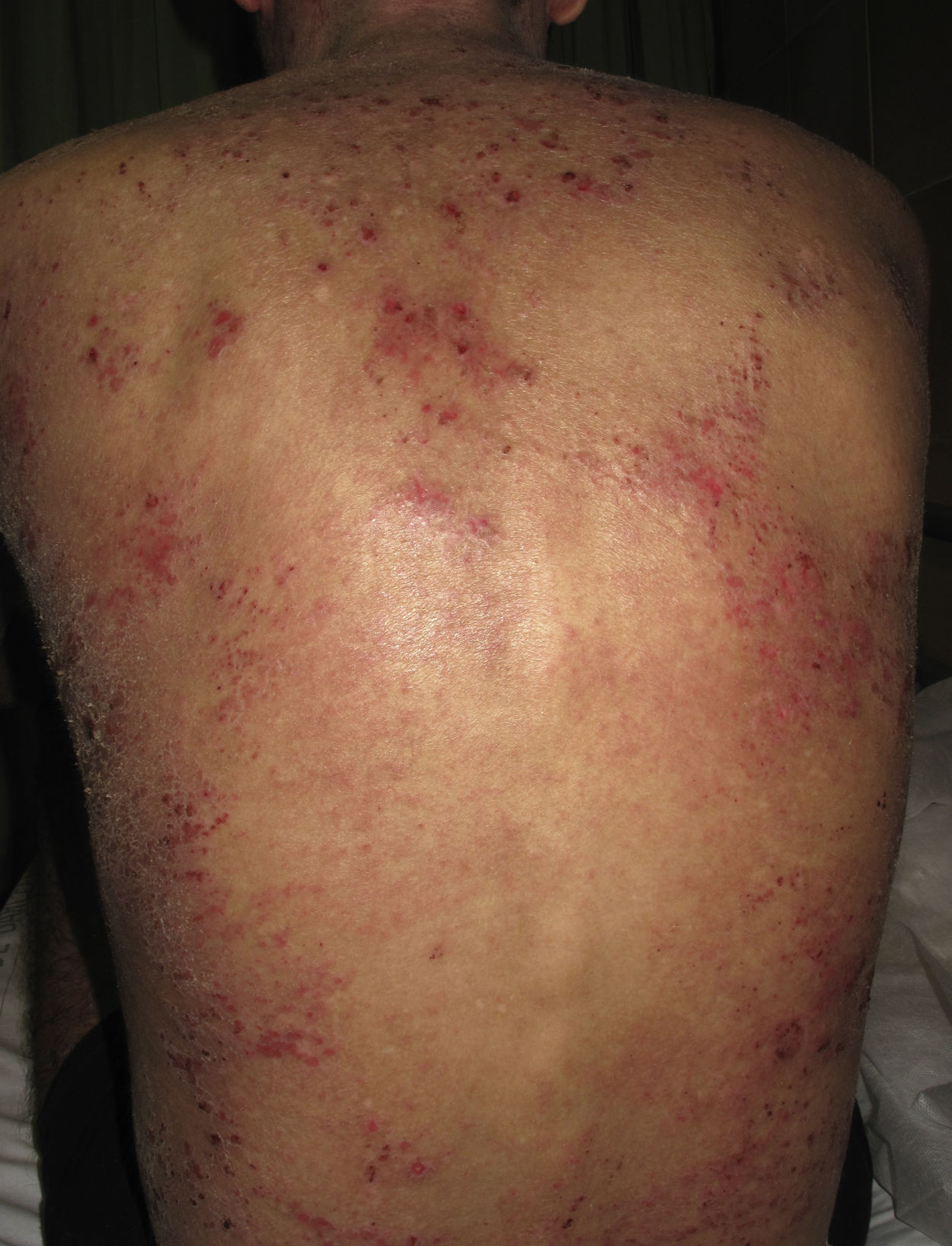
Atopic dermatitis (AD) is an inflammatory skin disease characterized by pruritic eczematous lesions that can affect large surface areas of the body (Fig. 1). Etiologic and pathogenic mechanisms include epithelial dysfunction, alterations to gut microbiota, and inadequate immune activation with overexpression of interleukin 4 (IL-4), IL-13, and IL-31.1 Severe AD requires systemic treatment with oral corticosteroids and immunosuppressive agents, but the long-term use of these drugs can cause considerable adverse effects. Despite the prevalence of AD, no new drugs have been developed in recent decades. The US Food and Drug Administration, however, recently approved 2 novel drugs, dupilumab and crisaborole, for use in AD, and other drugs have shown promising results in preliminary studies.
Dupilumab blocks the IL-4 receptor α subunit, thereby inhibiting IL-4 and IL-13 signaling and reducing not only the production of immunoglobulin E but also inflammatory responses mediated by type 2 helper T cells. Two randomized placebo-controlled clinical trials of dupilumab in patients with moderate to severe AD, SOLO-1 (n=671) and SOLO-2 (n=708), found that dupilumab 300mg administered subcutaneously every week or every 2 weeks resulted in an investigator's global assessment (IGA) score of 0 or 1 (clear or almost clear) at 16 weeks in 38% of patients (vs. 10% for placebo) in SOLO-1 and 36% (vs. 8% for placebo) in SOLO-2 (P<.001).2 It was also associated with a greater percent reduction in the Eczema Area and Severity Index (EASI) score (67% to 72% vs. 30% to 37% for placebo, P<.001) and better results on all the clinical evaluation, quality of life, and pruritus scales. Adverse effects were similar in both groups, although dupilumab was inexplicably associated with a higher incidence of conjunctivitis.
Topical crisaborole 2% is a nonsteroidal anti-inflammatory drug that inhibits phosphodiesterase (PDE) 4 (mainly PDE4B), reducing the release of proinflammatory cytokines, such as tumor necrosis factor, IL-12, and IL-23. Two clinical trials in patients older than 2 years with mild to moderate AD, AD:301 (n=763) and AD:302 (n=764), found crisaborole to be superior to placebo in terms of reducing IgA after 28 days (AD:301: 32.8% vs. 25.4%, P=.038; AD:302: 31.4% vs. 18.0%, P<.001) and improving pruritus.3 In addition, adverse effects were minimal.
One interesting drug currently being investigated in clinical trials is nemolizumab. Nemolizumab is a monoclonal anti-IL-31 receptor antibody that has been shown to be superior to placebo in a phase II trial of 264 patients with moderate to severe AD. It was more effective in improving pruritus (percentage improvement from baseline of 63% in the high-dose group vs. 21% for placebo), although it did not reduce the body surface area affected.4 Another promising drug is topical tofacitinib 2%, which inhibits janus kinases 1 and 3 and the activity of IL-2, IL-4, and other proinflammatory cytokines. The results of a phase IIa trial of 69 adults with mild to moderate AD showed that topical tofacitinib 2% was significantly superior to placebo in terms of improving EASI score (−81.7% vs. −29.9%) and pruritus. There were also few treatment-related effects.5
Dupilumab and crisaborole have emerged as novel dermatological treatments, and dupilumab in particular seems poised to revolutionize the treatment of moderate to severe AD. Further studies of IL-31, IL-13, and JAK inhibitors will determine their efficacy and safety. Patients will benefit from safe, effective drugs that are better tolerated than corticosteroids and other immunosuppressive agents.
Please cite this article as: Morgado-Carrasco D, Fustà-Novell X, Riera-Monroig J, Iranzo P. FR-Después de décadas sin novedades, nuevos fármacos prometen revolucionar el tratamiento de la dermatitis atópica. Actas Dermosifiliogr. 2018;109:443–444.