Erythroderma is an inflammatory skin syndrome that involves desquamation and erythema of more than 90% of the body surface area. It represents a final clinical endpoint for many adult dermatological conditions. The most frequent cause of erythroderma is psoriasis followed by eczematous conditions, drug-induced reactions, pityriasis rubra pilaris and cutaneous T-cell lymphomas. Diagnostic approach must include a thorough history and clinical examination. If the etiology of erythroderma is uncertain multiple skin biopsies may enhance diagnostic accuracy. The initial management of erythroderma must include a nutrition expert evaluation, fluid imbalance assessment, maintaining skin barrier function, sedative antihistamines and exclusion of secondary bacterial infection. We present a practical review of the etiology, diagnosis, and treatment of this entity.
La eritrodermia es un síndrome inflamatorio de la piel caracterizado por descamación y eritema en más del 90% de la superficie corporal. Representa la etapa final de muchas enfermedades dermatológicas en el adulto. La causa más frecuente es la psoriasis, le siguen las enfermedades eccematosas, las reacciones medicamentosas, la pitiriasis rubra pilaris y los linfomas cutáneos de células T. El abordaje diagnóstico debe incluir una historia y examen físicos exhaustivos. Si se desconoce la etiología de la eritrodermia es posible que múltiples biopsias a lo largo del curso de la enfermedad aumenten las posibilidades de un diagnóstico correcto. El abordaje inicial de la eritrodermia debe incluir la evaluación de un experto en nutrición, la valoración del balance hidroelectrolítico, medidas para mantener la función de barrera de la piel, antihistamínicos con efecto sedante y la exclusión de infecciones bacterianas secundarias. Presentamos una revisión práctica de la etiología, diagnóstico y tratamiento de esta entidad.
Erythroderma is the clinical finding of generalized erythema and scaling of the skin. This condition, especially when fulminant, is potentially life-threatening and has been associated with high mortality in hospitalized patients.1,2 The term erythroderma was first used by Ferdinand Von Hebra in 1868 in his work “On Diseases of the Skin” to describe generalized skin redness and scaling.3 A variety of diseases and exogenous factors can cause this syndrome. Although rare, it remains a relevant and difficult for dermatologists to treat this disease. The objective of this article is to review the general principles, the clinical aspects and the pathogenesis of erythroderma in adults, as well as to provide a concise guide for its diagnostic and therapeutic approach.
DefinitionErythroderma also called generalized exfoliative dermatitis or exfoliative erythroderma, is a severe inflammatory skin syndrome characterized by generalized erythema and desquamation comprising ≥ 90% of the body surface area.4 Generally, erythroderma is the preferred term for this syndrome.4,5 Previously, some authors used the term “red man syndrome” to refer to idiopathic erythroderma (which must not be confused with the cutaneous reaction associated to rapid intravenous infusion of vancomycin).6
EpidemiologyErythroderma is a rare condition. Most published studies are retrospective and do not address overall incidence.7,8 A retrospective study from China reported that erythroderma accounted for 13 of every 100,000 dermatologic patients.9 Recently, an incidence of 9.4 cases/year was reported in a retrospective study from Portugal.10 Excluding children, the average age of onset varies from 41 to 61 years; although it usually affects patients over 45 years of age.11,12 Studies favor a male predominance with a male-to-female ratio of 2-4:1.13
PathogenesisImplicated fisiopathogenic mechanisms depend upon the underlying cause. Common pathogenic pathways among different etiologies are still a question of debate.6 It is believed that this syndrome derives from a complex interaction between cytokines and cellular adhesion molecules. IL-1, IL-2, IL-8, intercellular adhesion molecule 1 (ICAM-1), tumor necrosis factor and interferon gamma have all been involved in the pathogenesis of erythroderma.4,7,8 Interactions between these cytokines result in an increased epidermal cellular division. This increased mitotic rate shortens the transit time of cells through the epidermis, thus resulting in cutaneous exfoliation. Scales are normally retained by the skin and contain amino acids, proteins and nucleic acids which are lost during desquamation.14,15 It has been calculated that this desquamative process may increase protein loss by 25-30% in psoriatic erythroderma and by 10-15% in other causes.15
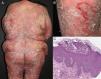
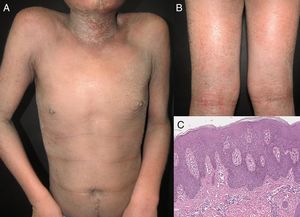
EtiologyErythroderma represents a final clinical endpoint for many dermatological diseases.16 As observed in a recent retrospective study, the relative incidence of different etiologies may vary among populations due to genetic, geographic, and social disparities.10 Most studies indicate that erythroderma is more commonly associated with an exacerbation of a pre-existing dermatosis; therefore, the patient's medical history is crucial for a correct diagnosis. Psoriasis is the most frequent cause of exfoliative dermatitis, which in some studies represents 25-50% of cases (Figure 1).10,17–19 Most patients have a history of localized disease before the onset of exfoliative dermatitis, which develops more frequently with long-standing psoriasis (more than 10 years after diagnosis).9,10,20,21 Psoriatic erythroderma has been associated with certain triggers, including the sudden withdrawal of topical or systemic corticosteroids and methotrexate, phototoxicity, or systemic infection.22
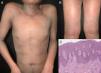
It has been reported that exfoliative dermatitis related to atopic dermatitis varies from 4.76 to 23.9% (Figure 2).19,23 A history of non-atopic eczema has been found in 5.12% to 25.3% of patients with erythroderma.24–26
In case series, drug-related reactions represent the second most frequent cause of erythroderma, ranging from 11.3 to 21.6% of cases.9,10,20 The list of drugs that cause erythroderma is long and continuously grows. Previous authors have reported that drugs with the greatest erythroderma-inducing potential are anti-epileptic medications and allopurinol.9,10,20,22 Carbamazepine is the most common anti-epileptic drug related to this syndrome and it has been postulated that this may be a result of genetic sensitivity or its frequent prescription.11Antifimic drugs have been associated with erythroderma in HIV-seropositive patients,27,28 as well as with traditional Chinese herbs used as analgesics.9,29 Other common drugs related to this condition are phenytoin, beta-lactam antibiotics, sulfonamides, phenobarbital, sulfasalazine and proton pump inhibitors.10,30 Non-ionic contrast material has also been reported as an inducer of this syndrome.31 Exfoliative dermatitis has been related to a severe form of pityriasis rubra pilaris in 1.25% to 8.2% of cases.11,24
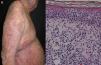
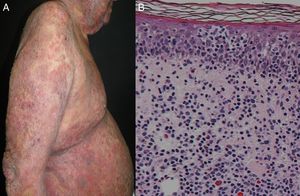
Most series report 1% of erythroderma cases have a neoplasic or paraneoplastic etiology.8,23,32,33 It has been implicated in laryngeal, thyroid, breast, lung, esophageal, gastric, hepatocellular, tongue, gallbladder, colon, fallopian tube and prostate cancers.4,9,34–42 But it is more often related to hematologic malignancies and cutaneous T-cell lymphomas, which constitute 25 to 40% of cases.4 Within this subgroup, mycosis fungoides (Figure 3), and Sézary syndrome are more frequent.43,44 Acute and chronic leukemia, reticular cell sarcoma, and malignant histiocytosis have also been implicated.10,45,46
In 6.51% to 36% of patients, no precise etiology can be identified.47 Some authors have described the progression of chronic idiopathic erythroderma to cutaneous T-cell lymphoma.19 Uncommon causes of adult exfoliative dermatitis include congenital icthyoses,6 staphylococcal scalded skin syndrome,6 immunobullous diseases,48 connective tissue diseases such as dermatomyositis,49 chronic actinic dermatitis,50 sarcoidosis,51 Norwegian scabies,52 Langerhans cell histiocytosis,53 irradiation, graft-vs-host disease, Ofuji papulo-erythroderma and Omenns’ syndrome.18,54–58
Clinical ManifestationsErythroderma has a gradual and insidious onset except for drug-induced cases.24 This condition begins as patches of erythema that enlarge and coalesce to eventually affect most of the skin surface. It is associated with a variable degree of scaling that typically appears 2-6 days after the onset of erythema. The skin is usually bright red, dry, warm and indurated. Most patients complain of skin pain or pruritus. In acute phases, scales may appear large and crusted, whereas in chronic states they tend to be smaller and drier.4,12 The type of scale may suggest the underlying etiology: fine scales are usually found in eczematous conditions, crusted scales in immunobullous diseases, exfoliative scales in drug reactions and bran-like scales in seborrheic dermatitis.6 In chronic erythroderma, patients may develop crusted erosions and secondary lichenification because of severe scratching; hyper- or hypopigmentation may also be present. Nails may become thick, dry, brittle, shiny, and show ridging.4,12 A recent clinical study by Mahabaleshwar et al. reported the following nail changes in erythroderma patients: discoloration (40%), ridges (36%), pitting (20%), onycholysis (18%), shiny nails (4%) and paronychia (2%).20 Palmoplantar keratoderma appears in approximately 30% of patients. Classically, marked keratoderma is associated with pityriasis rubra pilaris, yet some reports have found that palmoplantar keratoderma and nail changes are predictive clinical signs of psoriasis.6,9,10,22,25
Rare hematologic syndromes may mimic clinical manifestations of erythrodermic psoriasis such as idiopathic hypereosinophilic syndrome.59 Pityriasis rubra pilaris typically shows islands of sparing, orange-colored palmoplantar keratoderma and hyperkeratotic follicular papules on extensor surfaces.60 Violaceous papules and reticulated buccal mucosal lesions may indicate an underlying diagnosis of lichen planus.61 Patients with Norwegian scabies may develop heavy crusts on the palms and soles, as well as subungual hyperkeratosis.52,60 Gottron's papules, heliotrope rash, poikiloderma, periungual telangiectasias and muscle weakness may support the diagnosis of erythrodermic dermatomyositis.62,63 Moist and crusted lesions on the face and upper trunk can be an early manifestation of pemphigus foliaceous. Non-scarring alopecia may appear in 20% of patients with chronic erythroderma, and ocular complications such as bilateral ectropion and purulent conjunctivitis may be particularly prominent in chronic erythroderma secondary to Sézary syndrome.6 Some studies have described sparing of the nose and paranasal areas, described as the “nose sign”.64
Systemic ManifestationsPatients suffering exfoliative dermatitis will often shiver and complain of feeling cold. A recent literature analysis by Cesar et al. established pruritus as the most common symptom.10 Generalized peripheral lymphadenopathy may be present, prompting histologic and molecular examination to rule out hematologic malignancy. Other features described in this condition include facial, pedal or pretibial edema, hypothermia, cachexia, hepatomegaly, splenomegaly,6,10 and fever in about half of the patients.10,29
The clinician must be aware of systemic manifestations that could potentially complicate disease evolution. Exfoliative dermatitis may lead to systemic complications including fluid and electrolyte imbalance, high-output cardiac failure, acute respiratory distress syndrome, and secondary infections (erythrodermic skin is commonly colonized by Staphylococcus aureus).6,13,65–67 Staphylococcal sepsis may occur due to inflamed and excoriated skin, especially in HIV-positive patients or with an underlying hematological malignancy.68,69
HistopathologyIn erytrhodermic patients clinical and histopathological correlation can be difficult to attain,24 yet highly trained pathologists can provide a precise diagnosis.10 Skin samples are usually obtained with 4mm punch biopsies and previous series report that multiple biopsies over time can enhance diagnostic accuracy.13 Previous studies report skin biopsies to be useful in 53-66% of erythroderma cases.10,70,71 Frequent histopathologic findings in erythroderma include hyperkeratosis, acanthosis, spongiosis, and perivascular inflammatory infiltrate.72 A recent retrospective study by Megna et al. on 82 erythrodermic patients reported a diagnosis of psoriasis in 23.2% of subjects, strongly supported by the presence of acanthosis, diffuse parakeratosis, diffuse hypogranulosis and the presence of neutrophils on epidermis and dermis. Spongiotic dermatitis was found in 20.7% of patients; encountered features included exocytosis, superficial lymphocytic infiltrate, spongiosis, irregular acanthosis and dermal eosinophilic infiltrate. Drug-induced erythroderma was diagnosed in 8.5% of subjects with biopsies showing lymphocytes and colloid bodies, in addition to hydropic degeneration within the epidermal basal layer. Cutaneous T-cell lymphoma (CTCL)/mycosis fungoides (8.5% in this study) biopsies showed lymphocytic microabscesses, in addition to epidermal and dermal atypical lymphocytes. It is important to highlight that in a considerable number of cases (39.1%), the diagnosis was inconclusive not matching the final diagnosis.73 These results support the fact that skin biopsies are a mandatory first step in the required workup of erythrodermic patients.
The majority of studies report that nodal biopsies frequently demonstrate dermatopathic lymphadenopathy,10,29,74 yet lymph node biopsies may be the key to diagnostic exclusion of lymphomas or uncommon diseases such as multicentric Castleman's disease.75 The presence of atypical lymphocytes should prompt immunohistochemistry and T-cell receptor gene rearrangement studies. Sézary syndrome is supported by the presence of T-cells lacking mature T-cell antigens (CD3+, CD4+, CD7-) and T-cell receptor gene clonality.76 Immunofluorescence should be considered if histopathology suggests immunobullous disease, graft-vs-host disease, and connective tissue disorders.26
Laboratory and ImagingLaboratory findings of erythrodermic patients are frequently nonspecific. Common laboratory abnormalities described in previous studies (in order of frequency) include an elevated erythrocyte sedimentation rate (96.1%), leukocytosis (48.5%), eosinophilia (39.8%), and anemia (30.1%).10 Allergic conditions may present increased serum IgE. When suspecting drug-induced erythroderma, the eosinophil count is necessary in DRESS syndrome.7,33,49,77,78 A recent study found a correlation between the presence of eosinophilia and malignancy-related erythroderma.10 Other findings include elevated uric acid and creatinine levels, as well as reduced serum protein levels.19,24 Serum electrolytes can be used to monitor fluid loss. Liver and kidney function tests may be altered in erythroderma associated with severe drug reactions. Specific tests to diagnose Sézary syndrome include Sézary cell count analysis.79 Possible screening tests for connective tissue diseases include antinuclear antibodies, extractable nuclear antigen, rheumatoid factor, anti-DNA antibodies and complement levels. HIV virus testing may be mandatory in high-risk populations with erythroderma.80
Multiple blood cultures may be necessary to exclude Staphylococcal sepsis based on the fact that blood cultures may be contaminated secondary to cutaneous Staphylococcal colonization.66 When skin superinfections are suspected, fungal cultures, PCR for herpes simplex virus and the varicella-zoster virus can be useful. Diagnosis of crusted scabies can be made by examining scrapings obtained from burrows under the microscope.52 Imaging studies such as a chest radiograph, computed tomography, and magnetic resonance may aid in the diagnosis of paraneoplastic erythroderma.
DiagnosisBeing a syndromatic entity, the diagnosis of erythroderma is easily made with the clinical finding of generalized erythema and desquamation involving ≥ 90% of the skin surface area.4 Defining the underlying disease represent a challenge for physicians and must include a profound clinicopathological correlation. We describe the characteristics of the main differential diagnoses in Table 1.
Features of common causes of erythroderma in adults.
| Etiology | Reported frequency | Clinical clues | Diagnostic hints | Dermoscopy | Histopathology |
|---|---|---|---|---|---|
| Psoriasis | 25 - 50% | Previous psoriatic plaques, palmoplantar keratoderma, nail changes, arthritis, scalp involvement, seborrheic dermatitis-like features | -Most common cause -Long-standing psoriasis (more than 10 years from diagnosis) -Withdrawal of systemic or topical (extensive long-term use) corticosteroids, methotrexate or cyclosporine | Whitish scales, dotted vessels, regularly arranged homogeneous reddish background | Psoriasiform epidermal hyperplasia with confluent parakeratosis layered with neutrophils, hypogranulosis, and dilated tortuous papillary blood vessels, Munro microabscesses, spongiform pustules of Kogoj |
| Spongiotic dermatitis | 5.12% - 25.3% | Fine scales, lichenification, severe pruritus, oozing skin, erythematous papules and plaques | Look for a history of: -Atopic dermatitis (9%) -Contact dermatitis (6%) -Seborrhoeic dermatitis (4%) | Atopic dermatitis: yellowish scales/serocrusts, Patchily distributed dotted vessels | Superficial perivascular dermal infiltrate with eosinophils, overlying spongiosis |
| Drug-induced | 11.3 - 21.6% | Exfoliative scale preceded by morbilliform eruption, face edema, pruritus | -Look for intake of antiepileptics, allopurinol, beta-lactamics, sulfonamides, Chinese herbs, NSAIDs -Commonly resolves 2-6 weeks after discontinuation of offending drug -Eosinophilia -Liver enzyme elevation -Creatinine elevation | Perivascular infiltrate with eosinophils, interface dermatitis with necrotic keratinocytes | |
| Pityriasis rubra pilaris | 1.25% - 8.2% | Hyperkeratotic follicular papules, islands of sparing, orange-colored palmoplantarkeratoderma | -Cephalocaudal spread -Acute onset -Sixth decade of life -Ectroprion | Whitish scaling, orange blotches, scattered dotted vessels, islands of non-erythematous skin | Epidermal hyperplasia with horizontal and vertical alternating orthokeratosis and parakeratosis, follicular plugs with “shoulder parakeratosis” |
| T-cell lymphomas (Sézary syndrome and Mycosis fungoides) | 1% | Fissured painful palmoplantarkeratoderma, severe pruritus, hepatosplenomegaly, nail hypertrophy, ectropion | -Lymphadenopathy -Alopecia -Leonine facies -Hepatosplenomegaly -Periphereal blood: sezary cells (cerebriform nuclei), CD4:CD8 ratio of 10 or more | Mycosis fungoides: serpiginous vessels with spermatozoon-like shape, whitish–pinkish background | Nuclear atypia in lymphocytes, clustering of atypical cells within epidermis, clonal T-cell population, minimal spongiosis, may show unspecific inflammatory infiltrate |
| Other etiologies | |
|---|---|
| Dermatophytosis | Look for chronic use of topical corticosteroids. Hyphae within stratum corneum |
| Scabies | Burrows, nodules on the genitalia, scale in the webspaces, widespread crusted plaques, thick nails, ketaoderma. |
| Lichen planus | Look for pruritic, violaceous papules that favor the extremities. |
| Immunobullous disease | Bilsters, erosions and ulcers |
| Dermatomyositis | Gottron papules, heliotrope sign, poikiloderma Muscle weakness |
| Paraneoplasic | Failure to thrive |
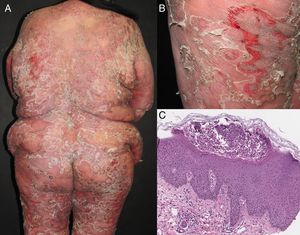
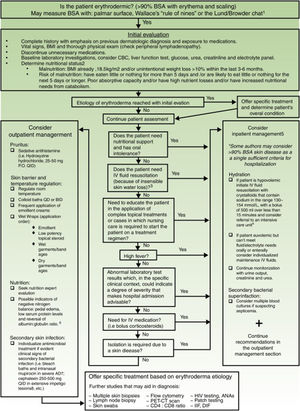
Exfoliative dermatitis is a dermatological emergency and severe cases require in patient care.80,81 The initial diagnostic approach and general principles of management for erythrodermic patients are described in detail in Figure 4.
Initial diagnostic approach and general principles of management for erythrodermic patients. BSA: Body surface area; BMI: Body mass index; CBC: complete blood count; ie: for example; PO: Per os (taken orally); QID: Quarter in die (4 times a day); QD: Quaque die (once a day); BID: Bis in die (twice a day); PET-CT: positron emission tomography-computed tomography; HIV: Human immunodeficiency virus; ANAs: Antinuclear antibodies; IFF: Indirect immunofluorescence. Sources and comments: 1.- Scarisbrick et al91; 2.- NICE: Clinical guideline for nutrition support in adults (2017 uptdate)92; 3.- Hypovolaemia criteria: Sistolic blood pressure < 100mmHg, Heart rate> 90 BPM, Capillary refill time> 2seconds, respiratory rate> 20 breaths per minute, passive leg raising suggests fluid responsiveness93; 4.- For further management of IV fluids: NICE: Clinical guideline for intravenous fluid therapy in adults in hospital (2013)93; 5.- Martínez-Morán et al81; 6.- Kanthraj et al15; 7.- Eichenfield et al94; 8.- Stevens et al.95
Treatment approach should include discontinuation of any unnecessary medications and appropriate workup to exclude an underlying malignancy. Bed rest and sedation should be used when necessary. Initial management of all types of erythroderma is similar, even without an etiologic diagnosis. Regulating environmental temperature is crucial since patients with this condition loose homeostatic body functions that prevent cooling or overheating.80 Skin barrier function can be improved with colloid baths and wet compresses on not more than a quarter of the body at a time,80 along with emollient creams and low-potency topical corticosteroids.82 High-potency topical corticosteroids and topical tacrolimus are not recommended since systemic absorption is enhanced by increased skin permeability.83,84 The initial approach to therapy must also include nutrition and fluid assessment as described in Figure 4.
Oral, intramuscular or intravenous sedative antihistamines can alleviate scratching, thus preventing secondary skin infections while relieving pruritus and anxiety (eg, hydroxyzine hydrochloride, 25–50mg P.O. QID).82
There is scarce high evidence-based data to determine treatment recommendations regarding erythrodermic psoriasis. A panel of experts suggested cyclosporine (Evidence IIB) or infliximab (Evidence IIB) might be the most rapidly acting agents. Other first-line choices are acitretin (Evidence IB) or methotrexate (Evidence III), although they usually work more slowly. A second-line treatment based on case series is Etanercept (Evidence IIB). Use of systemic steroids is controversial as withdrawal may precipitate an erythrodermic flare. Evidence for these recommendations was graded using levels of evidence developed by Shekelle et al.85
First-line treatment choices for adults with severe atopic dermatitis, particularly after failure with topical treatment, include narrow-band UVB phototherapy (Evidence IIB) or systemic immunosuppressant. Oral cyclosporine has been evaluated in randomized trials and systematic reviews showing to be an adequate short-term treatment (Evidence I-IIB). The evidence grading scale used in these recommendations was the Strength of Recommendation Taxonomy (SORT).86 Recent randomized clinical trials with dupilumab, an interleukin (IL)-4 receptor alpha antagonist, indicate it may be an alternative systemic therapy for long-standing severe atopic dermatitis in adults.87
Treatment recommendations for pityriasis rubra pilaris are based merely on case reports and small case series, to date no randomized controlled trials are available. First-line therapies are oral retinoids and methotrexate. Second-line therapies may include TNF-alpha inhibitors, systemic steroids, cyclosporine or azathioprine.88,89
Patients with idiopathic erythroderma who fail to respond to topical treatments may be treated with empiric regimens such as systemic corticosteroids or other immunosuppressants such as methotrexate and cyclosporine; however, evidence for this approach is scarce.9,11,22,47
Specific treatment regimens for common causes of erythroderma are described in detail in Table 2.
Specific treatment regimens for erythroderma with known etiology.
| Etiology | Treatment | Dose | Absolute contraindications | Important relative contraindications |
|---|---|---|---|---|
| Psoriasis | Cyclosporine (First line) | Initial mean dose 4 mg/kg/day slowly reduced after remission by 0.5 mg/kg every 2 weeks | Decreased renal function, uncontrolled hypertension, hypersensitivity, active malignancy | Controlled hypertension, age <18 years or>64 years, active infection, live attenuated vaccine, immunodeficiency, pregnancy (C), concomitant immunosuppressive drug |
| Infliximab (First line) | 5 mg/kg i.v. at week 0, 2, 6, and later every 8 weeks | Hypersensitivity, active infections, concurrent use of anakinra | Congestive heart failure, family history of demyelinating diseases, increased risk of malignancy | |
| Acitretin (First line, slow acting) | 0.3–0.75 mg/kg | Pregnancy (X), lactation, non-compliance with contraception | Leukopenia, hepatic or renal dysfunction, dyslipidemia, hypoithyroidism | |
| Methotrexate (First line, slow acting) | 7.5–15 mg/week | Pregnancy (X) and lactation | Hepatic disease, decreased renal function, immunodeficiency, severe hematologic abnormality, active infectious disease or potential reactivation of TB | |
| Etanercept | 50mg subcutaneous injection twice a week, reduce 50 mg/week after 3 months | Hypersensitivity, active or chronic infections, concurrent use of anakinra | Congestive heart failure, family history of demyelinating disease, increased risk of malignancy | |
| Phototherapy | UVB-NB: Initial dosing according to skin type (130-400 mJ/cm2) or MED (50% of MED). Subsequent dosage increase by 15-65 mJ/cm2 or ≤10% of initial MED. Treatment 3-5 times/week | Pemphigus and pemphigoid, lupus erythematosus with photosensitivity, xeroderma pigmentosa | Photosensitivity/photosensitizing medication, history of skin cancer, history or family history of melanoma, physical impairment, history of arsenic intake or ionizing radiation therapy, poor compliance (Menter et al) | |
| Adalimumab | 80mg at week 0, 40mg at week 1, later 40mg every 2 weeks | Hypersensitivity, active infections, concurrent use of anakinra | Congestive heart failure, family history of demyelinating disease, increased risk of malignancy | |
| Ustekinumab | 45/90mg (according to the weight) at week 0.4 and later every 12 weeks | Hypersensitivity, active serious infection | Increased risk of malignancy, avoid pregnancy | |
| Systemic corticosteroids | - | - | Avoid, as their withdrawal can result in a pustular flare or erythroderma that may be life-threatening | |
| Atopic dermatitis | Cyclosporin (FIrst line) | Initial mean dose 5 mg/kg/day, slowly reduced after remission by 0.5 mg/kg every 2 weeks | Decreased renal function, uncontrolled hypertension, hypersensitivity, active malignancy | Controlled hypertension, age <18 years or>64 years, active infection, live attenuated vaccine, immunodeficiency, pregnancy (C), concomitant immunosuppressive drug |
| Phototherapy (First line) | Narrowband UVB (311–313 nm) | Pemphigus and pemphigoid, lupus erythematosus with photosensitivity, xeroderma pigmentosa | Photosensitivity/photosensitizing medication, history of skin cancer, history or family history of melanoma, physical impairment, history of arsenic intake or ionizing radiation therapy, poor compliance | |
| Methotrexate | 10–25 mg/week slowly reduced after remission | Pregnancy (X) and lactation | Hepatic disease, decreased renal function, immunodeficiency, severe hematologic abnormality, active infectious disease or potential reactivation of TB | |
| Mycophenolate mofetil | 1–2 g/day slowly reduced after remission | Pregnancy (D), drug allergy | Lactation, peptic ulcer, hepatic/renal disease, concomitant use of azathioprine | |
| Azathioprine | 100–200 mg/day slowly reduced after remission | Pregnancy (D), hypersensitivity, active infection, myelosuppression | Use of allopurinol, prior use of alkylating agents, hepatic disease | |
| Intravenous immunoglobulins | 2 g/kg/month for 3–6 months | Anaphylaxis secondary to previous infusions | Congestive heart failure, renal failure, IgA deficiency, rheumatoid arthritis. | |
| Systemic corticosteroids | Prednisone 1 mg/kg/24 h, then gradually decreased | Systemic fungal infections, herpes simplex keratitis, hypersensitivity | Hypertension, CHF, prior psychosis, active TB, positive tuberculin test, osteoporosis, cataracts, glaucoma, pregnancy (C), diabetes mellitus, gastric disease | |
| Drug-induced | Systemic corticosteroids | Prednisone 1 mg/kg/24 h, then gradually decreased | Systemic fungal infections, herpes simplex keratitis, hypersensitivity | Hypertension, CHF, prior psychosis, active TB, positive tuberculin test, osteoporosis, cataracts, glaucoma, pregnancy (C), diabetes mellitus, gastric disease |
| Intravenous immunoglobulins | High dose (1 g/kg/day for 3 days) | Anaphylaxis secondary to previous infusions | Congestive heart failure, renal failure, IgA deficiency, rheumatoid arthritis | |
| Pityriasis rubra pilaris | Acitretin (First line) | 0.3–0.75 mg/kg/day slowly reduced after remission | Pregnancy (X), lactation, non-compliance with contraception | Leukopenia, hepatic or renal dysfunction, dyslipidemia, hypothyroidism |
| Methotrexate (First line) | 10–25 mg/week slowly reduced after remission | Pregnancy (X) and lactation | Hepatic disease, asses for active infectious disease or potential reactivation of TB | |
| Systemic corticosteroids | Prednisone 1 mg/kg/24 h, then gradually decreased | Systemic fungal infections, herpes simplex keratitis, hypersensitivity | Hypertension, CHF, prior psychosis, active TB, positive tuberculin test, osteoporosis, cataracts, glaucoma, pregnancy (C), diabetes mellitus, gastric disease |
Erythroderma secondary to drug reactions improves or resolves within 2-6 weeks of drug discontinuation. Exfoliative dermatitis associated with psoriasis and eczema may improve within several weeks or months, yet lesions may recur after the first episode in15% of patients. When related to CTCL or another malignancy, erythroderma may persist and be refractory. Thirty percent of subjects with idiopathic erythroderma can exhibit complete remission or 50% partial remission.13
Prognostic studies are scarce and show conflicting results. Early studies report significant mortality secondary to systemic complications.33,45 Low mortality has been reported in studies from recent decades, probably as a result of advances in hospital care and more therapeutic options.43,90 However, a recent retrospective population-based cohort study from Denmark found that 30.8% of patients with erythrodermic psoriasis and 39.6% with erythroderma, died within the first 3 years following hospital admission.2 This high mortality was comparable with older studies.
ConclusionErythroderma is a syndromatic entity and determining its etiology may be a challenge for dermatologists. Clinical features are frequently nonspecific and the dermatologist must search for cause-oriented clues. Multiples biopsies along the course of the disease could potentially enhance diagnostic accuracy. The initial management of erythroderma must include nutrition expert evaluation, fluid imbalance assessment, maintaining skin barrier function, sedative antihistamines and exclusion of secondary bacterial infection. This condition can be life-threatening and may require hospitalization.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Cuellar-Barboza A, Ocampo-Candiani J, Herz-Ruelas ME. Eritrodermia en el adulto: un enfoque práctico para el diagnóstico y tratamiento. Actas Dermosifiliogr. 2018;109:777–790.