Health care managers and hospital pharmacists are increasingly compelling prescribers to use medication substitutes. This policy becomes particularly evident when the agents are biologics with shared indications based on their assumed clinical equivalence and efficiency (cost-effectiveness), and in these cases the involvement of clinicians in decision making is often minimal or nonexistent. Lacking head-to-head clinical trials comparing various drugs, the prescriber can use indirect comparisons to define 2 or more agents as clinically equivalent therapeutic alternatives. This denomination of clinical equivalence does not imply that 2 such medications are truly therapeutically equivalent, or therapeutic equivalents, as this type of equivalence is defined by the absence of statistically significant differences between the drugs on all measures of effect in most patients, meaning that neither one is preferable to the other in different situations. Although real patients are not entirely comparable to those in clinical trials, the choice of a biologic agent to treat psoriasis is largely based on the findings of such trials. A recently published meta-analysis shows that not all the biologics currently available to treat moderate to severe psoriasis can be considered therapeutic equivalents, in spite of the authors’ claim to the contrary; indeed, infliximab and etanercept can in no way be considered equivalent therapeutic alternatives based on the data provided. Biologics do display real differences with respect to efficacy at different time points and in the time required to onset of effect. In any case, therapeutic decisions should be made by an experienced clinician and tailored to each individual patient.
Existe una tendencia creciente entre los gestores sanitarios y los farmacéuticos hospitalarios a insistir en la sustitución de fármacos, y en especial biológicos que comparten indicación terapéutica en función de una pretendida «equivalencia clínica» y consideraciones de eficiencia (eficacia/coste), a menudo con escasa o nula participación de los clínicos. Cuando no se dispone de ensayos clínicos comparativos (head-to-head) de diversos fármacos, se puede emplear el análisis comparativo indirecto para definir diferentes fármacos como «alternativas de tratamiento equivalentes». Que 2 fármacos se consideren «alternativas de tratamiento equivalentes» no demuestra que sean «equivalentes terapéuticos» o «terapéuticamente equivalentes»; para ello es preciso que en la mayoría de los pacientes no existan diferencias significativas (en todos los aspectos) que hagan que uno sea preferible al otro en determinadas situaciones. Aunque los pacientes en la vida real no sean totalmente comparables con los que participan en ensayos clínicos, la elección de un agente biológico en el tratamiento de la psoriasis viene determinada en gran medida por los resultados de los mismos. Empleando los datos de un reciente metaanálisis se comprueba que, en contra de la afirmación de sus autores, los tratamientos biológicos disponibles para la psoriasis moderada a grave no pueden considerarse alternativas terapéuticas equivalentes (en ningún caso por lo que respecta al par infliximab/etanercept). Existen diferencias reales en cuanto a eficacia en diferentes momentos y velocidad de inicio del efecto de los diferentes agentes biológicos disponibles, y en cualquier caso la decisión terapéutica debe ser tomada por un clínico experimentado de forma individualizada para cada paciente.
There is growing interest in assessing the relative effectiveness of alternative treatments that can—as in the case of biosimilars—offer cost savings and greater accessibility for patients, two objectives that should figure among the priorities of prescribing physicians. In the case of generic drugs, clinical equivalence is directly correlated with the presence of an identical active pharmaceutical ingredient (chemical) and proven pharmacological equivalence (bioavailability, etc.). In the case of biosimilars, however, there are obvious difficulties in extrapolating indications and determining interchangeability. Notwithstanding these difficulties, health care managers and hospital pharmacists are increasingly proposing the substitution of the prescribed biologics with other agents having the same indication. This is being justified by the assertion of a supposed clinical equivalence and considerations of efficiency (cost effectiveness).
Dermatologists and other specialists view this trend with growing concern, seeing it as incompatible with the need for individual treatment tailored to each patient. A further concern is that such substitutions may be based on assessments carried out by medical professionals lacking appropriate clinical competence in this area and that the analyses used are sometimes invalidated by conceptual or methodological errors. The problem is illustrated by a recent article that concluded, on the basis of a meta-analysis, that all currently available biologic agents for the treatment of psoriasis are “clinically equivalent” because the 95% CI of the absolute risk differences for achieving a greater than 75% improvement over baseline on the Psoriasis Area and Severity Index (a PASI 75 response) did not reach the “threshold for clinical relevance” of 25% fixed by the authors.1
In the absence of head-to-head comparative trials, the comparative effectiveness of different drugs can only be inferred indirectly through meta-analysis of data from the available clinical trials.
In order to define 2 or more agents as clinically equivalent therapeutic alternatives we must first define the concept of clinical relevance. However, a certain degree of confusion exists between this concept (which determines the efficacy outcome of clinical importance that should be assessed—for example, patient survival, a decrease in blood pressure, clearing of psoriasis, a specific PASI score, or a predefined percentage of reduction over baseline PASI) and other concepts including the following:
Absolute risk reduction (also called risk difference), which is the difference between the percentage of patients in the intervention group and the control group who achieved the outcome measure at a predefined point in the trial.
Effect size, which quantifies the magnitude of the difference between 2 or more groups in a clinical trial, and which is generally expressed either as an odds ratio (OR) or the number needed to treat to achieve the efficacy outcome measure (NNT). The NNT is the reciprocal of the absolute risk reduction.
The delta value, or difference in response rates between the 2 groups being compared. This value determines the null hypothesis (no difference or inferiority, etc.) making it possible to calculate the size of the sample required in a clinical trial for a determined probability of a type i or α error—that is, rejection of a true null hypothesis or a false positive—or a type ii or β error—that is, failure to reject a false null hypothesis or a false negative.
Probably owing to their training, which has not been clinical or in the field of dermatology, the authors of the recent meta-analysis do not altogether understand the meaning of the term minimal clinically important difference2 (also called the threshold for clinical significance). This term refers to a concept quite unrelated to that of the delta value used to calculate the NNT in clinical trials to rule out the null hypothesis with a specific degree of statistical significance, and to that of the delta value used in indirect comparisons of treatments, which is discussed below.
By definition, a clinically important difference or clinically significant threshold is the minimal absolute or relative improvement in the efficacy outcome measure that can be detected by the patient and/or the physician (thus making it clinically relevant). This outcome could, for example, be an absolute PASI value or a relative improvement in the PASI score; however, it can never be a rate or a percentage of a population (such as the rate of PASI 75 response) or the risk difference between the arms of a clinical trial or between interventions in a meta-analysis.
Perhaps the authors of the article in question have confused two concepts: clinical importance and effect size. Effect size quantifies the difference between 2 groups (for example, treatment and placebo) and can thus be said to represent a real measure of the importance of the difference observed. It should not be confused with statistical significance, which is the probability that the difference observed between the 2 groups may be a chance accident due to sampling. This probability is expressed as a P value. The value of P depends essentially on 2 factors: effect size and sample size. Thus, a statistically “significant” result can be obtained when the effect is very large even with a small sample, or when the sample is very large even when the effect is small. In meta-analyses, information is provided about the effect size (for example, in the form of an OR and its margin of probable error, the 95% CI). When the highest and lowest CI of the OR or the NNT (compared to placebo) of 2 drugs do not overlap, or when the CI of the relative risk does not cross 1 (in comparisons of 2 drugs), it can be said that the effect size of each drug is different (and consequently that the 2 drugs are not clinically equivalent in terms of efficacy). This is what happens in meta-analyses of the biologic agents used in the treatment of psoriasis,3–5 and no useful information is added by an author arbitrarily defining a “threshold of clinical importance” of 10%, 25%, or 30%: the effect sizes are either different or they are not.
We are witnessing a growing trend in the field of clinical pharmacology towards the use of indirect comparative analysis. One example of this is the model proposed by Bucher et al.6 for defining clinically equivalent therapeutic alternatives. Calculators have been devised for this purpose7 and meta-analyses are used to compare the results of different clinical trials with respect to a reference drug (for a given outcome at a specific endpoint).8 To compare results expressed as risk differences, the investigator defines an arbitrary delta value, which is considered to be the maximum clinically significant difference (which is not the same as the “minimal clinically important difference” or the “threshold of clinical significance”). To offer an analogous example, one treatment may have an annual cost €500 higher than another and exactly the same effectiveness, but if €1000 is defined as the minimal clinically important difference they can be considered “clinically equivalent therapeutic alternatives” from the point of view of cost despite the fact that one is more expensive than the other. The delta value is always arbitrary; it is usually fixed between 10% and 15% when absolute risk reductions expressed in percentages are used, although much depends on the risk difference over placebo detected in the meta-analysis.
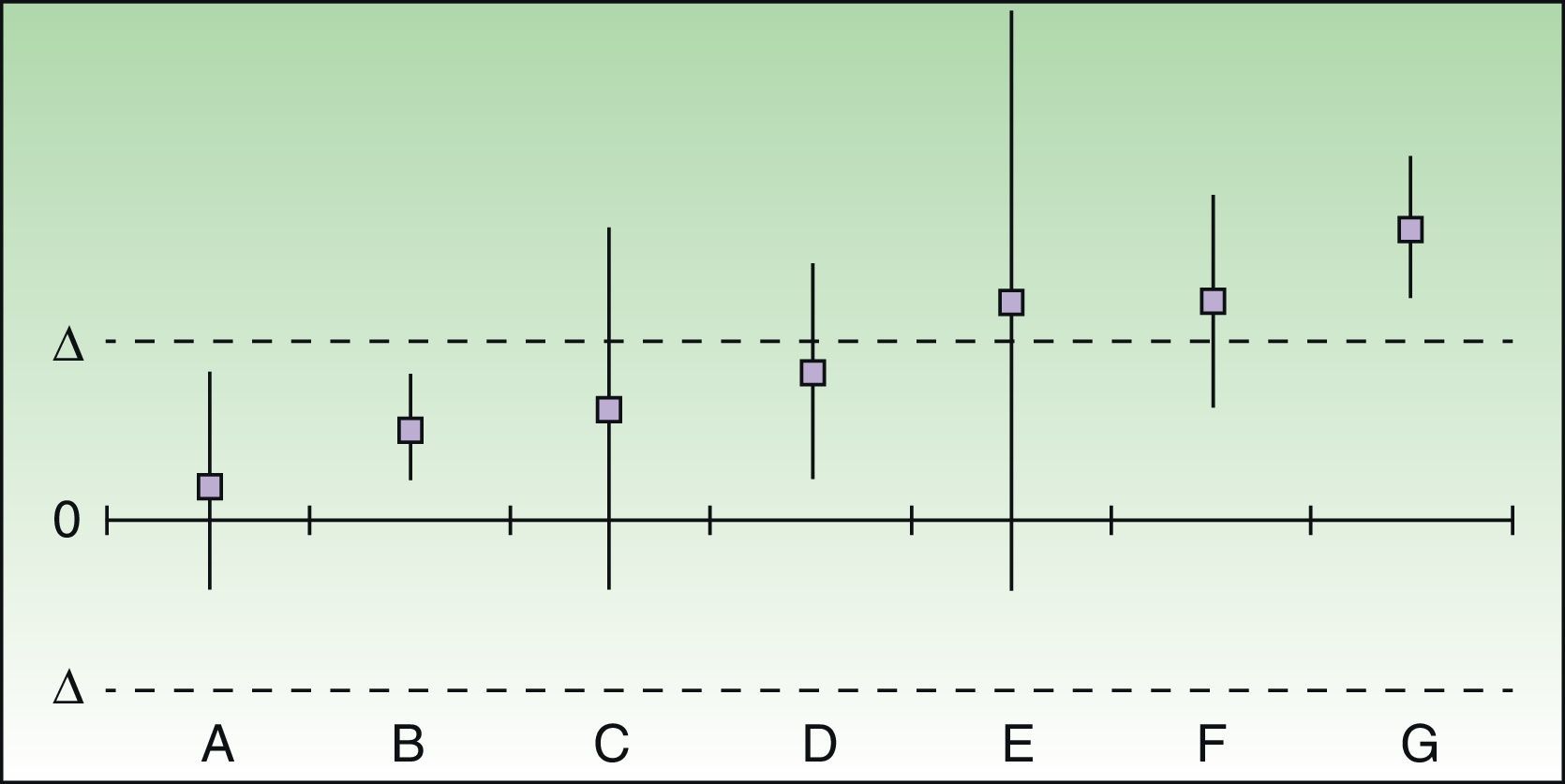
The results of the analysis of equivalence take into account the differences between the risk differences of each treatment over placebo and their respective 95% CIs, which are plotted on a graph in which 0 represents no difference and delta is the preset cutoff value. This graph represents the relative positions of the drugs (Fig. 1).
Assessment and positioning of 2 drugs as clinically equivalent therapeutic alternatives. In cases A and B, the 2 drugs can be considered to be clinically equivalent therapeutic alternatives even if treatment failure would imply serious or irreversible harm to the patient. In the doubtful or inconclusive cases (C-E) the 2 drugs cannot be considered to be equivalent therapeutic alternatives if failure would imply serious or irreversible harm to the patient, but they could conditionally be accepted as equivalent if this were not the case. F and G, for which the difference in risk difference exceeds the predefined delta value, can never be considered to be clinically equivalent therapeutic alternatives.
The authors of a recent article on the treatment of psoriatic arthritis with biologic agents used a delta value of 16%, which was half of the risk difference obtained in the meta-analysis for the primary outcome (a 50% improvement using the American College of Rheumatology Criteria).9 However, it is highly questionable whether two drugs, one of which has a probability of response half that of the other, can be said to be “clinically equivalent.”
In the case of psoriasis, a condition in which risk differences for a PASI 75 response of between 60% and 78% can be expected at week 24 with most biologic agents, a delta value of 10% (about 15% of the overall risk difference) might be reasonable.
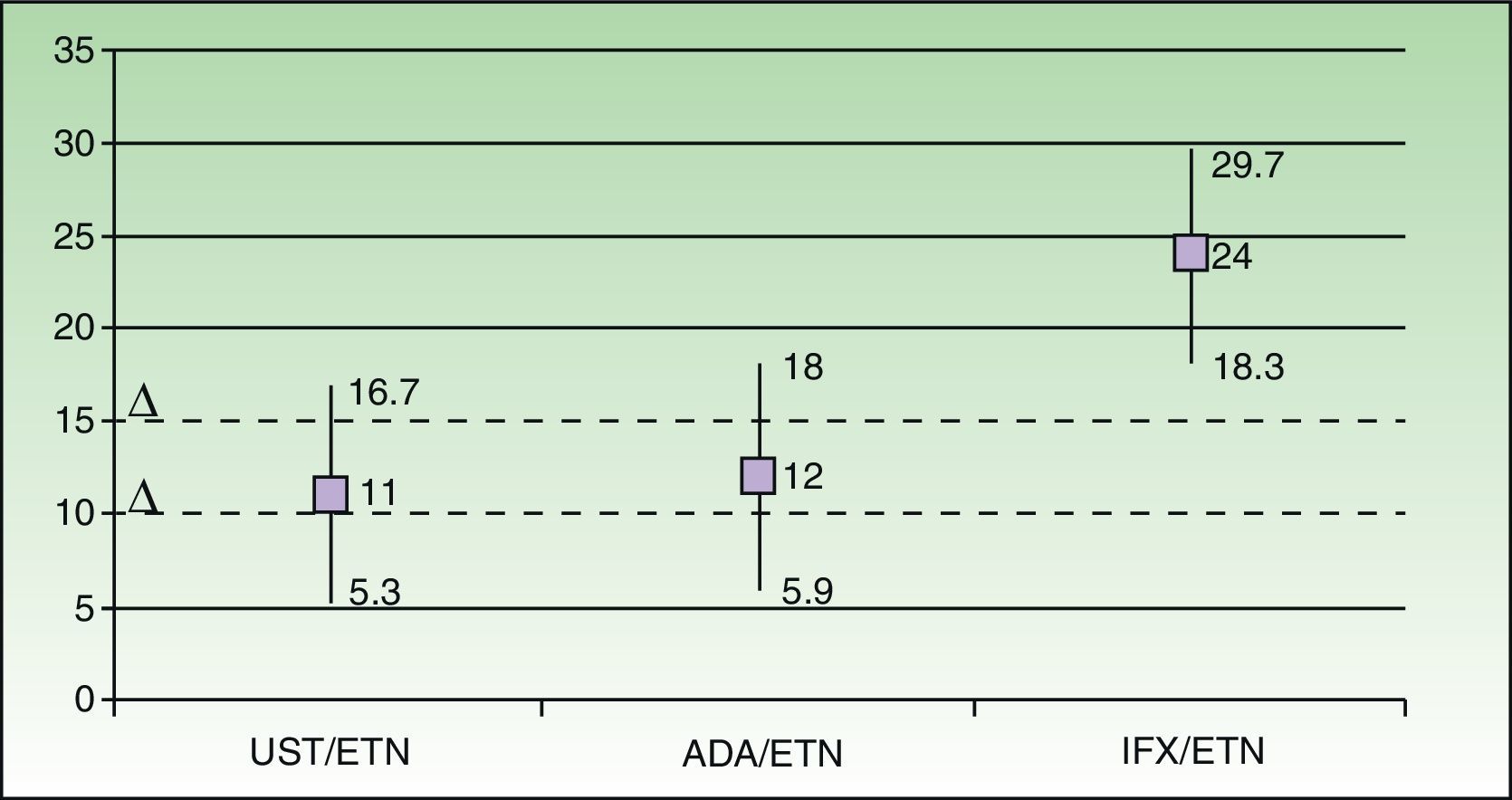
Using data from the meta-analysis carried out by Galván-Banqueri et al.,1Fig. 2 shows that the available biologic therapies for the treatment of moderate to severe psoriasis cannot be considered clinically equivalent therapeutic alternatives (particularly in the case of infliximab/etanercept).
Assessment and positioning of the biologic agents currently available for the treatment of psoriasis based on the meta-analysis carried out by Galván-Banqueri et al.1 and using a delta value of 10%. In the indirect comparison, ustekinumab is superior to etanercept (risk difference relative to etanercept [RD] 11% [95% CI, 5.3-16.7]), and to adalimumab (RD, 12%; IC 95%, 5.9-18) and infliximab (RD 24%; IC 95%, 18.3-29.7). If we use a delta value of 10%, the pairs ustekinumab/etanercept (UST/ETN) and adalimumab/etanercept (ADA/ETN) would correspond to case F in Fig. 1, and infliximab/etanercept (IFX/ETN) would correspond to case G. Using a delta value of 15%, the 3 cases would be D, D, and G, respectively. In no case can it be said that all the biologic agents indicated for moderate to severe psoriasis are clinically equivalent therapeutic alternatives.
The denomination of 2 drugs as being “clinically equivalent” in this way does not demonstrate that they are in fact therapeutic equivalents, because therapeutic equivalence would imply the absence in most patients of any statistically significant differences between the drugs in all of the measures of effect that would make one preferable to the other in certain situations.
We fully agree with Galván-Banqueri et al.1 that the choice of a biologic agent in psoriasis is largely determined by the drug's relative safety profile, contraindications, and pharmacoeconomic considerations, but there are also real differences in efficacy3–5 and speed of onset of effect.10 In any event, the therapeutic decision must be taken by an experienced clinician on a case-by-case basis, taking into account numerous factors, including weight, comorbidities (including arthritis), and the convenience of administration for the patient.2 Perhaps in the near future most patients will achieve complete or nearly complete clearing of psoriasis in response to treatment, a response that will clearly be important. When that happens we will no longer have to worry about defining a threshold of clinical significance in this disease that has such a negative impact on the quality of life of our patients.
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their place of work concerning the publication of patient data and that all patients included in this study were appropriately informed and gave their written informed consent.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe author has been remunerated for his work as a consultant and a speaker by Abbvie, Boehringer, Janssen, Merck-Serono, MSD, Novartis y Pfizer. He has participated in clinical trials sponsored by Abbvie, Amgen, Janssen, Lilly, MSD, Novartis, Pfizer, and VBL.
Please cite this article as: Puig L. Los tratamientos biológicos de la psoriasis moderada a grave no son alternativas terapéuticas equivalentes. Actas Dermosifiliogr. 2014;105:483–486.

![Assessment and positioning of the biologic agents currently available for the treatment of psoriasis based on the meta-analysis carried out by Galván-Banqueri et al.1 and using a delta value of 10%. In the indirect comparison, ustekinumab is superior to etanercept (risk difference relative to etanercept [RD] 11% [95% CI, 5.3-16.7]), and to adalimumab (RD, 12%; IC 95%, 5.9-18) and infliximab (RD 24%; IC 95%, 18.3-29.7). If we use a delta value of 10%, the pairs ustekinumab/etanercept (UST/ETN) and adalimumab/etanercept (ADA/ETN) would correspond to case F in Fig. 1, and infliximab/etanercept (IFX/ETN) would correspond to case G. Using a delta value of 15%, the 3 cases would be D, D, and G, respectively. In no case can it be said that all the biologic agents indicated for moderate to severe psoriasis are clinically equivalent therapeutic alternatives. Assessment and positioning of the biologic agents currently available for the treatment of psoriasis based on the meta-analysis carried out by Galván-Banqueri et al.1 and using a delta value of 10%. In the indirect comparison, ustekinumab is superior to etanercept (risk difference relative to etanercept [RD] 11% [95% CI, 5.3-16.7]), and to adalimumab (RD, 12%; IC 95%, 5.9-18) and infliximab (RD 24%; IC 95%, 18.3-29.7). If we use a delta value of 10%, the pairs ustekinumab/etanercept (UST/ETN) and adalimumab/etanercept (ADA/ETN) would correspond to case F in Fig. 1, and infliximab/etanercept (IFX/ETN) would correspond to case G. Using a delta value of 15%, the 3 cases would be D, D, and G, respectively. In no case can it be said that all the biologic agents indicated for moderate to severe psoriasis are clinically equivalent therapeutic alternatives.](https://static.elsevier.es/multimedia/15782190/0000010500000005/v1_201405281018/S1578219014001255/v1_201405281018/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)


