply once a day for 1–2 weeks, gradually reducing until discontinued. |
| Calcipotriol | - Preferably use once a day for at least two weeks (to assess effectiveness). Typically, treatments last 1–2 months.- Generally, avoid use if lesions are extensive (BSA>30%).- Can be used as a corticosteroid-sparing treatment. |
| Calcipotriol/Betamethasone dipropionate | - Particularly consider for scalp psoriasis.- Preferably use once a day for 4 weeks. |
| Calcineurin inhibitors | - Particularly consider for psoriasis in sensitive areas (face, folds, genital area).- Preferably use twice a day for short periods, and intermittently if used for extended periods.- Can be used as a corticosteroid-sparing treatment. |
| Anthralin | - Particularly consider for thick plaques and few lesions. |
| Tar | - Particularly consider for thick plaques and few lesions. |
| Salicylic acid | - No clear evidence on its effectiveness. |
Guide on the use of topical corticosteroids based on localization and patient age according to the Fingertip Unit (FTU).a
| Localization | 3–6 months | 1–2 years | 3–5 years | 6–10 years | >10 years |
|---|---|---|---|---|---|
| Face and neck | 1 | 1.5 | 1.5 | 2 | 2.5 |
| Chest and abdomen | 1 | 2 | 3 | 3.5 | 7 |
| Back | 1.5 | 3 | 3.5 | 5 | 7 |
| Arm and hand | 1 | 1.5 | 2 | 2.5 | 4 |
| Hand and fingersb | – | – | – | – | 1 |
| Leg and Foot | 1.5 | 2 | 3 | 4.5 | 8 |
A FTU is described as “the amount of cream/ointment squeezed from a tube with a 5mm diameter nozzle, applied from the distal fold of the palmar surface of the index fingertip, approximately 0.5g.
An RCT in pediatric patients with moderate-to-severe plaque psoriasis found that twice-daily topical calcipotriol for more than 8 weeks was significantly superior to placebo in reducing redness and scaling and achieving a higher percentage of PGA 0/1 according to physicians.38 However, there were no significant differences in PASI changes (baseline vs post-treatment), lesion thickness or extent reduction, or PGA 0/1 according to patients.38 Adverse events were generally mild, with skin irritation being the most common of all.38 Observational studies have shown improvement/resolution rates of 57.2% up to 100% with topical calcipotriol, with good cosmetic acceptance and a favorable safety profile.11,35–37,42,52
Topical calcipotriol can act as a corticosteroid-sparing agent and may also be applied to sensitive skin to minimize the risk of local side effects associated with topical corticosteroids.
Calcipotriol/betamethasone dipropionateThis combination, in pediatric patients with moderate-to-severe plaque psoriasis, used as a standard 4-week regimen once a day with subsequent dose titration, significantly improved PASI, an effect sustained for up to 48 weeks.48 It also improved CDLQI, PGA, BSA, pain, and itching, with a favorable safety profile.48 Other observational studies report plaque improvement/resolution rates of 32.1% up to 80% with its use.35–37
Topical calcineurin inhibitorsEvidence for these drugs in pediatric plaque psoriasis is limited, but they appear to be effective, with lesion improvement/resolution rates exceeding 50%.35,53 Their main adverse effects include pruritus and local irritation, and they may increase the risk of skin infections and acne.53
AnthralinSeveral observational studies have evaluated the efficacy and safety of anthralin in short treatments lasting approximately 8–12 weeks for moderate-to-severe pediatric plaque psoriasis.35,46,54,55 Improvements in PASI of 69.3%, lesion improvement/resolution rates ranging from 3.7% to 81%, and positive changes in CDLQI have been documented. The reported adverse reaction was irritation.
Salicylic acidTopical treatment with salicylic acid has not demonstrated clear efficacy in observational studies, and evidence for its use is limited.35–37
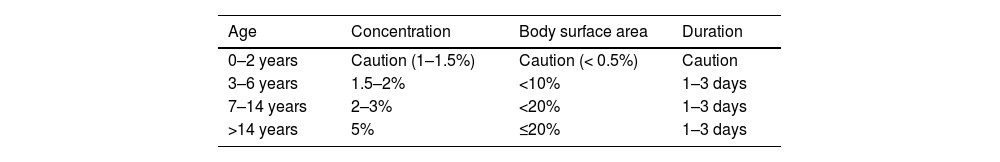
The topical use of salicylic acid carries a low risk of systemic chronic or acute intoxication, with symptoms such as oral discomfort, headache, dizziness, tinnitus, nausea, or hyperventilation.56–58 These symptoms may occur after topical treatment over large body areas (>20%)57,59,60 especially in children under 12 years and in patients with renal or hepatic failure.56,60,61 Therefore, caution is advised when prescribing salicylic acid (Table 9).
TarTar is often used in combination with phototherapy for the management of pediatric plaque psoriasis.62 Evidence on its use as monotherapy is limited, but it may be effective.35 Although tar has a good safety profile, it is cosmetically unsatisfactory.
Systemic treatmentSystemic treatment is indicated for moderate-to-severe pediatric psoriasis.
Several systemic therapies have demonstrated efficacy in pediatric plaque psoriasis, but currently, only biologic therapies have regulatory approval.
Of note, methotrexate (MTX), acitretin, and cyclosporine (CsA) are not indicated for individuals under 18 years old according to regulatory clincal practice guidelines.
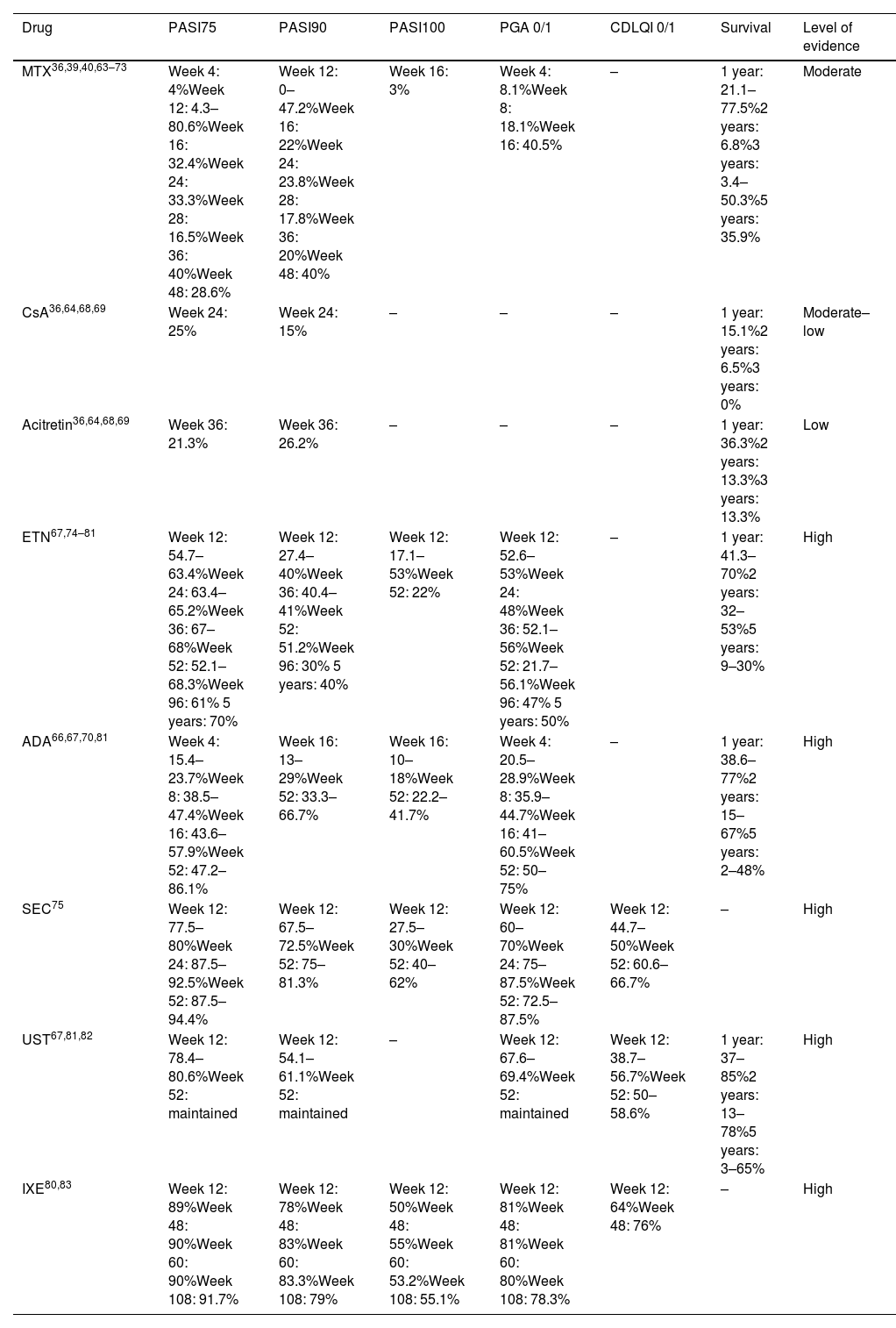
Table 10 illustrates the efficacy of systemic treatments, and Table 6 provides a usage guide. Risk management and monitoring of these therapies are shown in Table 11.
Efficacy of systemic treatments in pediatric psoriasis.
| Drug | PASI75 | PASI90 | PASI100 | PGA 0/1 | CDLQI 0/1 | Survival | Level of evidence |
|---|---|---|---|---|---|---|---|
| MTX36,39,40,63–73 | Week 4: 4%Week 12: 4.3–80.6%Week 16: 32.4%Week 24: 33.3%Week 28: 16.5%Week 36: 40%Week 48: 28.6% | Week 12: 0–47.2%Week 16: 22%Week 24: 23.8%Week 28: 17.8%Week 36: 20%Week 48: 40% | Week 16: 3% | Week 4: 8.1%Week 8: 18.1%Week 16: 40.5% | – | 1 year: 21.1–77.5%2 years: 6.8%3 years: 3.4–50.3%5 years: 35.9% | Moderate |
| CsA36,64,68,69 | Week 24: 25% | Week 24: 15% | – | – | – | 1 year: 15.1%2 years: 6.5%3 years: 0% | Moderate–low |
| Acitretin36,64,68,69 | Week 36: 21.3% | Week 36: 26.2% | – | – | – | 1 year: 36.3%2 years: 13.3%3 years: 13.3% | Low |
| ETN67,74–81 | Week 12: 54.7–63.4%Week 24: 63.4–65.2%Week 36: 67–68%Week 52: 52.1–68.3%Week 96: 61% 5 years: 70% | Week 12: 27.4–40%Week 36: 40.4–41%Week 52: 51.2%Week 96: 30% 5 years: 40% | Week 12: 17.1–53%Week 52: 22% | Week 12: 52.6–53%Week 24: 48%Week 36: 52.1–56%Week 52: 21.7–56.1%Week 96: 47% 5 years: 50% | – | 1 year: 41.3–70%2 years: 32–53%5 years: 9–30% | High |
| ADA66,67,70,81 | Week 4: 15.4–23.7%Week 8: 38.5–47.4%Week 16: 43.6–57.9%Week 52: 47.2–86.1% | Week 16: 13–29%Week 52: 33.3–66.7% | Week 16: 10–18%Week 52: 22.2–41.7% | Week 4: 20.5–28.9%Week 8: 35.9–44.7%Week 16: 41–60.5%Week 52: 50–75% | – | 1 year: 38.6–77%2 years: 15–67%5 years: 2–48% | High |
| SEC75 | Week 12: 77.5–80%Week 24: 87.5–92.5%Week 52: 87.5–94.4% | Week 12: 67.5–72.5%Week 52: 75–81.3% | Week 12: 27.5–30%Week 52: 40–62% | Week 12: 60–70%Week 24: 75–87.5%Week 52: 72.5–87.5% | Week 12: 44.7–50%Week 52: 60.6–66.7% | – | High |
| UST67,81,82 | Week 12: 78.4–80.6%Week 52: maintained | Week 12: 54.1–61.1%Week 52: maintained | – | Week 12: 67.6–69.4%Week 52: maintained | Week 12: 38.7–56.7%Week 52: 50–58.6% | 1 year: 37–85%2 years: 13–78%5 years: 3–65% | High |
| IXE80,83 | Week 12: 89%Week 48: 90%Week 60: 90%Week 108: 91.7% | Week 12: 78%Week 48: 83%Week 60: 83.3%Week 108: 79% | Week 12: 50%Week 48: 55%Week 60: 53.2%Week 108: 55.1% | Week 12: 81%Week 48: 81%Week 60: 80%Week 108: 78.3% | Week 12: 64%Week 48: 76% | – | High |
MTX: methotrexate; CsA: cyclosporine; ETN: etanercept; ADA: adalimumab; SEC: secukinumab; UST: ustekinumab; IXE: ixekizumab; PASI: Psoriasis Area Severity Index; PGA: Physician Global Assessment; CDLQI: Children's Dermatology Life Quality Index.
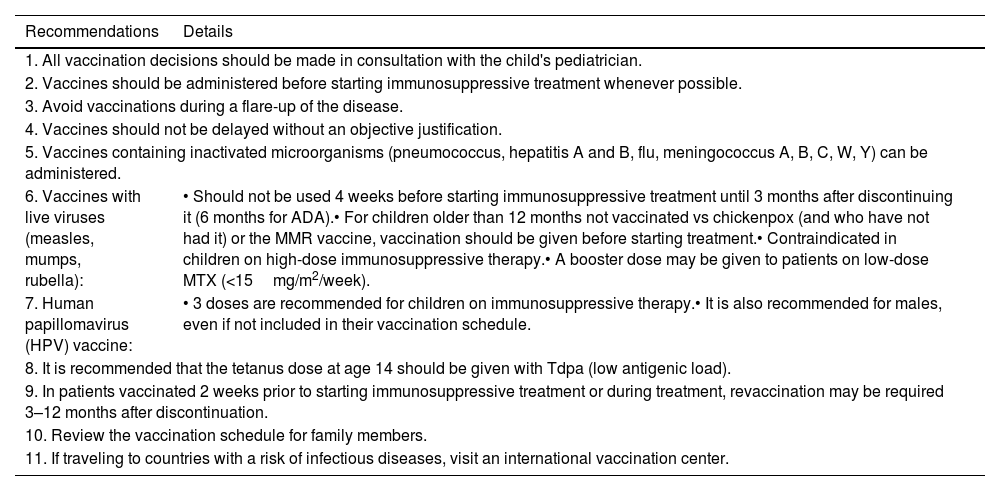
Vaccination recommendations for children with psoriasis on immunosuppressive therapies.
| Recommendations | Details |
|---|---|
| 1. All vaccination decisions should be made in consultation with the child's pediatrician. | |
| 2. Vaccines should be administered before starting immunosuppressive treatment whenever possible. | |
| 3. Avoid vaccinations during a flare-up of the disease. | |
| 4. Vaccines should not be delayed without an objective justification. | |
| 5. Vaccines containing inactivated microorganisms (pneumococcus, hepatitis A and B, flu, meningococcus A, B, C, W, Y) can be administered. | |
| 6. Vaccines with live viruses (measles, mumps, rubella): | • Should not be used 4 weeks before starting immunosuppressive treatment until 3 months after discontinuing it (6 months for ADA).• For children older than 12 months not vaccinated vs chickenpox (and who have not had it) or the MMR vaccine, vaccination should be given before starting treatment.• Contraindicated in children on high-dose immunosuppressive therapy.• A booster dose may be given to patients on low-dose MTX (<15mg/m2/week). |
| 7. Human papillomavirus (HPV) vaccine: | • 3 doses are recommended for children on immunosuppressive therapy.• It is also recommended for males, even if not included in their vaccination schedule. |
| 8. It is recommended that the tetanus dose at age 14 should be given with Tdpa (low antigenic load). | |
| 9. In patients vaccinated 2 weeks prior to starting immunosuppressive treatment or during treatment, revaccination may be required 3–12 months after discontinuation. | |
| 10. Review the vaccination schedule for family members. | |
| 11. If traveling to countries with a risk of infectious diseases, visit an international vaccination center. | |
MTX is the most widely used classic systemic drug for the management of pediatric psoriasis.36,39,40,64–66,68–73, It can be administered orally or parenterally on a weekly basis. Typically used as monotherapy, it may be combined with biologic therapies in highly individualized cases.73Tables 6 and 10 describe its main characteristics.
Several SLRs have described the efficacy of MTX (at various doses and regimens) in treatments lasting 6 weeks to 4 years36,68,69 finding up to a 90% significant improvement/clearance rate in pediatric plaque psoriasis. A RCT reported the following outcomes for MTX (0.1–0.4mg/kg/week) at 16 weeks: PASI75 32.4%, PGA 0/1 40.5%, PASI90 22%, PASI100 3%, CDLQI −5.0, and Pediatric Quality of Life Inventory (PedsQL) 1.9.66 The rate of adverse events was 76%, with severe adverse events occurring in 5%.66 Data at 52 weeks confirmed the results observed at 16 weeks.70 Observational studies up to 5 years align with these findings.65,71 MTX also improves quality of life40,65 and is highly effective in children with associated PsA.84 MTX survival rates are estimated at 77.5%, 50.3% and 35.9% at 1, 3, and 5 years, respectively (significantly lower vs those achieved with biologics).72
The safety profile of MTX is very similar to that described in adults.39,64,65,71
CyclosporineCsA is another classic systemic drug used in pediatric psoriasis, particularly for special locations and severe forms. It is typically administered orally, though parenteral use is possible.
SLRs and observational studies report PASI75 and PASI90 response rates of 25% and 15%, respectively after 24 weeks of treatment, with a safety profile similar to that of adults.36,39,64,68,69
AcitretinThis retinoid has been studied in pediatric patients with pustular psoriasis, generalized guttate psoriasis, erythrodermic psoriasis, and specific locations.36,68,69 However, evidence for its use in plaque psoriasis is limited.36,68,69
Although no serious safety concerns have been reported in published studies, retinoids may cause significant musculoskeletal adverse events with chronic high-dose use.85,86 Specifically, they have been associated with premature epiphyseal closure, hyperostosis, anterior spinal ligament calcification, periosteal bone formation, and decreased bone mineral density.85,86 Additionally, acitretin is teratogenic.87
Biologic therapiesBiologics approved for moderate-to-severe pediatric psoriasis include etanercept (ETN), adalimumab (ADA), ustekinumab (UST), secukinumab (SEC), and ixekizumab (IXE). These therapies are the only ones supported by high-quality clinical trials and short- to mid- to long-term data (Table 10). Their indications and monitoring requirements are based on these findings.
Given their safety and efficacy profiles in pediatric patients, biologics are increasingly considered first-line systemic therapies.
ETN has shown significant superiority over placebo in pediatric psoriatic patients (≥4 years) with ≥10% BSA.77 At 12 weeks, ETN achieved PASI75 in 57%, PASI90 in 27%, and PGA 0/1 in 53%. This improvement persisted at week 36, with PASI75 in 65–68%, PASI90 in 38–41%, and PGA 0/1 in 53% up to 56%.77 Results were sustained at 2 (PASI75 61%, PASI90 30%, PGA 0/1 47%)78 and 5 years (PASI75 70%, PASI90 40%, PGA 0/1 50%).79 ETN also significantly improves quality of life.76–79 Although the efficacy of ETN is consistent across age groups it is lower in overweight/obese patients vs those with normal weight.79 ETN was generally well tolerated, with infections being the most common adverse events and severe adverse events being a rare finding.76–79
On the other hand, ADA has shown efficacy in children (≥4 years) with severe plaque psoriasis.66,70 ADA at 0.4mg/kg and 0.8mg/kg doses achieved the following outcomes at 16 weeks: PASI75 43.6% and 57.9%, PASI90 13% and 29%, PASI100 10% and 18%, PGA 0/1 41.0% and 60.5%, and CDLQI −4.9 and −6.6.66 ADA at 0.8mg/kg was significantly superior to MTX in PASI75 and PedsQL but showed no differences in PGA 0/1, PASI90, PASI100, and CDLQI. The relates of adverse event were 77% and 68%, respectively, with severe adverse events in 13% and 3%.66 Infections were the most common adverse events.66 Data at 52 weeks confirmed observations from 16 weeks.70
A RCT evaluated SEC at low (75/75/150mg), higher doses (75/150/300mg), and ETN 0.8mg/kg (max. 50mg) in children (≥6 years), nearly 100% of whom had plaque psoriasis with a PASI≥20.75 At 12 weeks, both doses of SEC were significantly superior to placebo, with approximately 30% of patients achieving PASI100 with SEC. Compared to ETN, both SEC doses were significantly superior in achieving PASI90 and Investigator's Global Assessment (IGA) 0/1, but no differences were observed in PASI75 or PASI100 between groups at 12 weeks.75 At 24 weeks, data for low-dose SEC vs high-dose SEC vs ETN were as follows: IGA 0/1, 87.5% vs 75% vs 48%; PASI75, 92.5% vs 87.5% vs 63.4%. At 52 weeks, results were PASI90, 75–81.3% vs 77.8–80% vs 51.2%; PASI100, 40–62% vs 47.5–55.6% vs 22%; IGA 0/1, 72.5–87.5% vs 72.5–75% vs 56.1%; and CDLQI 0/1, 66% vs 66.7% vs 44.4%.75 The safety profile of SEC was similar to that of other biologic therapies. Most adverse events reported were mild and transient, with infections being the most common one.75 Another open-label, phase III RCT without a comparator group showed similar results at 24 weeks.88
The CADMUS Study analyzed UST in adolescents (12–17 years) with moderate-to-severe plaque psoriasis over 52 weeks. UST was administered at its standard and half-doses and compared to a placebo. At 12 weeks, both UST regimens were significantly superior, with up to 61% of patients achieving PASI9082. Similarly, ay 52 weeks, 50% and 58.6% of patients receiving medium and standard doses of UST achieved CDLQI 0/1. This study found no differences in clinical efficacy based on weight (<60kg, 60–100kg) or serum UST concentrations (within each dose group). UST was well-tolerated, with most adverse events being mild and transient, the most common being infections82.
Regarding IXE, the IXORA-PEDS trial analyzed children (≥6 years) with various types of moderate-to-severe psoriasis, comparing IXE with placebo and ETN.80 Compared with placebo, at 12 weeks, IXE was significantly superior in terms of PASI75, PASI90, PASI100, PGA 0/1, and CDLQI/DLQI 0/1, among other outcomes.80 Compared with ETN in patients with severe psoriasis, at 12 weeks, IXE was significantly superior in achieving PASI90, PASI100, and PGA 0. However, there were no differences in PASI75 or PGA 0/1.80 By week 48, 90% of patients had achieved PASI75; 83%, PASI90; 55%, PASI100; and 76%, CDLQI/DLQI 0/1.80 At week 60, the rates of patients achieving PASI75, PASI90, and PASI100 were 90%, 83.3%, and 53.2%, respectively. By week 108, these rates were 91.7%, 79%, and 55.1%. Safety data at weeks 12 and 108 indicated that IXE was generally well-tolerated. The rate of serious adverse events was low (7.7% at week 108), with infections being the most common.80,89
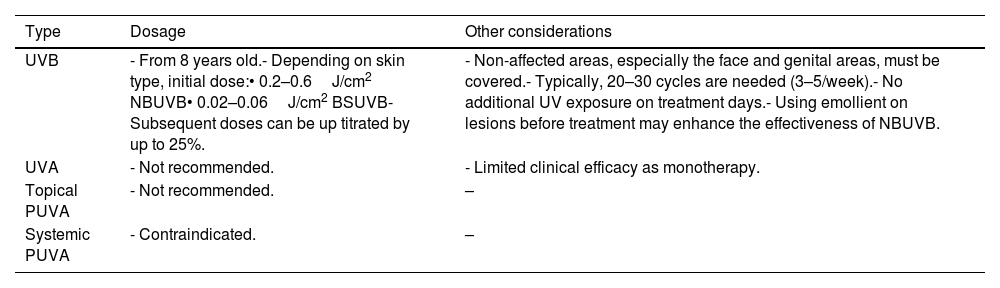
PhototherapyPhototherapy is indicated for children≥8 years with moderate-to-severe psoriasis (refer to Table 12 for guidelines). However, access to phototherapy can be limited due to logistical constraints in certain hospitals.
Guide on the use of phototherapy/heliotherapy in pediatric psoriasis patients.
| Type | Dosage | Other considerations |
|---|---|---|
| UVB | - From 8 years old.- Depending on skin type, initial dose:• 0.2–0.6J/cm2 NBUVB• 0.02–0.06J/cm2 BSUVB- Subsequent doses can be up titrated by up to 25%. | - Non-affected areas, especially the face and genital areas, must be covered.- Typically, 20–30 cycles are needed (3–5/week).- No additional UV exposure on treatment days.- Using emollient on lesions before treatment may enhance the effectiveness of NBUVB. |
| UVA | - Not recommended. | - Limited clinical efficacy as monotherapy. |
| Topical PUVA | - Not recommended. | – |
| Systemic PUVA | - Contraindicated. | – |
UVB: ultraviolet B light; NBUVB: narrowband ultraviolet B light; UVA: ultraviolet A light; PUVA: psoralen and ultraviolet A light therapy; J: Joule; cm: centimeter.
A 2021 a SLR and meta-analysis focused on narrow-band ultraviolet B (UVB) phototherapy, including 10 prospective and retrospective observational studies with nearly 300 pediatric psoriatic patients treated with a mean 17–57 sessions90. An overall efficacy rate of 80% was estimated, defined as excellent or good response if lesion clearance was ≥75%. However, no association was ever found between efficacy and cumulative or maximum mean doses in the review90. Erythema was the most common adverse event.
Treatment strategies in pediatric psoriasisMild pediatric psoriasisFor the management of mild pediatric psoriasis, first-line therapies include topical therapy or phototherapy/heliotherapy, barring contraindications.
It is important to remember that, except for corticosteroids, topical treatments do not have an approved indication in their product labels.
The selection of a treatment should follow the previously discussed criteria. At this point, experts also emphasize the importance of collaborating with and considering the opinion of the patient's parents or guardians/caregivers.
Many authors consider corticosteroids as the topical treatment of choice.13 If a topical corticosteroid is prescribed, its potency should be adapted to the extent and site of application. Medium- and high-potency corticosteroids are generally preferred, except on the face, folds (including the genital area), and diaper area, where low-potency corticosteroids are recommended. Due to the risk of adverse events, topical corticosteroids should be used at the lowest effective dose and never for long periods of time, especially high-potency corticosteroids. Patients requiring long-term treatment should transition to other types of drugs. For example, topical corticosteroids are recommended once daily for 1–2 weeks, followed by gradual tapering over 2–6 weeks until discontinuation.13–15
For scalp psoriasis, the combination of calcipotriol/betamethasone dipropionate in a single formulation may also be considered.35,48 The recommended quantities of calcipotriol must not be exceeded, taking into account the body surface area to be treated; therefore, it is not a suitable option for extensive psoriasis.91 Calcineurin inhibitors are useful for facial and flexural psoriasis.41 Anthralin and tar preparations can be considered for patients with limited thick plaques.91
Phototherapy/heliotherapy may be considered for patients with more extensive lesions and palmoplantar psoriasis.90
These therapies should be administered until the therapeutic goal has been achieved.
Moderate-to-severe pediatric psoriasisFor patients with moderate-to-severe pediatric psoriasis:
- •
For mild psoriatic patients who do not achieve therapeutic goals with topical treatments or phototherapy/heliotherapy, adherence and treatment compliance should first be evaluated before confirming therapeutic failure.
- •
If no contraindications exist, classic systemic therapies and biologic therapy as monotherapy are the treatments of choice.
- •
Biologic therapies, which have demonstrated safe and effective in clinical trials and have been specifically approved for moderate-to-severe pediatric psoriasis, should be considered the first choice.
- •
Topical therapies should always be considered as coadjuvant therapy for both classic systemic treatments and biologics, at least, at the initiation of systemic therapy while waiting for its effects. They can also be used for residual lesions.
- •
Phototherapy may be considered (e.g., for patients with extensive lesions) as monotherapy or coadjuvant therapy to systemic treatment.
- •
Combination therapy with a classic systemic drug and a biologic can be considered in very selected cases (e.g., to reduce or minimize the risk and impact of immunogenicity).
Poor adherence or improper use of topical therapies is common in pediatric psoriasis.92 Various causes have been described, such as corticophobia, fear of adverse events, low cosmetic acceptance, lack of understanding/knowledge about the mode of application, forgetfulness, etc. Therefore, adherence and compliance should be evaluated before confirming therapeutic failure, and possible errors should be corrected if found. In such cases, the doctor-patient relationship, including interaction with parents or guardians, is fundamental. Effective verbal and non-verbal communication, active listening, and collaboration contribute to an optimal relationship and improve adherence to treatments.92 It is also essential to inform, explain, and educate both patients and their parents or guardians about medication use to improve outcomes.92
For moderate-to-severe pediatric psoriasis, systemic therapy selection should follow previously defined criteria. It is important to adhere to the product label indications (which vary by drug, age, or weight of the patient) and consider the quality of the available evidence.38,66,70,75–80,82,83,93–95 In this regard, biologic therapies, along with acitretin, are the only treatments approved for moderate-to-severe pediatric psoriasis. In terms of evidence, biologic therapies have shown high skin clearance rates in high-quality studies, which are sustained over time. Therefore, and in line with the most recent international consensus documents,13 biologics are considered first-line therapy.
As in adults, in pediatric psoriatic patients, topical treatments can be used as coadjuvant therapy. Their use is recommended at the initiation of systemic therapy due to their rapid action, while awaiting the effect of the main treatment, and later for residual lesions.
Lastly, phototherapy90 and the combination of a classic systemic drug, such as MTX, with a biologic therapy may also be considered in very selected cases.
Pediatric psoriasis in special locations ad severe forms of psoriasisAlthough limited, we describe below the available evidence on the efficacy of pharmacological therapies and phototherapy in special locations (Table 4 of the annex of the supplementary data) and severe forms of pediatric psoriasis. Psoriasis in special locations is considered moderate-to-severe psoriasis. Of note, the severity of pediatric psoriasis is also influenced by its functional or psychological impact (e.g., school absenteeism, stigma, etc.) or its impact on the patient's quality of life.
Scalp psoriasisIt is estimated that between 10% up to 48% of pediatric psoriatic patients experience scalp involvement, either in isolation or associated with other lesions.91–96
Regarding treatment evidence, data from the IXORA-PEDS study conducted on 171 children demonstrated that IXE achieved PASI100 response in 47% of patients at 12 weeks, 76% at 48 weeks, 73.4% at 60 weeks, and 90% at 108 weeks.80,83 Similarly, data from the Child-Capture registry and other observational studies showed that the combination of calcipotriol and betamethasone improved outcomes in pediatric patients with scalp psoriasis.45,89,97 Published studies reported an improvement in the PSI of 19.2% at 6 weeks, increasing up to 29.5% at 12 weeks, with sustained efficacy at week 48.45 The percentages of IGA 0/1 achieved with the combination ranged from 55% up to 85% at 8 weeks.89,97 Further evidence is required for other therapies.
Facial psoriasisFacial lesions are more common in children than in adults, affecting up to 50% of cases and being the only clinical sign in, approximately, 4% of patients.91,96
Topical therapies, particularly tacrolimus, calcitriol, and corticosteroids, have shown efficacy in generally low-quality studies, at least, in the short-to-mid term.36,37,41 The application of tacrolimus 0.1% ointment twice daily for 6 months resulted in significant clinical improvement, though relapses were frequent shortly after treatment discontinuation41. Further evidence is needed for other therapies.
Flexural psoriasis including genital psoriasisUp to 10% of pediatric psoriatic patients may experience flexural psoriasis.91,96
Several SLRs and observational studies have shown that some topical treatments may be effective in the short-to-mid term with an acceptable safety profile, although the evidence level is generally low.35,80 The application of tacrolimus 0.1% ointment twice daily for 6 months produced significant lesion improvement in flexural psoriasis. After 30 days of treatment, 88% of patients experienced excellent improvement from baseline, and 12% achieved complete remission of symptoms. However, relapses were frequent after discontinuation.41 Topical corticosteroids and calcitriol have also demonstrated efficacy.35,37 Isolated cases have reported effectiveness with tar-based treatments.35 However, current evidence does not support the efficacy of salicylic acid for pediatric genital psoriasis.35 In a high-quality RCT with 171 children, IXE achieved 85% genital psoriasis clearance at 12 weeks vs 36% with placebo (p<0.001). Moreover, in the IXE arm, genital psoriasis clearance rates were 90% and 88.9% at 48 and 60 weeks, respectively.80,83 Further evidence is needed for other therapies.
Palmoplantar psoriasisPalmoplantar psoriasis is relatively uncommon in pediatric patients.
Evidence on pharmacological treatments for pediatric palmoplantar psoriasis is limited.
Evidence on pharmacological treatments for pediatric palmoplantar psoriasis is very limited. In the IXORA-PEDS RCT with IXE, 69% of patients achieved a PASI 0 at 12 weeks, 74% at 48 weeks, 81.8% at 60 weeks, and 90% at 108 weeks.80,83 Small observational studies have demonstrated the efficacy of phototherapy in this location.98,99 Cases suggesting that acitretin and phototherapy could be effective have been reported too.90,100 Further evidence is needed to support the efficacy of other therapies.
Guttate psoriasisGuttate psoriasis is the second most common form of pediatric psoriasis.91.96
Many of the results discussed for plaque psoriasis could potentially be extrapolated to guttate psoriasis.36,69 More specifically, phototherapy has shown good outcomes in a small RCT with 20 pediatric patients and other observational studies.90,93 Further evidence is needed to support the efficacy of other therapies.
Nail psoriasisAlthough less common than in adults, nail involvement has been documented in 7% up to 40% of pediatric psoriatic patients.91,96
The impact of various therapies on nail involvement has been scarcely studied. A RCT with IXE found no statistically significant differences in the Nail Psoriasis Severity Index (NAPSI) at 12 weeks vs placebo. However, at 48, 60, and 108 weeks, 50%, 65.3%, and 68.1% of patients, respectively, achieved NAPSI0.80,83 Further evidence is needed for other therapies.
Pustular psoriasisThis severe form of psoriasis is rare in children and even less common in adults.91–96
Acitretin—monotherapy or in combination with phototherapy—may be effective with an acceptable safety profile in patients with pustular and erythrodermic psoriasis.36,68,69 Nine months of treatment reported PASI75 and PASI90 responses in 21.3% and 26.2% of patients, respectively.64 Small case series have also shown that MTX, CsA, phototherapy, and ETN may be effective.68 Further evidence is needed for other therapies.
Erythrodermic psoriasisErythrodermic psoriasis is considered a severe form of psoriasis in children.
Although evidence quality is low, acitretin and CsA may be effective in these patients.36,64,68,69,101,102 Further evidence is needed for other therapies.
ComorbiditiesIn relation to the prevention and presence of comorbidities in pediatric psoriatic patients:
- •
The presence of comorbidities is a common finding and can impact the child and even interfere with the indication, safety or efficacy of treatments. Therefore, comorbidities are a topic of special relevance in the management of patients with pediatric psoriasis.
- •
It is the responsibility of the dermatologist to screen for comorbidities.
- •
Comorbidity screening should be performed regularly, considering the patient's medical history and physical examination. When deemed appropriate, additional tests will be requested.
- •
If a comorbidity is suspected, the patient will be referred to the corresponding health professional depending on local organizational characteristics.
- •
If necessary, multidisciplinary care will be provided with shared decision-making between the dermatologist and other specialists/health professionals.
- •
If necessary, inform, explain, and educate patients and parents or guardians/caregivers about the possibility of comorbidities emerging and the need to prevent many of them.
- •
It is the responsibility of everyone involved in the management of patients with pediatric psoriasis to promote health and prevention.
Psoriasis is a multisystem inflammatory disease. Both adults and children can present comorbidities that may impact their health, quality of life, educational, and psychosocial development or interfere with the safety and efficacy of treatments.18 It has been described that in children with psoriasis, the risk of presenting comorbidities is twice as high vs children without the disease.103 Although evidence is limited, the appearance of comorbidities could be more frequent in children with more severe disease.104 Among the most frequent and/or impactful are obesity, depression, or PsA.105
In line with other international consensus documents,8 regular screening of comorbidities is recommended, at least, for the most frequent ones or those that may have a greater impact on the patient and/or treatments.
On the other hand, experts are aware of the lack of time and logistical resources (e.g., pediatric-specific materials) in some consultations to address comorbidity in the routine clinical practice (both screening and prevention). To simplify and facilitate this work, we present Tables 13 and 14, which illustrate the characteristics and impact of the main comorbidities and show a series of recommendations for their prevention, screening, and other practical considerations.
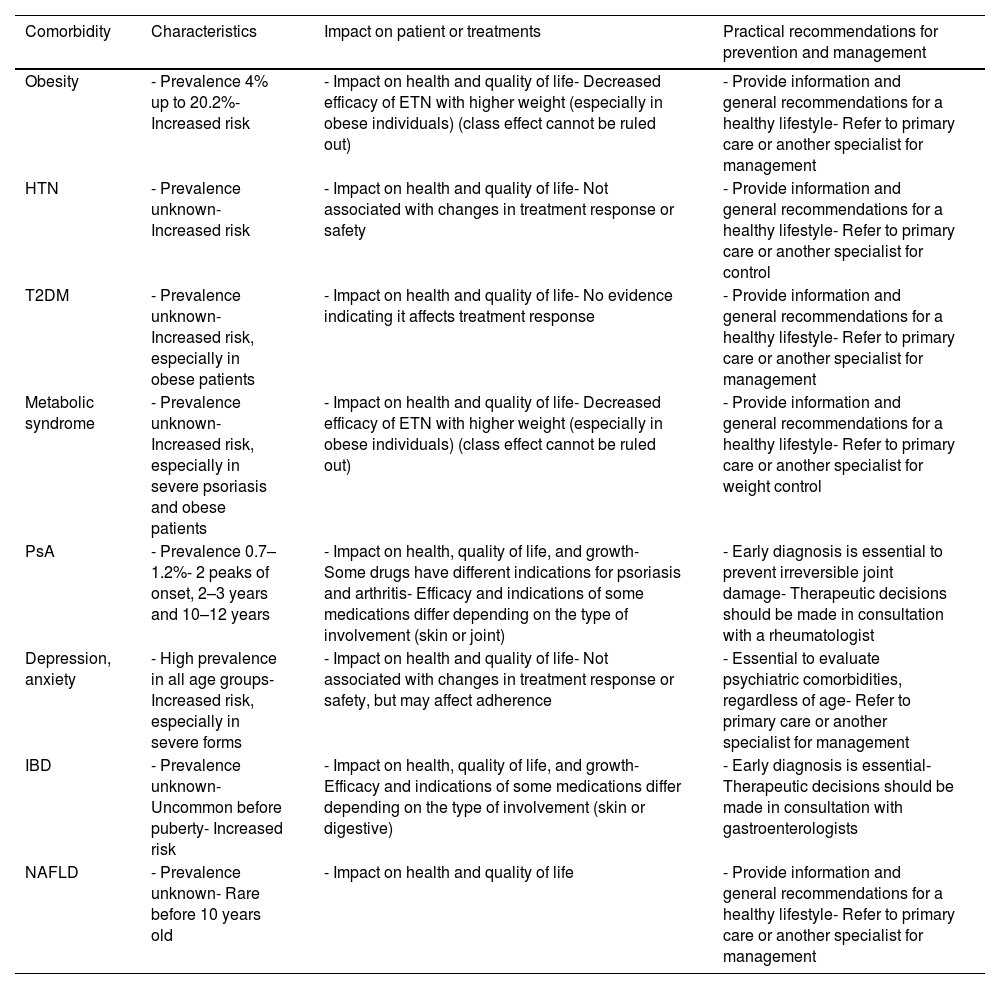
Summary of the main comorbidities, their impact on patient or treatments, and practical recommendations for prevention and management.
| Comorbidity | Characteristics | Impact on patient or treatments | Practical recommendations for prevention and management |
|---|---|---|---|
| Obesity | - Prevalence 4% up to 20.2%- Increased risk | - Impact on health and quality of life- Decreased efficacy of ETN with higher weight (especially in obese individuals) (class effect cannot be ruled out) | - Provide information and general recommendations for a healthy lifestyle- Refer to primary care or another specialist for management |
| HTN | - Prevalence unknown- Increased risk | - Impact on health and quality of life- Not associated with changes in treatment response or safety | - Provide information and general recommendations for a healthy lifestyle- Refer to primary care or another specialist for control |
| T2DM | - Prevalence unknown- Increased risk, especially in obese patients | - Impact on health and quality of life- No evidence indicating it affects treatment response | - Provide information and general recommendations for a healthy lifestyle- Refer to primary care or another specialist for management |
| Metabolic syndrome | - Prevalence unknown- Increased risk, especially in severe psoriasis and obese patients | - Impact on health and quality of life- Decreased efficacy of ETN with higher weight (especially in obese individuals) (class effect cannot be ruled out) | - Provide information and general recommendations for a healthy lifestyle- Refer to primary care or another specialist for weight control |
| PsA | - Prevalence 0.7–1.2%- 2 peaks of onset, 2–3 years and 10–12 years | - Impact on health, quality of life, and growth- Some drugs have different indications for psoriasis and arthritis- Efficacy and indications of some medications differ depending on the type of involvement (skin or joint) | - Early diagnosis is essential to prevent irreversible joint damage- Therapeutic decisions should be made in consultation with a rheumatologist |
| Depression, anxiety | - High prevalence in all age groups- Increased risk, especially in severe forms | - Impact on health and quality of life- Not associated with changes in treatment response or safety, but may affect adherence | - Essential to evaluate psychiatric comorbidities, regardless of age- Refer to primary care or another specialist for management |
| IBD | - Prevalence unknown- Uncommon before puberty- Increased risk | - Impact on health, quality of life, and growth- Efficacy and indications of some medications differ depending on the type of involvement (skin or digestive) | - Early diagnosis is essential- Therapeutic decisions should be made in consultation with gastroenterologists |
| NAFLD | - Prevalence unknown- Rare before 10 years old | - Impact on health and quality of life | - Provide information and general recommendations for a healthy lifestyle- Refer to primary care or another specialist for management |
HTN: hypertension; ETN: etanercept; PsA: psoriatic arthritis; T2DM; type 2 diabetes mellitus; IBD: inflammatory bowel disease; NAFLD: Non-Alcoholic Fatty Liver Disease.
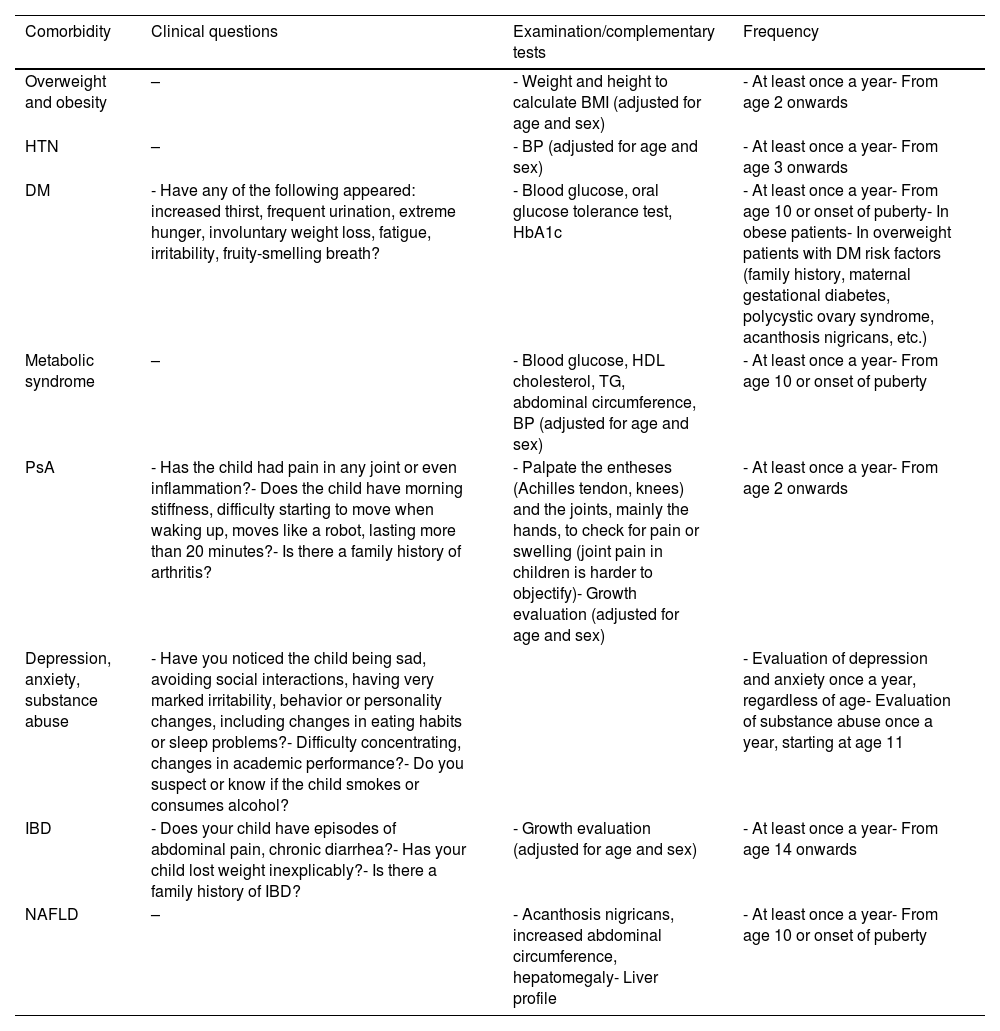
Recommendations for screening pediatric comorbidities in dermatology consultations.
| Comorbidity | Clinical questions | Examination/complementary tests | Frequency |
|---|---|---|---|
| Overweight and obesity | – | - Weight and height to calculate BMI (adjusted for age and sex) | - At least once a year- From age 2 onwards |
| HTN | – | - BP (adjusted for age and sex) | - At least once a year- From age 3 onwards |
| DM | - Have any of the following appeared: increased thirst, frequent urination, extreme hunger, involuntary weight loss, fatigue, irritability, fruity-smelling breath? | - Blood glucose, oral glucose tolerance test, HbA1c | - At least once a year- From age 10 or onset of puberty- In obese patients- In overweight patients with DM risk factors (family history, maternal gestational diabetes, polycystic ovary syndrome, acanthosis nigricans, etc.) |
| Metabolic syndrome | – | - Blood glucose, HDL cholesterol, TG, abdominal circumference, BP (adjusted for age and sex) | - At least once a year- From age 10 or onset of puberty |
| PsA | - Has the child had pain in any joint or even inflammation?- Does the child have morning stiffness, difficulty starting to move when waking up, moves like a robot, lasting more than 20 minutes?- Is there a family history of arthritis? | - Palpate the entheses (Achilles tendon, knees) and the joints, mainly the hands, to check for pain or swelling (joint pain in children is harder to objectify)- Growth evaluation (adjusted for age and sex) | - At least once a year- From age 2 onwards |
| Depression, anxiety, substance abuse | - Have you noticed the child being sad, avoiding social interactions, having very marked irritability, behavior or personality changes, including changes in eating habits or sleep problems?- Difficulty concentrating, changes in academic performance?- Do you suspect or know if the child smokes or consumes alcohol? | - Evaluation of depression and anxiety once a year, regardless of age- Evaluation of substance abuse once a year, starting at age 11 | |
| IBD | - Does your child have episodes of abdominal pain, chronic diarrhea?- Has your child lost weight inexplicably?- Is there a family history of IBD? | - Growth evaluation (adjusted for age and sex) | - At least once a year- From age 14 onwards |
| NAFLD | – | - Acanthosis nigricans, increased abdominal circumference, hepatomegaly- Liver profile | - At least once a year- From age 10 or onset of puberty |
BP: blood pressure; BMI: body mass index; HTN: hypertension; PsA: psoriatic arthritis; DM: diabetes mellitus; HDL: high-density protein; TG: triglycerides; NAFLD: Non-Alcoholic Fatty Liver Disease; IBD: inflammatory bowel disease.
Overweight is defined as a body mass index (BMI)≥85th percentile but <95th percentile (for age and sex), and obesity as BMI≥95th percentile (for age and sex).
The prevalence of overweight and obesity varies greatly depending on the study, but in general, it is very high in pediatric psoriasis. It has been reported that up to 17.6% of patients with pediatric psoriasis are overweight and 4% up to 20.2% are obese.105,106 The risk of a child with psoriasis having obesity is 4.29 times higher than a child without the disease, and this risk increases with the severity of psoriasis.106 Obesity in pediatric psoriatic patients tends to be central and generally develops around 8 years of age.11 The impact on the child's health—it is a cardiovascular risk factor—and quality of life is significant and can negatively affect health outcomes in adulthood. Moreover, it has been found that the efficacy of ETN is lower in overweight/obese patients vs children with a normal weight.79
Obesity is considered particularly relevant among the comorbidities of children with psoriasis, and therefore, regular screening should be conducted. Additionally, information about the impact of this comorbidity should be provided, along with general recommendations for a healthy lifestyle to prevent it.
HypertensionThe prevalence of hypertension (HTN) is poorly studied in pediatric psoriasis, but some studies have found a specific association.107,108 Like obesity, HTN can impact the child's health and future health as an adult.
It is recommended to follow the approved guidelines for HTN screening in children and adolescents109 and provide information on the impact of this comorbidity along with general recommendations for a healthy lifestyle to prevent it.
Type 2 diabetes mellitusAlthough the prevalence of diabetes mellitus (DM) is poorly analyzed in pediatric psoriasis, studies have found an association between pediatric psoriasis and insulin resistance and T2DM, especially in obese patients.108,110 This disease and its treatments can have a very negative impact on the health and quality of life of pediatric patients.
It is recommended to follow the approved guidelines for DM screening in children and adolescents,111 and provide information on the impact of this comorbidity along with general recommendations for a healthy lifestyle to prevent it.
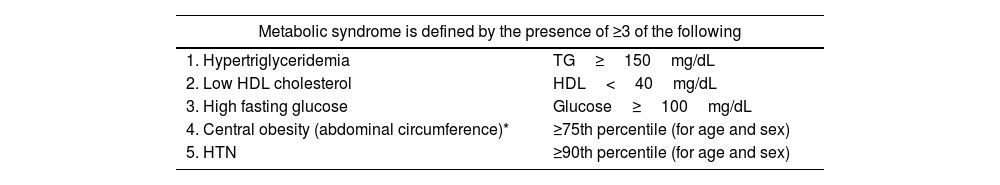
Metabolic syndromeMetabolic syndrome includes a group of cardiovascular risk factors, with various definitions, most of which apply to children from 10 years of age. One of the most widely used definitions is shown in Table 15.112
Definition of metabolic syndrome in pediatric patients.
| Metabolic syndrome is defined by the presence of ≥3 of the following | |
|---|---|
| 1. Hypertriglyceridemia | TG≥150mg/dL |
| 2. Low HDL cholesterol | HDL<40mg/dL |
| 3. High fasting glucose | Glucose≥100mg/dL |
| 4. Central obesity (abdominal circumference)* | ≥75th percentile (for age and sex) |
| 5. HTN | ≥90th percentile (for age and sex) |
TG: triglycerides; mg: milligrams; dL: deciliters; HDL: high-density lipoprotein; HTN: hypertension.
* Abdominal circumference in children is measured with a non-elastic measuring tape, with the child standing, feet together, arms at the sides, and the abdomen relaxed. The tape is placed around the abdomen at the level of the navel, midway between the last rib and the iliac crest, without pressing, while the child takes a deep breath, and the measurement is taken during exhalation.115
Although prevalence data is scarce, different studies suggest an increased risk of metabolic syndrome in pediatric patients with psoriasis vs children without this disease.113 Like the previously described comorbidities, it has an important impact on the child's health and can affect their health as an adult.
One of the criteria included in the definition is central obesity. Fat distribution significantly influences the development of the metabolic complications of obesity.114 Abdominal circumference has been recognized as the best clinical indicator of visceral fat accumulation,115 and various studies have shown that it is a more appropriate measure for metabolic syndrome and cardiovascular risk.116
It is recommended to follow the guidelines outlined in the previous comorbidities and provide information on the impact of this comorbidity, along with general recommendations for a healthy lifestyle to prevent it.
Psoriatic arthritisThe prevalence of PsA is lower in children vs adults with psoriasis, with an estimated prevalence of 0.7% in all children with psoriasis, increasing up to 1.2% by the time they reach 18 years old.117 Although PsA can appear in children of any age, there are 22 peaks of onset: one at 2–3 years and another at 10–12 years.118 Disability and the impact on the child's quality of life can be very significant.104 Moreover, although many of the drugs used for both diseases are the same, their efficacy may differ depending on the type of involvement.
Pediatric patients with PsA exhibit symptoms of inflammatory arthritis, such as pain, swelling, and joint stiffness at rest or upon waking. Younger children with PsA, especially girls, tend to have oligoarticular disease and/or dactylitis. Older children, particularly boys, often present enthesitis and axial involvement.8,118,119
Based on this information, it is essential to conduct regular screening and, if diagnosis is confirmed, to adopt a multidisciplinary approach to the patient.
Depression, anxiety, and substance abuseIt is estimated that up to 70% of pediatric psoriatic patients experience some psychological-psychiatric issues, which can appear at any age.120 More specifically, it has been described that pediatric psoriatic patients have a 25% up to 30% increased risk of developing depression and/or anxiety vs children without psoriasis.121
The impact of psoriasis on the mental health of children is equal to or even greater than that of diabetes, epilepsy, or atopic dermatitis.122 A qualitative study showed that 65% of children experienced some form of stigmatization or even bullying due to psoriasis.25 Others have shown that psoriasis in children can lead to mood changes, low self-esteem, social isolation, and risk behaviors.123
In adult patients, a strong correlation between psoriasis and the use of alcohol and tobacco has been found. Although this may also occur in adolescents and young adults with psoriasis, there is currently insufficient data to prove it.
However, given the prevalence of mental health problems and substance use in this population, the recommendations for mental health screening124 should be followed.
Inflammatory bowel diseaseVarious studies have shown that the risk of inflammatory bowel disease (IBD) in children with psoriasis is 3–4 times higher than in children without psoriasis.1,8,125 Although it can appear at any age, it is uncommon before puberty.126 Like PsA, its impact on the child and treatments can be significant, so it should be regularly ruled out.
Non-alcoholic fatty liver diseaseNon-alcoholic fatty liver disease (NAFLD) is a term that includes both simple steatosis and non-alcoholic steatohepatitis. It is characterized by excessive accumulation of liver fat not due to an autoimmune, metabolic, or infectious process. Its prevalence in pediatric psoriatic patients is not well known. In the general population, the prevalence of NAFLD increases with age, being exceptional in children<3 years old and rare in children<10 years old.127 The symptoms associated with NAFLD are nonspecific, such as fatigue or abdominal pain. This disease is associated with acanthosis nigricans (present in 33% up to 50% of cases), increased abdominal circumference, and hepatomegaly (in up to 50% of cases).
Due to the association of NAFLD with overweight, obesity, and insulin resistance, regular screening for this condition in pediatric patients is recommended.
Drugs in the study phaseApremilastA phase II RCT analyzed the use of apremilast 20–30mg twice a day (depending on age and weight) in children aged 6–17 years with moderate-to-severe plaque psoriasis.128 On week 16 of treatment, the mean changes in PASI for adolescents with 20mg apremilast were −69.6, and with 30mg apremilast, −66.5. In children on 20mg apremilast, the changes in PASI at week 16 were −79.3. The drug was generally well tolerated. The data being presented from phase III studies with apremilast (NCT03701763, NCT06088199, NCT04175613) are consistent with these results.
GuselkumabCurrently, a phase III RCT (NCT03451851) is evaluating the safety and efficacy of subcutaneous guselkumab vs ETN and placebo in pediatric patients aged 6–18 years with moderate-to-severe plaque psoriasis.
RisankizumabA phase III RCT (NCT04435600) is in the pipeline evaluating subcutaneous risankizumab vs UST for pediatric participants aged 6–17 years with moderate-to-severe psoriasis.
TildrakizumabA phase III RCT is ongoing with tildrakizumab (NCT03997786), placebo, and active control in children aged 6 to <18 years with moderate-to-severe plaque psoriasis.
BrodalumabAn open study is analyzing the safety, tolerability, and pharmacokinetics of brodalumab in children aged 6–17 years with severe plaque psoriasis (NCT03240809).
BimekizumabA study is underway to evaluate the safety, efficacy, and pharmacokinetics of 2 doses of bimekizumab in adolescents with moderate-to-severe plaque psoriasis (NCT04718896).
TofacitinibA recent open study including 47 patients with pediatric psoriasis showed significant improvement in efficacy and quality of life with tofacitinib. At week 12, 55.32% of patients achieved PASI75, and 70.21% reached PASI75 at week 36.129 No severe adverse events were observed.
NetakimabA phase III RCT is currently underway to evaluate the safety, efficacy, pharmacokinetics, and immunogenicity of netakimab in children (6–18 years) with plaque psoriasis (NCT06640517).
DeucravacitinibData on other Janus kinase (JAK) inhibitors, specifically tyrosine kinase 2 (TYK2) inhibitors such as deucravacitinib, are still being tested in children (phase III RCT NCT04772079).
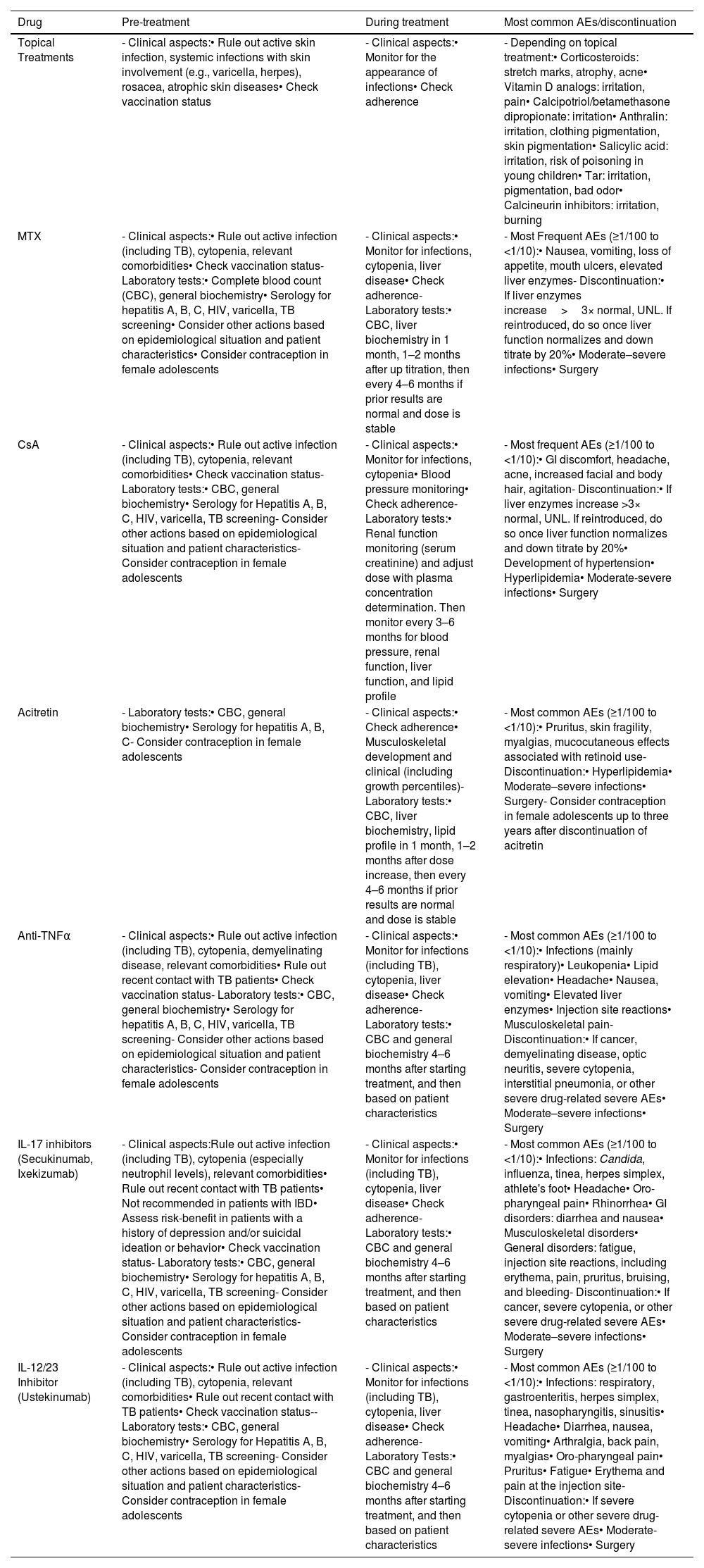
Risk managementFollow-upAlthough it depends on the type of treatment, adverse events from topical treatments are not very common37 and are generally mild (though they vary depending on the drug), especially when following the guidelines provided in the drug prescribing information. Systemic adverse events from these therapies are rare, and monitoring is primarily clinical (Table 16).
Risk management with the use of different therapeutic options for pediatric patients with moderate-severe psoriasis.
| Drug | Pre-treatment | During treatment | Most common AEs/discontinuation |
|---|---|---|---|
| Topical Treatments | - Clinical aspects:• Rule out active skin infection, systemic infections with skin involvement (e.g., varicella, herpes), rosacea, atrophic skin diseases• Check vaccination status | - Clinical aspects:• Monitor for the appearance of infections• Check adherence | - Depending on topical treatment:• Corticosteroids: stretch marks, atrophy, acne• Vitamin D analogs: irritation, pain• Calcipotriol/betamethasone dipropionate: irritation• Anthralin: irritation, clothing pigmentation, skin pigmentation• Salicylic acid: irritation, risk of poisoning in young children• Tar: irritation, pigmentation, bad odor• Calcineurin inhibitors: irritation, burning |
| MTX | - Clinical aspects:• Rule out active infection (including TB), cytopenia, relevant comorbidities• Check vaccination status- Laboratory tests:• Complete blood count (CBC), general biochemistry• Serology for hepatitis A, B, C, HIV, varicella, TB screening• Consider other actions based on epidemiological situation and patient characteristics• Consider contraception in female adolescents | - Clinical aspects:• Monitor for infections, cytopenia, liver disease• Check adherence- Laboratory tests:• CBC, liver biochemistry in 1 month, 1–2 months after up titration, then every 4–6 months if prior results are normal and dose is stable | - Most Frequent AEs (≥1/100 to <1/10):• Nausea, vomiting, loss of appetite, mouth ulcers, elevated liver enzymes- Discontinuation:• If liver enzymes increase>3× normal, UNL. If reintroduced, do so once liver function normalizes and down titrate by 20%• Moderate–severe infections• Surgery |
| CsA | - Clinical aspects:• Rule out active infection (including TB), cytopenia, relevant comorbidities• Check vaccination status- Laboratory tests:• CBC, general biochemistry• Serology for Hepatitis A, B, C, HIV, varicella, TB screening- Consider other actions based on epidemiological situation and patient characteristics- Consider contraception in female adolescents | - Clinical aspects:• Monitor for infections, cytopenia• Blood pressure monitoring• Check adherence- Laboratory tests:• Renal function monitoring (serum creatinine) and adjust dose with plasma concentration determination. Then monitor every 3–6 months for blood pressure, renal function, liver function, and lipid profile | - Most frequent AEs (≥1/100 to <1/10):• GI discomfort, headache, acne, increased facial and body hair, agitation- Discontinuation:• If liver enzymes increase >3× normal, UNL. If reintroduced, do so once liver function normalizes and down titrate by 20%• Development of hypertension• Hyperlipidemia• Moderate-severe infections• Surgery |
| Acitretin | - Laboratory tests:• CBC, general biochemistry• Serology for hepatitis A, B, C- Consider contraception in female adolescents | - Clinical aspects:• Check adherence• Musculoskeletal development and clinical (including growth percentiles)- Laboratory tests:• CBC, liver biochemistry, lipid profile in 1 month, 1–2 months after dose increase, then every 4–6 months if prior results are normal and dose is stable | - Most common AEs (≥1/100 to <1/10):• Pruritus, skin fragility, myalgias, mucocutaneous effects associated with retinoid use- Discontinuation:• Hyperlipidemia• Moderate–severe infections• Surgery- Consider contraception in female adolescents up to three years after discontinuation of acitretin |
| Anti-TNFα | - Clinical aspects:• Rule out active infection (including TB), cytopenia, demyelinating disease, relevant comorbidities• Rule out recent contact with TB patients• Check vaccination status- Laboratory tests:• CBC, general biochemistry• Serology for hepatitis A, B, C, HIV, varicella, TB screening- Consider other actions based on epidemiological situation and patient characteristics- Consider contraception in female adolescents | - Clinical aspects:• Monitor for infections (including TB), cytopenia, liver disease• Check adherence- Laboratory tests:• CBC and general biochemistry 4–6 months after starting treatment, and then based on patient characteristics | - Most common AEs (≥1/100 to <1/10):• Infections (mainly respiratory)• Leukopenia• Lipid elevation• Headache• Nausea, vomiting• Elevated liver enzymes• Injection site reactions• Musculoskeletal pain- Discontinuation:• If cancer, demyelinating disease, optic neuritis, severe cytopenia, interstitial pneumonia, or other severe drug-related severe AEs• Moderate–severe infections• Surgery |
| IL-17 inhibitors (Secukinumab, Ixekizumab) | - Clinical aspects:Rule out active infection (including TB), cytopenia (especially neutrophil levels), relevant comorbidities• Rule out recent contact with TB patients• Not recommended in patients with IBD• Assess risk-benefit in patients with a history of depression and/or suicidal ideation or behavior• Check vaccination status- Laboratory tests:• CBC, general biochemistry• Serology for hepatitis A, B, C, HIV, varicella, TB screening- Consider other actions based on epidemiological situation and patient characteristics- Consider contraception in female adolescents | - Clinical aspects:• Monitor for infections (including TB), cytopenia, liver disease• Check adherence- Laboratory tests:• CBC and general biochemistry 4–6 months after starting treatment, and then based on patient characteristics | - Most common AEs (≥1/100 to <1/10):• Infections: Candida, influenza, tinea, herpes simplex, athlete's foot• Headache• Oro-pharyngeal pain• Rhinorrhea• GI disorders: diarrhea and nausea• Musculoskeletal disorders• General disorders: fatigue, injection site reactions, including erythema, pain, pruritus, bruising, and bleeding- Discontinuation:• If cancer, severe cytopenia, or other severe drug-related severe AEs• Moderate–severe infections• Surgery |
| IL-12/23 Inhibitor (Ustekinumab) | - Clinical aspects:• Rule out active infection (including TB), cytopenia, relevant comorbidities• Rule out recent contact with TB patients• Check vaccination status-- Laboratory tests:• CBC, general biochemistry• Serology for Hepatitis A, B, C, HIV, varicella, TB screening- Consider other actions based on epidemiological situation and patient characteristics- Consider contraception in female adolescents | - Clinical aspects:• Monitor for infections (including TB), cytopenia, liver disease• Check adherence- Laboratory Tests:• CBC and general biochemistry 4–6 months after starting treatment, and then based on patient characteristics | - Most common AEs (≥1/100 to <1/10):• Infections: respiratory, gastroenteritis, herpes simplex, tinea, nasopharyngitis, sinusitis• Headache• Diarrhea, nausea, vomiting• Arthralgia, back pain, myalgias• Oro-pharyngeal pain• Pruritus• Fatigue• Erythema and pain at the injection site- Discontinuation:• If severe cytopenia or other severe drug-related severe AEs• Moderate-severe infections• Surgery |
AEs: adverse events; UNL: upper normal limit; IBD: inflammatory bowel disease; TB: tuberculosis; TNF: tumor necrosis factor; HIV: human immunodeficiency virus.
Of note, regarding the use of topical corticosteroids, children—especially younger ones—have thinner skin and a proportionally larger skin surface area, making the risk of systemic absorption of these drugs higher than in adults. Therefore, the appropriate corticosteroid (lower potency) should be selected, and application over large areas and for prolonged periods should be avoided.
On the other hand, classic systemic treatments and biological therapies generally present a safety profile similar to that observed in adults,36 so the recommended follow-up is very similar (Table 11). One of the most common adverse events with these therapies is respiratory infections, which are also very common in healthy children. Pediatric infections are caused by different germs than in adults, and the clinical presentations are also particular. Therefore, in case of fever or infection, it is important to conduct a medical evaluation. For mild infections, it is not mandatory to discontinue treatment, but for moderate and severe infections, it is recommended to suspend treatment until clinical improvement.
VaccinationChildren with pediatric psoriasis, especially those on classic systemic treatments and biological therapies, may be at a higher risk of infections. Therefore, updating the child's vaccination schedule, as well as that of their close contacts, is considered essential.
In general, the vaccines recommended according to the current vaccination schedule of each region should be administered, with some specifications in line with recommendations issued for pediatric patients who are immunocompromised due to other diseases and immunosuppressive treatments (Table 11).
ConclusionsPsoriasis is one of the most common dermatological diseases in the pediatric population. As it happens in adults, there are various therapeutic modalities available for its treatment. However, this subgroup of the population presents differential characteristics related to the disease per se, the impact on the child/adolescent, as well as in pharmacological management that should be taken into consideration.
This consensus document provides dermatologists with a consultation and support guide for making therapeutic decisions for pediatric psoriasis patients, all based on the best available evidence and expert opinion to this date.
FundingThis project was funded by the Spanish Academy of Dermatology and Venereology (AEDV).
Conflicts of interestA. Pérez-Ferriols received payments or fees for conferences, presentations, lectures, manuscript drafting, or educational events from Ampersand Integral Projects, Organon Salud, Takeda Pharmaceutical, Hispanic Osteoporosis Foundation, Iberian Imed Institute Foundation, Clover Solutions, Permanyer Advertising; expert opinion fees from Laboratorios Viñas; support for attending meetings and/or travel from Lilly, Jansen.
A. Batalla received payments for his participation in clinical trials for: Sanofi, Amgen, Abbvie, Leo Pharma, Lilly, Pfizer, Novartis, Kymab, Galderma.
A. Vicente received consultancy fees from Abbvie, Amgen, Amryt, Boehringer Ingelheim, Bristol-Myers Squibb, Lilly, Novartis, Pierre Fabre, and Sanofi Genzyme. B. Pérez received consultancy fees from UCB, Janssen, and Lilly, and payments or fees for conferences, presentations, lectures, manuscript drafting, or educational events from UCB, Janssen, and Lilly.
J.M. Carrascosa received grants or contracts from Janssen, Novartis, and Almirall; consultancy fees from Abbvie, Novartis, Leo Pharma, Sanofi, Janssen, UCB, BI, Lilly, and Almirall; payments or fees for conferences, presentations, lectures, manuscript drafting, or educational events from Abbvie, Novartis, Leo Pharma, Sanofi, Janssen, UCB, BI, Lilly, and Almirall. L. García-Fernández received consultancy fees from Abbvie; payments or fees for conferences, presentations, lectures, manuscript writing, or educational events from Abbvie. N. Eiris received consultancy fees from Abbvie, Janssen, Novartis, Lilly, UCB, Almirall, and Leo Pharma.
P. De la Cueva received consultancy fees from Abbvie, Almirall, Amgen, Boehringer, Celgene, Janssen, Leo Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, and UCB. R. de Lucas declared no conflicts of interest whatsoever.