Immune-mediated skin diseases are triggered by a complex interaction between individual genetic susceptibility and environmental factors. Genetic and epidemiologic data from numerous sources suggest a link between diseases that appear to have little in common, such as Crohn disease, ulcerative colitis, lichen planus, dermatitis herpetiformis, and psoriasis. One possible association that has been identified is that between psoriasis and dermatitis herpetiformis. The association is supported by the fact that both diseases share genetic polymorphisms in several immunoregulatory genes, and that patients with psoriasis have a higher prevalence of celiac disease than the general population (4.34% vs 1%-2%).1
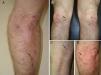
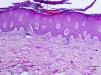
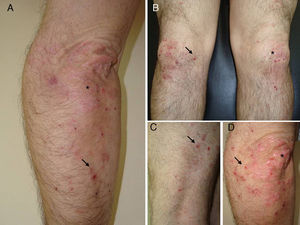
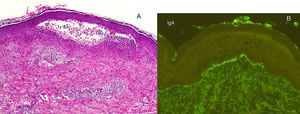
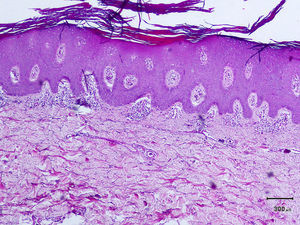
A 50-year-old man, with mild plaque psoriasis on his elbows and knees for over 20 years but no other relevant past medical history, consulted for the progressive appearance of intensely pruritic bullous lesions around the psoriatic plaques on the extensor surfaces of his arms and legs in the previous months. Examination revealed small, symmetrically distributed, morphologically identical blisters on an erythematous base; the blisters measured 3 to 7mm in diameter and contained a clear fluid (Fig. 1). The patient reported no other symptoms related to other body systems. The rest of the skin examination was unremarkable. Hematoxylin-eosin staining of a biopsy specimen from 1 of the blisters showed numerous neutrophilic infiltrates in the dermal papillae and a blister at the dermoepidermal junction. Direct immunofluorescence study of healthy perilesional skin revealed granular deposits of immunoglobulin (Ig) A in the papillary dermis (Fig. 2), and biopsy of a plaque from 1 of the elbows confirmed the diagnosis of psoriasis (Fig. 3). Laboratory tests detected anti-tissue transglutaminase antibodies at a titer of over 100; the results were negative for antiendomysial and anti-gliadin antibodies. The rest of the results, including glucose-6-phosphate dehydrogenase levels, were normal or negative. The patient was diagnosed with concomitant dermatitis herpetiformis and plaque psoriasis. Biopsy of the small intestine confirmed the diagnosis of celiac disease. The patient was started on a gluten-free diet and oral sulfone at a dose of 100 mg/day. Three months later, the bullous lesions had disappeared completely and the psoriasis score, assessed using the Psoriasis Area and Severity Index, had improved from 3 to 1.
A, Histology of a bullous lesion showing numerous neutrophilic infiltrates in the dermal papillae and a blister at the dermoepidermal junction (hematoxylin-eosin, original magnification x20); these 2 findings are suggestive of dermatitis herpetiformis. B, Direct immunofluorescence study of healthy perilesional skin showing granular immunoglobulin A deposits in the papillary dermis; this finding is also characteristic of dermatitis herpetiformis.
The coexistence of plaque psoriasis lesions and dermatitis herpetiformis is rare and has only been described in anecdotal reports. Numerous authors, however, believe that there is an association between psoriasis and celiac disease. It has been observed, for example, that over 16% of patients with psoriasis have IgG and IgA anti-gliadin antibodies, IgA antitransglutaminase antibodies, and IgA antiendomysial antibodies.2 Other studies have demonstrated improvements in psoriatic lesions in patients who followed a gluten-free diet, with no additional pharmacologic treatment, for 3 to 6 months.3 The same patients experienced flare-ups when gluten was reintroduced to their diet. It is noteworthy that in the same study, there was no improvement in psoriasis in patients who followed the gluten-free diet but who did not have celiac disease–associated antibodies. Based on these observations,2 several authors recommend screening for celiac disease in patients with psoriasis.4 Psoriasis has also been associated with other IgA-mediated autoimmune diseases, namely, linear IgA pemphigus, IgA necrotic renal glomerular vasculitis, and IgA nephropathy.5 One of the most interesting recent genetic findings is the increased frequency of the Ig heavy-chain HS1,2-A enhancer *2 allele in patients with dermatitis herpetiformis, plaque psoriasis, and psoriatic arthritis, suggesting a differential immune response induction in patients with psoriasis compared to the general population.6 There have also been reports of other common genetic factors (polymorphisms in the interleukin-23 receptor gene and class II human leukocyte antigen haplotypes) that are thought to predispose to an excessive or poorly regulated immune response and a chronic proinflammatory state.7–9
Taken together, the above data support the existence of a common underlying immune disorder involving IgA dysregulation in these skin diseases. The mechanisms involved, however, are not clear.
According to several authors, however, the concomitant presence of psoriasis and celiac disease (and hence dermatitis herpetiformis) is probably fortuitous due to the high prevalence of both diseases in the general population. Because most of the data supporting a possible association between celiac disease and psoriasis are derived from isolated cases, it is not possible to claim a conclusive association between the diseases. Nonetheless, the improvement seen in our patient, and in similar patients, following the adequate management of celiac disease suggests that controlled studies should be performed to investigate the true nature of the association between celiac disease and psoriasis.
Please cite this article as: Agusti-Mejias A, et al. Coexistencia de dermatitis herpetiforme y psoriasis en placas, ¿dos manifestaciones cutáneas de la enfermedad celiaca? Actas Dermosifiliogr.2011;102:471-473.