Psoriasis is a recurrent, chronic inflammatory disorder that mainly affects the skin and joints1. In view of the scarcity of studies on peripheral lymphocyte subpopulations in Brazilian psoriasis patients, we performed cytofluorometric analysis of peripheral blood mononuclear cells (PBMC) in psoriatic patients and healthy controls in order to characterize the lymphocyte subpopulations and certain molecules involved in cell activation and migration.
The study was approved by the Ethics Committee of Escola de Medicina e Cirurgia (UNIRIO, MEC, Brazil) and all patients signed an informed consent.
Twenty-five individuals were recruited from the Dermatology Department of HUGG/UNIRIO/MEC-Brazil. Seventeen had chronic plaque psoriasis and there were 8 healthy controls. None of the psoriasis patients was receiving systemic treatment. The physician's global assessment score2–4 was 4 in 6 cases, 5 in 6 cases, and 6 in 5 cases.
A 20-ml blood sample was drawn from each patient and PBMCs were separated using the Ficoll-Hypaque gradient (Sigma Chemical Co, St.Louis, USA). Five microliters of each monoclonal antibody—anti-CD3, anti-CD4, anti-CD8, anti-CD11a (Immunotech, Beckman Coulter, France), anti-CD25, anti-CD69, and anti-CLA (BD Biosciences, California, USA)—were added according to the combinations listed in the table 1 table 1. Laboratory procedures were performed according to the manufacturer's instructions5. The nonparametric Mann-Whitney and Kruskal-Wallis tests were used for the statistical analysis, which was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, California, USA).
Set of monoclonal markers
| Monoclonal markers | Analysis | Source | ||
| CD3-PC5/CD4-PE/CD8-FITC | CD3 in total lymphocytes | CD4 into CD3 | CD8 into CD3 | Immunotech, Beckman Coulter, France |
| CD4-PC5/CD8-FITC/CD25-PE | CD25 in total lymphocytes | CD25 into CD4 | CD25 into CD8 | BD Biosciences, CA, USA |
| CD4-PC5/CD8-PE/CD69-FITC | CD69 in total lymphocytes | CD69 into CD4 | CD69 into CD8 | BD Biosciences, CA, USA |
| CD4-PC5/CD8-PE/CLA-FITC | CLA in total lymphocytes | CLA into CD4 | CLA into CD8 | BD Biosciences, CA, USA |
| CD4-PC5/CD8-PE/CD11a-FITC | CD11a in total lymphocytes | CD11a into CD4 | CD11a into CD8 | Immunotech, Beckman Coulter, France |
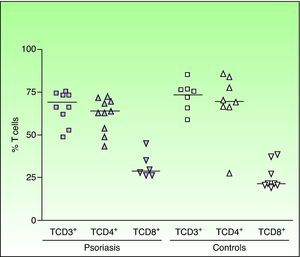
Analysis of the lymphocyte subpopulations (CD3+ or total T cells, CD4+ or helper T cells, and CD8+ or cytotoxic T cells) revealed no significant differences between patients with psoriasis and healthy controls. The relative percentages of each lymphocyte subpopulation in patients and controls are shown in Figure 1. Lymphocyte activation was determined through analysis of CD25 and CD69 expression (figs. 2 A and B). Higher percentages of activated (CD25+ and CD69+) cells were detected in the 2 lymphocyte subpopulations (CD4+ and CD8+) in psoriatic patients; the difference compared to controls was significant for the percentage of CD25+ cells in the CD8+ T-cell subpopulation (mean [SD], 39.2% (26.9); median, 35.1%; P <.05), and there was a trend to elevation of the CD25+ CD4+ subpopulation. There was also a trend to elevation in the percentage of CD69+ cells in both T-cell subpopulations (CD4+: mean, 16.0% [19.6]; median, 7.8%; CD8+: mean, 2.0% [1.47]; median, 2.23%) in psoriatic patients when compared to controls (fig. 2B). Migration of circulating T lymphocytes to the skin was studied through an analysis of CLA and CD11a expression. Compared to control subjects, patients with psoriasis presented an increase in the percentage of CD4+ T cells expressing CLA (control group: mean, 19.30% [13.13]; median, 14.67%; psoriasis group: mean, 38.86% [20.67]; median, 39.76%; P<.05). However, this was not observed in the CD8+ T cells, although increased CLA expression was detected in 4 of the 10 patients with high levels of CD8+ lymphocytes (fig. 3A). A significant increase in the percentage of cells with CD11a expression was observed in psoriasis patients compared to controls in both CD4+ (mean, 82.17% [29.91]; median, 99.45%; P < .01) and CD8+ T cells (mean, 84.96 [29.77]; median, 99.75; P < .05) (fig. 3B).
Quantification of T lymphocyte subpopulations (CD4+ and CD8+ T cells) in the peripheral blood of patients with chronic plaque psoriasis. The results are expressed as percentage of positive cells. The horizontal bars represent the median values of the results. Each point represents an individual.
A. Elevated expression of molecules related to cutaneous cell migration (cutaneous lymphocyte associated antigen [CLA]) B. Endothelial tissue inflammation (CD11a) in CD4+ and CD8+ T cells in psoriasis patients and controls. Each point represents an individual. The horizontal bars represent the median values.
Although our study is somewhat limited by the small sample size, the results showed that there was no change in the proportion of CD4+ and CD8+ T cells in the PBMC population in psoriatic patients when compared with general population, as has been reported in previous studies6. However, we observed qualitative differences between the 2 groups in the expression of activation molecules CD25 and CD69. Although the results were significant only for CD25 expression in CD8+ T cells, there was a trend to increased expression of CD25 and CD69 in both CD4+ and CD8+ T cells, findings not reported by other authors7. The presence of activation molecules has been detected in the initial stages of psoriasis, even prior to the onset of clinically apparent lesions; these molecules are therefore presumably involved in lymphocyte recruitment and migration8,9.
Our data indicate that, although there is no significant increase or variation in the relative percentages of circulating mononuclear cells in psoriatic patients, these cells are qualitatively different because they express activation molecules that are involved in the initiation and progression of psoriasis lesions.
We are grateful to Ana Cristina de Almeida for her valuable suggestions, to Gustavo Estef Lino da Silveira for the linguistic revision, and to the medical staff of Serviço de Dermatologia-HUGG/UNIRIO, Rio de Janeiro, Brazil. C Porto-Ferreira is a graduate student at Programa de Pós-Graduação em Ciências Médicas, FCM-UERJ, Rio de Janeiro, Brazil. AM Da-Cruz is a research fellow from FAPERJ, Rio de Janeiro, Brazil.









![A. Elevated expression of molecules related to cutaneous cell migration (cutaneous lymphocyte associated antigen [CLA]) B. Endothelial tissue inflammation (CD11a) in CD4+ and CD8+ T cells in psoriasis patients and controls. Each point represents an individual. The horizontal bars represent the median values.](https://static.elsevier.es/multimedia/00017310/0000010200000005/v1_201304241325/S000173101100041X/v1_201304241325/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)



