The association between dipeptidyl peptidase 4 inhibitors (DPP-4i) and bullous pemphigoid (BP) has been demonstrated in several studies. The main aim of this study was to estimate the use of DPP-4i treatment in patients diagnosed with BP in our setting.
MethodsWe selected patients histologically diagnosed with BP in our department between October 2015 and October 2018 and performed a retrospective chart review to assess clinical and epidemiological data and direct immunofluorescence (DIF) patterns.
ResultsOf the 70 patients diagnosed with BP during the study period, 50% were diabetic and 88.57% of these were being treated with a DPP-4i when diagnosed with BP. The most common DPP-4i was linagliptin (used in 18.6% of patients), followed by vildagliptin (17.1%). The median latency period between initiation of DPP-4i treatment and diagnosis of BP was 27.5 months for all treatments, 16 months for linagliptin, and 39 months for vildagliptin (log rank < 0.01). A negative DIF result was significantly more common in patients not being treated with a DPP-4i. The DIF pattern most strongly (and significantly) associated with DPP-4i treatment was linear immunoglobulin G deposits along the dermal-epidermal junction. DPP-4i treatment was withdrawn in 87% of patients and 96% of these achieved a complete response.
ConclusionsDPP-4i treatment is very common in patients with BP in our setting. The latency period between start of treatment and onset of BP seems to be shorter with linagliptin than with other types of gliptins. Patients receiving DPP-4i treatment may show different DIF patterns to those not receiving treatment.
La asociación entre los inhibidores de la dipeptidil peptidasa 4 (iDPP-4) y el penfigoide ampolloso (PA) se ha demostrado en varios estudios. El objetivo principal de este estudio era estimar el uso del tratamiento con iDPP-4i en pacientes diagnosticados de PA en nuestro entorno.
Material y métodosSeleccionamos pacientes diagnosticados histológicamente de PA en nuestro departamento entre octubre de 2015 y octubre de 2018. Realizamos una revisión retrospectiva para evaluar los datos clínicos-epidemiológicos y los patrones de inmunofluorescencia directa (IFD).
ResultadosDe los 70 pacientes diagnosticados con PA durante el período de estudio, el 50% eran diabéticos y el 88,57% de ellos estaban siendo tratados con un iDPP-4 en el momento del diagnóstico de PA. El iDPP-4 más frecuente era la linagliptina (utilizada en el 18,6% de los pacientes), seguida de la vildagliptina (el 17,1%). La mediana de tiempo de latencia entre el inicio del tratamiento con iDPP-4 y el diagnóstico de PA fue de 27,5 meses, siendo de 16 meses para la linagliptina y 39 meses para la vildagliptina (log Rank < 0,01). La IFD fue negativaUn resultado negativo de DIF fue significativamente más común en pacientes que no fueron tratados con un DPP-4i. El patrón DIF más fuertemente (y significativamente) asociado con el tratamiento con DPP-4i fueron los depósitos lineales de inmunoglobulina G a lo largo de la unión dermoepidérmica. El tratamiento con DPP-4i se retiró en el 87% de los pacientes y el 96% de ellos logró una respuesta completa.
ConclusiónEl tratamiento con DPP-4i es muy común en pacientes con BP en nuestro entorno. El período de latencia entre el inicio del tratamiento y el inicio de la PA parece ser más corto con linagliptina que con otros tipos de gliptinas. Los pacientes que reciben tratamiento con DPP-4i pueden mostrar patrones DIF diferentes a los que no reciben tratamiento.
Bullous pemphigoid is the most common autoimmune bullous disease in our setting.1 It is caused by the presence of circulating autoantibodies against proteins that make up the hemidesmosomes of the dermal-epidermal junction (the antigens BP180 and BP230) and is characterized by the formation of intensely pruritic urticarial plaques and tense blisters affecting the whole skin surface, with a predilection for the skinfolds. Mucous involvement is uncommon.2 The incidence of the disease has increased 2- to 4-fold during the last 20 years. This increase is associated with the administration of various drugs, such as spironolactone. An association was recently observed between bullous pemphigoid and administration of oral antidiabetic drugs belonging to the dipeptidylpeptidase-4 inhibitor (DPP-4i) family, which are also known as gliptins.3,4 This association was not observed in clinical trials before these drugs were marketed.
The objective of the present study was to estimate the prevalence of DPP-4i in patients with a histological diagnosis of bullous pemphigoid in the Dermatology Department of Hospital General Universitario de Valencia, Valencia, Spain between October 2015 and October 2018. We also evaluated whether there were differences in the time of onset of bullous pemphigoid according to the type of gliptin used.
Material and methodsWe designed a single-center, retrospective, descriptive, observational study to be performed in the Dermatology Department of Hospital General de Valencia, Valencia, Spain between October 2015 and October 2018. All patients with a histological diagnosis of bullous pemphigoid were included. The patients were selected continuously via a biopsy database in our department. We excluded patients with other bullous skin diseases or with cicatricial pemphigoid or paraneoplastic pemphigus.
Once the sample was selected, we performed a retrospective search of the electronic clinical history of each patient in order to record data for the study variables, as follows: sex, age, history of diabetes, current treatment with a DPP-4i, type of gliptin, months between prescription of the drug and consultation in our department because of symptoms, direct immunofluorescence pattern, and response to treatment according to whether the DPP-4i was withdrawn or not. The direct immunofluorescence patterns analyzed were linear deposition of immunoglobulin (Ig) G and complement factor 3 (C3) and deposition of IgM. All patients were treated with oral corticosteroids, and those in treatment with a DPP-4i were referred to their primary care physician to have the drug withdrawn and replaced by an antidiabetic agent from a different family. A complete response was defined as the total disappearance of the lesions, and a partial response was defined as the presence of lesions requiring treatment with oral or topical corticosteroids.
The statistical analysis was performed using the software application IBM SPSS Statistics, Version 21.0 (IBM Corp.). Continuous variables are expressed as median (interquartile range [IQR]). Qualitative variables are expressed as a whole number and percentage. Hypotheses for quantitative variables were tested using the Pearson χ2 test. Survival was analyzed using Kaplan-Meier plots of the time to onset of bullous pemphigoid (in months) and the different gliptins used. The log-rank test was used for comparisons. Statistical significance was set at P < .05.
ResultsA total of 70 patients were histologically diagnosed with bullous pemphigoid during the 3-year study period. Of these, 35 (50%) were men and 35 (50%) were women. The median age was 82 (38-97) years. Thirty-five patients (50%) had type 2 diabetes; of these, 31 (88.5%) were receiving a DPP-4i. Table 1 shows the demographic characteristics of the patients according to whether or not they were receiving gliptins.
Demographic Characteristics of Patients Diagnosed With Bullous Pemphygoid.
| Receiving DPP-4i | Not Receiving DPP-4i | P Value | |
|---|---|---|---|
| Total | 31 | 39 | |
| Sex | .09 | ||
| Male | 19 (54.3%) | 16 (47.3) | |
| Female | 12 (34.3%) | 23 (65.7%) | |
| Mean (SD) age, y | 77.71 (8.4) | 77.38 (12.9) | .1 |
| Clinical picture | .7 | ||
| Urticarial phase /eczema | 10 (32%) | 13 (33%) | |
| Bullous phase | 6 (19%) | 10 (25%) |
Abbreviation: DDP-4i, dipeptidyl peptidase-4 inhibitor.
At diagnosis, the most commonly used DPP-4i was linagliptin (13 patients [18.6%]), followed by vildagliptin (12 [17.1%]), sitagliptin (5 [7.1%]), and saxagliptin (1 [1.4%]). In 8 patients (11.4%), this was the second gliptin the patient had been prescribed. Six patients had previously been treated with sitagliptin, 1 with linagliptin, and 1 with vildagliptin, with a median time of treatment with the drug of 9 (4) months. Of the 31 patients receiving treatment with a DPP-4i, 25 (80.64%) were taking the drug in combination with metformin, 5 (16.13%) in monotherapy, and 1 (3.22%) in combination with sulfonylurea.
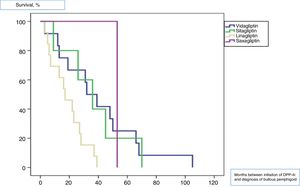
All patients were diagnosed with bullous pemphigoid after initiation of treatment with gliptins, with a median time of 27.5 (33) months. The median time from the initiation of treatment for each of the gliptins until onset of bullous pemphigoid lesions was 39 (13) months for vildagliptin, 36 (7) months for sitagliptin, 16 (3) months for linagliptin, and 53 (0) months for saxagliptin (log-rank, <.011). Fig. 1 shows the survival plots for the different types of gliptin with respect to onset of bullous pemphigoid.
Direct immunofluorescence testing was applied in 67 patients (95.7%). Table 2 shows the results of direct immunofluorescence testing according to the different patterns analyzed.
Direct Immunofluorescence Patterns in Patients Diagnosed with Bullous Pemphigoid.
| Pattern | Receiving DPP-4i | Not Receiving DPP-4i | P Valuea |
|---|---|---|---|
| Negative | 3 | 11 | .04 |
| Linear IgG positive/negative | 24/6 | 20/17 | .02 |
| Linear C3 positive/negative | 24/6 | 22/15 | .72 |
| Granular C3 positive/negative | 0/30 | 1/36 | .3 |
| IgA in inflammatory cells positive/negative | 3/27 | 4/33 | .9 |
| Linear IgA positive/negative | 3/27 | 1/37 | .2 |
| Fibrinogen in the dermis positive/negative | 0/30 | 1/36 | .3 |
| IgM in colloid bodies positive/negative | 1/29 | 1/36 | .8 |
| Linear IgM positive/negative | 1/29 | 1/36 | .8 |
Abbreviation: DDP-4i, dipeptidyl peptidase-4 inhibitor.
All of the 31 patients receiving treatment with a DPP-4i were advised to discontinue therapy. The DPP-4i was eventually withdrawn in 27 cases (87%). A complete response was achieved in 26 cases (83.8%) (1 patient died). Of the 4 patients in whom the drug was not discontinued, 3 (9.6%) achieved a partial response, and 1 was lost to follow-up.
DiscussionThe association between DPP-4i and bullous pemphigoid has been demonstrated in several case-control studies, although not in our setting. The underlying pathogenic mechanism remains unclear. We know that DPP-4 is a cell surface plasminogen receptor that is expressed in keratinocytes and in other types of cell, such as endothelial cells and T lymphocytes. Activation of DPP-4 in vitro leads to the formation of plasmin, a protease that cleaves BP-180 via the NC-16A domain. Furthermore, it has been demonstrated in vivo that inhibition of this receptor by DPP-4i can increase the activity of proinflammatory cytokines such as eotaxin/CCL11, thus leading to recruitment of eosinophils in the dermis and the formation of blisters.5
Over the 3-year period we studied, a total of 70 patients were diagnosed with bullous pemphigoid. Given the catchment population of Hospital General de Valencia (approximately 370 000 people), this incidence is approximately 3 times higher than that published in a French study on the incidence of bullous pemphigoid (21.7 cases per million people per year).6 There may be several explanations for this finding. In recent years, the number of cases of bullous pemphigoid has increased.6 Possible reasons include increased use of DPP-4i and other drugs. In addition, the French study was published almost 8 years ago; therefore, we might expect the incidence to have increased. Furthermore, it is difficult to estimate prevalence based only on the hospital catchment population, since some of the patients diagnosed with bullous pemphigoid in the last few years were from outside our area.
These drugs are thought to increase the risk of bullous pemphigoid by up to 2.8-fold, especially in the case of vildagliptin and, to a lesser extent, linagliptin.5,7 Moreover, small case series have reported a noninflammatory phenotype of bullous pemphigoid induced by DPP-4i.8,9 In their major case-control study, Kridin and Bergman7 found no differences in the clinical pattern of presentation of bullous pemphigoid (beyond greater involvement of the mucous membranes). Since the study was retrospective, the clinical description of the lesions was very poor, as was the explicit evaluation of the mucous membrane, and no differences were found in the cases we collected. While many of these drugs are combined with metformin (80.64% in the present cohort), this drug was not associated with an increased risk of bullous pemphigoid in the univariate or the multivariate analysis.7
The median latency period between initiation of therapy with these antidiabetic agents and disease onset varies considerably depending on the study, from 6 to 26.4 months.7 The median latency in our cohort was slightly higher (27.5 months), perhaps because we defined onset as the point at which the patients came to our department. Given that there may have been some delay until consultation, our results seem consistent with those reported elsewhere. We were unable to find studies that compared the latency of onset of bullous pemphigoid according to the type of gliptin used. In our cohort, linagliptin was the drug with which bullous pemphigoid appeared earliest. A review of the literature indicates that the latency periods reported with this gliptin are similar to those we report, although the cases are usually isolated.10,11
The direct immunofluorescence pattern is similar in bullous pemphigoid associated with DPP-4i and in cases in which these drugs are not involved. In the present cohort, a negative direct immunofluorescence result was statistically significantly associated with the absence of DPP-4i, whereas the presence of linear IgG at the dermal-epidermal junction was statistically significantly more common in cases of bullous pemphigoid associated with DPP-4i. Although infrequent, there have been reports of patients diagnosed with bullous pemphigoid in whom direct immunofluorescence is initially negative or indeterminate, thus necessitating a second biopsy to confirm the diagnosis.12 According to our cohort, false negatives were less common in patients treated with DPP-4i, although this was not confirmed with a second direct immunofluorescence assay. Furthermore, detection of linear IgG with or without C3 in these patients seems to be more sensitive in the diagnosis of bullous pemphigoid. This is a common finding in patients with bullous pemphigoid induced by drugs in general, and not only in those cases where the disease is induced by DPP-4i. We found no studies that evaluate the difference in direct immunofluorescence patterns of bullous pemphigoid according to the presence of these antidiabetic drugs.
Discontinuation of treatment was associated with complete clinical remission in most patients, thus confirming similar data reported elsewhere.13 In contrast, other authors did not find differences in the clinical response between patients who discontinued therapy with gliptins and those who continued therapy.14
The main limitation of our study is that data were collected retrospectively. This prevented us from evaluating the clinical characteristics of patients, since this information was not available in the clinical history in many cases. In addition, it would have been interesting to study the serological values of the patients’ anti–basement membrane antibodies and to determine whether there are differences between patients taking gliptins and patients who were not, even though other authors report no differences.7 We do not routinely use these analytical markers, except in patients with discrepant clinical-pathological data in whom direct immunofluorescence testing is negative. Our study has an advantage over other studies in that we avoided Berkson bias by selecting patients in whom bullous pemphigoid was diagnosed based on histology. The small sample is a disadvantage when attempting to draw robust conclusions, although our sample was very similar to or even larger than those of similar previously published studies.
In conclusion, the prevalence of treatment with DPP-4i in patients diagnosed with bullous pemphigoid is high. The direct immunofluorescence pattern observed in these patients may differ from that observed in those who are not receiving DPP-4i, with a higher frequency of positive results in direct immunofluorescence testing for the combination linear IgG-C3. We also observed that the latency period between initiation of the drug and onset of bullous pemphigoid was significantly shorter with linagliptin than with the other gliptins. Discontinuation of DPP-4i combined with the usual treatment for bullous pemphigoid results in a complete clinical response in almost all patients.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Magdaleno-Tapial J, Valenzuela-Oñate C, Esteban Hurtado Á, Ortiz-Salvador JM, Subiabre-Ferrer D, Ferrer-Guillén B, et al. Asociación entre penfigoide ampolloso e inhibidores de la dipeptidilpeptidasa-4: estudio de cohortes retrospectivo. Actas Dermosifiliogr. 2020;111:249–253.