Folliculitis decalvans (FD) is a type of neutrophilic primary cicatricial alopecia (PCA) whose pathogenic effect is associated with a scarring response to infection by Staphylococcus aureus in predisposed individuals. It affects young adults, mainly males, and is characterized by the presence of pruriginous or painful plaques located mainly on the vertex, with pustules, crusting, and tufting.
Recent years have seen growing interest in a type of FD with lichenoid characteristics that can appear simultaneously or during the course of the disease.
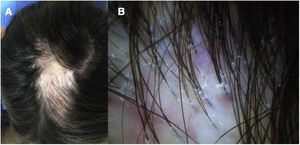
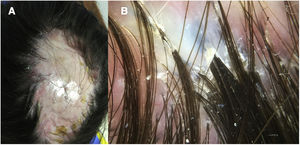
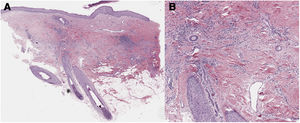
A 32-year-old woman consulted for a pruriginous bald patch on her scalp. The patch had been developing over the previous 12 years. It first appeared as a scarring plaque of alopecia on the parietal region, with irregular borders and accompanied by erythema, crusting, and perifollicular scaling (Fig. 1). Histology revealed lymphocytic scarring alopecia with scant focal neutrophils. A blood workup including a study of autoimmunity was unremarkable. With a clinical diagnosis of FD, the patient underwent treatment with various regimens of topical antibiotics and corticosteroids, intralesional corticosteroids, doxycycline, rifampicin/clindamycin, oral corticosteroids, and hydroxychloroquine. The flares initially stabilized, although the disease progressed toward the posterior region (Fig. 2). During follow-up, the patient experienced flares of solitary pustules at the edge of the patch, as well as follicular tufting. Given the absence of a response to therapy and the clinical-pathological correlation (symptoms suggestive of FD, but histology showing lymphocytic PCA), a second biopsy specimen was taken of the active lesions. This again revealed lymphocytic cicatricial alopecia (Fig. 3).
The North American Hair Research Society classification of PCA according to the predominant cell in the inflammatory infiltrate1 has led lichen planopilaris (LPP) and FD to be considered prototypes of lymphocytic and neutrophilic PCA, respectively. However, co-occurrence of characteristics of FD and LPP points to an FD-LPP phenotypic spectrum.
In 2017, Morais et al.2 reported a series of 13 patients with what they termed “lichen planopilaris with pustules”, based on the clinical presentation of erythema and perifollicular scaling, together with crusts, follicular tufts, and pustules. However, histopathology revealed that only 54% of patients had lichenoid perifolliculitis, whereas 77% had neutrophilic folliculitis and 62% tufts. More recently, Yip et al.3 reported 13 patients with characteristics of FD (tufts, pustules) and of LPP (erythema and perifollicular scaling), that occurred sequentially or simultaneously. Analysis of biopsy specimens of FD revealed a perifollicular infiltrate with neutrophils, lymphocytes, and plasma cells, whereas lymphocytic inflammation was observed in the lichenoid areas. Furthermore, Egger et al.4 reported on 7 patients, with clinical and trichoscopy findings of LPP, together with typical features of FD, such as follicular tufts, isolation of S. aureus in culture, and histologic characteristics such as compound follicular structures.
Various hypotheses have been put forward to explain the overlap between these 2 entities. Yip et al.3 pointed to the dysbiosis of the microbiome in FD as the trigger of collapse of the immune privilege of the hair follicle through autoantigens that could induce a lichenoid immune response. In addition, it has been proposed that signs classified as LPP could be a manifestation of a common final pathway, that the image is of an attenuated or forme frustre of FD modified by treatment,5 or that the fugacity of the pustules and, therefore, of the neutrophils in the inflammatory infiltrate could lead them to be missing in histology, thus paving the way for an erroneous diagnosis of LPP. In this sense, crusts are more constant, and their presence, together with follicular tufts, reinforces the diagnosis of FD as opposed to LPP. When the neutrophils disappear, the presence of plasma cells in the inflammatory infiltrate would support the diagnosis of FD,6,7 as well as the absence of interface dermatitis and epidermal hyperplasia.8,9
In the case we report, the patient's condition had lichenoid characteristics such as perifollicular scaling and erythema, as well as a lymphocytic infiltrate in the histology workup. However, the presence of crusts, follicular tufts, and the appearance of pustules during the course of the disease support a diagnosis of FD.
In practice, it can prove impossible to distinguish between LPP and a lichenoid form of FD in PCA with only erythema and perifollicular hyperkeratosis. Biopsy of an area with a lichenoid appearance can reveal a lymphocytic infiltrate. However, other histology data, together with the clinical signs suggestive of FD described above, enable the diagnosis to be corrected. Recognizing this lichenoid type of FD has important implications for therapy.
In conclusion, we report the case of a patient with FD characterized clinically and histologically by lichenoid traits. The case illustrates the difficulty in diagnosing this condition and the need to know the type of presentation in order to avoid a mistaken diagnosis of LPP and select appropriate therapy.
Conflicts of InterestThe authors declare that they have no conflicts of interest.