Cutaneous metastases appear in 0.6% to 10.4% of malignant tumors and account for 2% of all cutaneous tumors. Metastasis to the skin may arise from progression of a known primary tumor or provide the first sign of an unsuspected one. Acral metastases are particularly unusual. Most derive from bone tumors. Clinical signs vary and the lesions generally resemble infection or inflammation, leading to diagnostic delays. When metastasis involves the fingers, the primary tumor is usually lung carcinoma. In contrast, toe involvement usually derives from a tumor in the genitourinary tract. A pathologic diagnosis in these cases is necessary and will suggest the location of the primary tumor.
We report 2 cases of metastasis to the fingers. One is the first report of acral metastasis of a myoepithelial carcinoma of the breast. The other concerns acral metastasis as the first sign of lung carcinoma.
Las metástasis cutáneas aparecen en el 0,6-10,4% de los pacientes con tumores malignos y representan hasta el 2% de los tumores cutáneos. En algunos casos representan la primera manifestación de una neoplasia no conocida. Además pueden poner de manifiesto la progresión metastásica del tumor primario. Las metástasis de localización acral son particularmente raras. En la mayoría de los casos aparecen secundariamente a afectación ósea. Aunque la clínica es variable, generalmente se confunden con un proceso infeccioso o inflamatorio, retrasándose el diagnóstico. Cuando se localizan en los dedos de la mano la causa más frecuente es el carcinoma de pulmón, mientras que las localizadas en los dedos de los pies suelen deberse a tumores del tracto genitourinario. El estudio dermatopatológico en estos casos es fundamental para establecer el diagnóstico y orientar hacia el origen del tumor primario.
Presentamos 2casos clínicos de metástasis digital acral. El primero de ellos representa el primer caso de la literatura de metástasis acral de mioepitelioma maligno (carcinoma mioepitelial) de mama y el otro una metástasis acral como manifestación inicial de carcinoma de pulmón.
Between 0.6% and 10.4% of patients with malignant tumors develop cutaneous metastases, which can alert to the presence of an unknown tumor in up to 1% of cases. Based on data from different series, cutaneous metastases account for up to 2% of all tumors involving the skin.1 At times, they can be difficult to recognize because of their varying clinical presentations and histopathologic features. This variability can lead to delayed diagnoses of both the metastasis and the primary tumor.2 Acral cutaneous metastases are uncommon and there have been very few reports in the literature. Metastases involving the skin tend also to involve the bone.3
Case 1The first patient in this report is a 76-year-old woman with a history of rheumatoid arthritis and hypertension under treatment with valsartan, paracetamol, methotrexate, and torsemide. In July 2011, she underwent surgery for myoepithelial carcinoma of the breast with squamous differentiation, which was treated with surgical excision of the tumor and sentinel lymph node followed by tamoxifen. Computed tomography (CT) of the chest, abdomen, and pelvis showed no signs of distant metastasis. In July 2012, she developed local recurrence in the same breast. Follow-up examination identified a pulmonary nodule suggestive of neoplastic invasion and the patient was scheduled for removal of the lower right lobe; the diagnosis was metastatic myoepithelial carcinoma.
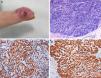
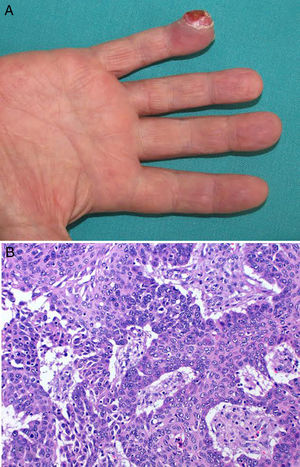
In March 2013, the patient presented with painful, fast-growing lesions of 2 months’ duration on the third finger of the right hand. The physical examination showed an erythematous plaque that mainly affected the finger pad and featured several papules, some of which were slightly eroded, in addition to small palpable nodules (Fig. 1A). The lesion was particularly painful on palpation. Given the suspicion of cutaneous metastasis, we performed a 4-mm punch biopsy of 1 of the nodules. Histopathologic examination showed solid nests with expansive borders that mainly affected the mid and deep dermis. The epidermis had been pushed up and rejected by the expansive growth, and there was no visible connection between the two. The nests were formed by round cells with central and excentric nuclei, lumpy chromatin, and granular cytoplasm (Fig. 1B). The immunohistochemical study showed positivity for actin and neuroendocrine markers (enolase, synaptophysin, and CD56) and negativity for CK7, CK20, EMA, CEA, GCDFP-15, and hormone receptors (Fig. 1 C and D). The X-ray study of the affected joint showed no alterations. In view of the patient's clinical situation, a palliative approach was decided on in conjunction with the oncology department. The patient died several months later.
A, Painful erythematous plaque on the finger containing several superficial nodules and papules, some of which are eroded. B, Proliferation of small cells with round, hyperchromatic nuclei with scant eosinophilic cytoplasm forming cords immersed in a basophilic matrix. Some mitotic figures are observed. There are no areas of squamous differentiation. C, Actin staining showing strong cytoplasmic positivity. D, S-100 staining showing strong cytoplasmic positivity.
The second patient was a 77-year-old man with a history of hypertension, dyslipidemia, and chronic obstructive pulmonary disease under treatment with atorvastatin, budesonide/inhaled formoterol, and valsartan/hydrochlorothiazide. He was an ex-smoker of over 150 pack-years and had quit in 2008 when operated on for cancer of the tongue.
He presented in October 2014 with a lesion of 20 days’ duration on the pulp of the little finger of his right hand. The lesion was painful to the touch and the patient reported no history of trauma.
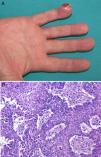
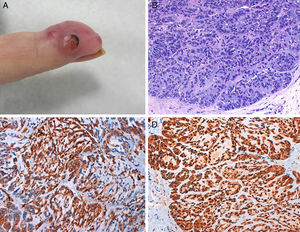
Physical examination showed an erythematous-pink tumor with a maximum diameter of 2cm on the pulp of the right hand with keratotic areas (Fig. 2A). A 4-mm punch biopsy was performed and histopathologic examination showed a partially ulcerated epidermis. In the dermis there was a proliferation of neoplastic cells with an epithelial appearance, together with abundant mitotic figures and nuclear atypia (Fig. 2B). The cells showed positive cytokeratin staining. On suspecting an acral metastasis, we performed a CT scan of the chest, abdomen, and pelvis, which showed a large tumor with a 6-cm diameter on the right upper lobe with bronchial involvement and mediastinal invasion; the scan also showed multiple intraparenchymal nodules on the left lung. The diagnosis was acral metastasis from non-small cell lung cancer. The phalanx of the affected finger was amputated as the X-ray study showed bone involvement. The oncology team decided to administer chemotherapy. The disease, however, progressed and the patient died several months later.
DiscussionAn estimated 23.9% of patients with breast cancer develop cutaneous metastases.1 This is the most common type of cutaneous metastasis seen in dermatology departments because of the high incidence of breast cancer. The most common sites for metastasis from this location are the chest and abdomen.4
The lesions generally appear as indurated nodules that tend to cluster together creating plaques that may become ulcerated.5 Other lesions include inflammatory or erysipeloid lesions, carcinoma en cuirasse, alopecia neoplastica of the scalp,6 telangiectatic carcinoma, and histiocytoid carcinoma of the eyelid.7 Epidermotropic metastases from breast cancer mimicking extramammary Paget disease are also seen, albeit infrequently.8
Cutaneous metastasis from lung cancer is the most common type of cutaneous metastasis in men, and, as occurred in our case, it can be the first sign of a primary tumor.9 Over 50% of cases are seen in patients with non-small cell lung cancers. The most common sites for metastasis in this setting are the chest, abdomen, upper lip, and back. The lesions generally appear as solitary or multiple erythematous-violaceous nodular lesions that tend to ulcerate.1 Acral metastases are rare, but the most common site is the tip of the nose, giving the appearance of what has been termed clown nose.10
The incidence of different types of cutaneous metastasis is directly linked to that of the most common cancers. In women, for example, the most common cutaneous metastasis is from breast cancer, the most common cancer in women, while in men, it is from lung cancer. Analysis of age-standardized incidence, however, shows that most common cutaneous metastases in men under 45 years of age are from melanoma.11
Acral metastases are uncommon clinical findings and account for just 0.1% of all metastatic lesions. They can be monostotic, polyostotic, unilateral, or bilateral (although they are rarely symmetric). They can sometimes affect the fingers or toes. Most metastases to the fingers and toes start in the bone and then spread to the skin rather than the other way round.12 The most common primary cancers that cause metastases to the fingers and toes are lung and endometrial cancer, respectively.13 Acral involvement in patients with metastatic breast cancer is rare. In a retrospective study of 4020 patients with metastatic cancer by Lookingbill et al.,1 of 212 women with cutaneous metastasis from breast cancer, none of them had acral metastases. This was also the case for patients with metastatic lung cancer.1
Histology is essential for diagnosing acral metastasis and investigating the site of the primary tumor via immunohistochemical studies.2 In the first patient described in the present report, the primary tumor and cutaneous metastases both corresponded to myoepithelial carcinoma of the breast. This histologic subtype of breast cancer is extremely rare. Microscopic findings include spindle cells with poorly defined borders forming a storiform pattern. Three cell types can be observed: clear cells, plasmacytoid cells, and epithelial cells. The biologic behavior of malignant myoepithelioma of the breast is not yet fully understood.14 In the case of skin involvement, there tends to be continuity between the 2 foci. To our knowledge, ours is the first report of acral involvement. The second patient in this report had non-small cell lung cancer that had large cells containing eosinophilic cytoplasm and expressed CK5/6.15
The differential diagnosis should include infections (bacterial, mycobacterial, viral), inflammatory disease (gout, rheumatoid arthritis, granulation tissue), benign tumors (pyogenic granuloma, glomus tumor, epidermoid cyst), primary malignant tumors (Bowen disease, epitheliomas, melanoma, eccrine porocarcinoma, sarcoma), and hematologic disease (leukemia, myeloma, Langerhans cell histiocytosis).12,13,16
We have presented 2 cases of acral cutaneous metastases from the breast and lungs. Of note in the first case, the patient with breast cancer, is the acral location of the metastasis, the noninvolvement of the distal phalanx, the rare histologic subtype (myoepithelial carcinoma of the breast), and its highly aggressive behavior. Of note in the second case is the fact that the finger lesion enabled detection of a primary lung tumor.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Baños-Arévalo AJ, López-Navarro N, Gallego-Domínguez E, Herrera E. Acral Metastasis of the Fingers: Report of 2 Cases. Actas Dermosifiliogr. 2018;109:e1–e4.