Biologic drugs, which are molecules designed to act on specific immune system targets, have been shown to be very effective in treating various dermatological, rheumatological, and systemic diseases. As a group, they have an acceptable safety profile, but their use has been associated with the onset of both systemic and organ-specific inflammatory conditions. True paradoxical reactions are immune-mediated disorders that would usually respond to the biologic agent that causes them. There is still debate about whether certain other adverse reactions can be said to be paradoxical. The hypotheses proposed to explain the pathogenesis of such reactions include an imbalance in cytokine production, with an overproduction of IFN-α and altered lymphocyte recruitment and migration (mediated in part by CXCR3), and the production of autoantibodies. Some biologic therapies favor granulomatous reactions. While most of the paradoxical reactions reported have been associated with the use of TNF-α inhibitors, cases associated with more recently introduced biologic therapies —such as ustekinumab, secukinumab, and ixekizumab—are increasingly common. The study of paradoxical adverse events not only favors better management of these reactions in patients receiving biologic therapy, but also improves our knowledge of the pathogenesis of chronic inflammatory diseases and helps to identify potential therapeutic targets.
Los fármacos biológicos son moléculas dirigidas frente a dianas específicas del sistema inmune que han demostrado una gran efectividad en diversas enfermedades dermatológicas, reumatológicas y sistémicas. A pesar de que en su conjunto presentan un perfil de seguridad adecuado, su uso se ha asociado al desarrollo de enfermedades inflamatorias, limitadas a un órgano o sistémicas. Hablamos de verdaderas reacciones paradójicas cuando estas patologías inmunomediadas normalmente responderían a ese mismo agente biológico que las induce, mientras que el resto de reacciones son aún controvertidas. Las hipótesis patogénicas propuestas para estos procesos incluyen un desbalance de citoquinas, con una sobreproducción de IFN-α y una alteración en el reclutamiento y la migración linfocitaria, mediada en parte por CXCR3, así como la producción de autoanticuerpos. Además, algunos de estos fármacos favorecerían la aparición de reacciones granulomatosas. Aunque las reacciones paradójicas se han descrito en la mayoría de casos para los fármacos anti-TNF-α, cada vez son más frecuentes aquellos asociados con terapias biológicas de más reciente aparición, como ustekinumab, secukinumab o ixekizumab. El estudio de estas reacciones no solo favorece un mejor manejo de los pacientes susceptibles de recibir tratamiento biológico, sino que permite mejorar el conocimiento patogénico de las enfermedades inflamatorias crónicas y de sus posibles dianas terapéuticas.
Psoriasis is a chronic immune-mediated disease with an estimated prevalence of 2.3%.1
It results from a dynamic interaction between a complex genetic predisposition2 and an aberrant immune and epidermal response.3,4 Biologic agents target key points in the pathogenic cycle. Currently, of particular note are those that selectively target 3 molecules, tumor necrosis factor (TNF) α, interleukin (IL) 12/23, and IL-17,4,5 with high efficacy rates in moderate and severe psoriasis, whether or not psoriatic arthritis (PsA) is present.6–8
Although the overall safety profile is acceptable, some patients develop adverse reactions that are not expected according to the mechanism of action. Such reactions have been denoted paradoxical reactions and comprise a de novo or worsening condition that would normally respond to these agents.9,10 Paradoxical reactions often resolve on discontinuation of the drug or switching to another biologic agent, but occasionally additional therapies are required. These can be topical for some mild or moderate dermatologic reactions, but in other cases, systemic immunosuppressants may be necessary.11,12
Search Strategy and Article SelectionA search was performed of the PubMed and EMBASE databases for articles published between 2000 and 2017, inclusive, in English or Spanish. To identify the most relevant articles, we filtered by reviews, clinical trials, and cases series. The search terms used were: “tumor necrosis factor alpha inhibitor,” “TNF,” “infliximab,” “etanercept,” “adalimumab,” “golimumab,” “ustekinumab,” “secukinumab,” “ixekizumab,” “psoriasis,” “paradoxical,” “induced,” and “adverse event.” Finally, the reference lists in the selected articles were reviewed to complete the search.
DefinitionParadoxical adverse reactions constitute a group of adverse effects of biologic therapy and are defined as a de novo or worsening immune-mediated underlying disease that would normally respond to the same therapeutic agent that is causing it.10 Before an adverse reaction is considered as paradoxical, a standardized tool should be applied to assess causality such as, for example, the Naranjo Adverse Drug Reaction Probability Scale (Table 1).13
Naranjo Adverse Drug Reaction Probability Scale: Tool for Evaluating Causality in Adverse Pharmacological Reactions.
| Yes | No | Unknown | |
|---|---|---|---|
| 1. Are there previous conclusive reports of this reaction? | +1 | 0 | 0 |
| 2. Did the adverse event appear after the suspect drug was administered? | +2 | −1 | 0 |
| 3. Did the adverse reaction improve when the drug was discontinued or a specific antagonist was administered. | +1 | 0 | 0 |
| 4. Did the adverse reaction appear when the agent was readministered? | +2 | −1 | 0 |
| 5. Are there alternative causes that could on their own have caused the reaction? | −1 | +2 | 0 |
| 6. Did the reaction reappear when a placebo was given? | −1 | +1 | 0 |
| 7. Was the drug detected in blood (or other fluids) in concentrations known to be toxic? | +1 | 0 | 0 |
| 8. Was the reaction more severe when the dose was increased, or less severe when the dose was decreased? | +1 | 0 | 0 |
| 9. Did the patient have similar reactions to the same or similar drugs in any previous exposure? | +1 | 0 | 0 |
| 10. Was the adverse event confirmed with any objective evidence? | +1 | 0 | 0 |
Total score: probability category, ≤0: doubtful; 1-4: possible; 5-8: probable; ≥9: definitive.
Most paradoxical reactions have been reported in association with anti-TNF-α therapy; however, it is likely that the number of cases will increase with other recently introduced biologic agents.14
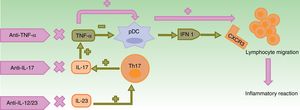
Many different immune-mediated entities have common pathogenic hypotheses. Psoriasis and other inflammatory diseases are the result of interaction of 3 key cytokines: TNF-α, type 1 interferon (IFN), and the IL-23/IL-17 axis, which are interlinked through a triangle in which pharmacologically targeting one leads to changes in the other 2.14
Alteration of TNF-α and IFN-α CounterregulationTNF-α, which is synthesized in various cell types, such as keratinocytes (Th1, Th17, and Th22 cells) and macrophages, stimulates T-cell infiltration, controls apoptosis, and acts on plasmacytoid dendritic cells (pDCs),4 present in both apparently normal skin and in skin lesions of patients with psoriasis3 and other autoimmune diseases.15 In normal conditions, TNF-α silences production of IFN-α by forcing pDC maturation.4,5,14
Blockade of TNF-α results in an excess of IFN-α, which stimulates and amplifies T-cell response and induces the inflammatory reaction (Fig. 1).3,14,16
Pathogenic mechanisms proposed to explain paradoxical reactions.
In normal conditions, TNF-α inhibits pDCs, which produce IFN-α. Use of anti-TNF-α molecules leads to an excess of IFN-α which, in turn, promotes expression of CXCR3 in T cells, thereby allowing migration to the inflamed tissue. The use of other biologic agents with different mechanisms of action such as ustekinumab (anti-IL-12/23) and secukinumab and ixekizumab (anti-IL-17A) indirectly leads to a decrease in TNF-α concentrations, with the aforementioned consequences.
CXCR3: CXC3 chemokine receptor; IFN: interferon; IL: interleukin; pDC: plasmacytoid dendritic cell; Th cell: T helper cell; TNF: tumor necrosis factor.
Increased INF-α induces overexpression of chemokine receptors, such as CXCR3, thus allowing T-cell migration to the skin or any other inflamed tissue.17,18 It also reduces transport of Th1 cells to the initial site of inflammation and these cells are mobilized to other sites, particularly in the skin. This could explain the onset of paradoxical psoriasis in patients with rheumatoid arthritis (RA).19,20
Recruitment of CXCR3+T cells is associated with expression of anti-myxovirus-resistance-protein A (MxA), which highlights, through immunohistochemical staining, local activity of IFN-α in psoriasiform lesions.15,21
IL-23/IL-17 AxisIL-23 induces survival and expansion of Th17 cells, which produce IL-17 and TNF-α. IL-17 also favors the production of TNF and IFN-γ, and recruitment of Th1 cells.14,22
The paradoxical reactions described with ustekinumab could be explained by reduced TNF-α levels caused by blockade of IL-23, with the aforementioned consequences.23
On the other hand, IL-17A blockage with secukinumab or ixekizumab might not be sufficient, given that IL-17F remains inactive.14,22 In support of this, secukinumab has not shown any benefit in patients with Crohn disease (CD). In this disease, loss of control over IL-17, implicated in defenses against fungi, may allow the participation of the fungal microbiota in intestinal inflammation.24
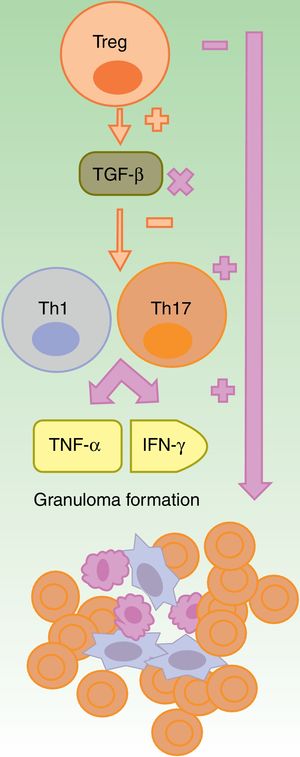
Granulomatous ReactionsThe formation of noncaseating granuloma, both in sarcoidosis and CD, requires Th1 response, with initial participation of IL-1β and IFN-γ, and another later response with participation of TNF-α.25 Anti-TNF-α agents could favor granulomatous reactions through imbalance between Th17 and Treg cells. The lack of efficacy of etanercept in granulomatous diseases and the paradoxical onset of such reactions with its use could be explained by partial neutralization of TNF26–28 and by lack of production of TGF-β, in absence of which there is an overproduction of IFN-γ and TNF-α (Fig. 2).27
Formation of sterile granulomas during therapy with anti-TNF-α agents.
The anti-TNF-α agents are partially effective, in general, in granulomatous diseases such as Crohn disease or sarcoidosis. It is suggested that this is due to an imbalance caused by their use, with an increase in Th17 cell function. Among this class, etanercept causes granulomatous reactions most often because, on the one hand only partial blockade of TNF-α occurs and, on the other, TGF-β is not produced thus allowing overproduction of IFN-γ and TNF-α, which are essential for aseptic granuloma development
IFN: interferon; TGF: transforming growth factor; Th: T helper cell; TNF tumor necrosis factor; Treg: T regulatory cell;.
TNF-α blockade could reduce elimination of cell waste after apoptosis. This would impede autoreactive T and B cell clearance and would facilitate the production of antibodies against nuclear antigens.29,30 In patients with RA, psoriasis, and PsA treated with infliximab and, less frequently, with etanercept and adalimumab, formation of antinuclear antibodies (ANA) (29%-76.7%) and anti-DNA-ds (10%-29%) has been reported. This immunogenicity does not influence the effectiveness of therapy and, generally, does not have any clinical repercussion,10,29 although the development of lupus-like reactions is possible.29,31
Genetic FactorsWhen psoriasiform lesions caused by biologic agents develop, a relationship with different polymorphisms of a single nucleotide has been detected. These polymorphisms impact the genes implicated in cytokine production, such as IL-23R and CTLA-4 or FBXL19. It is thus likely that paradoxical reactions occur in patients with an underlying genetic predisposition and that advances in this field will enable identification of those individuals at risk of developing such reactions.32
Paradoxical ReactionsTrue Paradoxical ReactionsThe term true paradoxical reaction applies to immune-mediated diseases in which the efficacy of the biologic therapy has been clearly established.
Psoriasis and Psoriasiform ReactionsAmong the first paradoxical reactions to be reported and also the most frequent, of note are psoriasiform reactions and psoriasis.
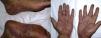
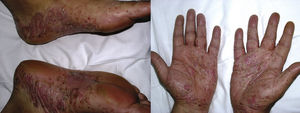
De novo psoriasis occurs in patients with different inflammatory diseases under biologic therapy9 with an incidence of 0.6% to 5.3% (Fig. 3).21 Of the 207 cases included in the review by Collamer and Battafarano,19 43% had AR, 26% seronegative spondyloarthropathy, and 20% inflammatory bowel disease (IBD). The most frequently implicated drug is infliximab, which accounts for 50% of cases,11,33 followed by adalinumab,34 and etanercept, with reactions to certolizumab35 or golimumab9,11,33 being infrequent. These reactions can occur at any time from initiation of treatment, although the mean latency is 14 months and more than two-thirds occur in the first year.33,35 Although there is a predominance of female patients, this appears to simply reflect the epidemiology of the underlying inflammatory diseases and clear risk factors or differences in terms of sex or age have not been established.36 Such reactions have also been reported in children. Concomitant use of another immunosuppressant such as methotrexate does not appear to modify the risk of these reactions.19
New-onset psoriasis can adopt any morphology. The review by Collamer and Battafarano19 establishes palmoplantar pustular psoriasis (56%) followed by plaque psoriasis (50%), and guttate psoriasis (12%) as common presentations with up to 15% of the patients affected by multiple forms at the same time. No prior history of psoriasis is present in 92% of cases. The review by Brown et al.33 only includes those cases of new-onset disease and describes the most frequent forms as plaque psoriasis (44.8%), followed by the palmoplantar pustular form (36.3%). Lesions tend to appear, in descending order of frequency, on the palms and soles, limbs, scalp, and trunk.33 Involvement of the scalp has also been associated with alopecia.37,38 There have also been reports of nail psoriasis, with characteristic changes such as decoloration, oncolysis, and pitting, as well as paronychia.11,19,39
The use of biologic agents can also lead to worsening of psoriasis already present before treatment.11,21,40,41 In these cases, there are no differences in terms of sex and the agent usually implicated is etanercept (62%), followed by infliximab (23%), and adalimumab (15%).21
Worsening of prior psoriasis can occur without any changes in morphology, with a highly variable latency (from 15 days to 32 months). When a change in the morphology of the lesions occurs, the most characteristic form is development of an exacerbation of guttate lesions in patients with plaque psoriasis, in which the plaque lesions are in remission. This worsening occurs from 15 days up to 18 months after start of treatment.21
The histological characteristics of paradoxical psoriasis are indistinguishable from spontaneous psoriasis; however, the presence, at times limited, of eosinophils or plasma cells may reflect drug-induced development.33,42,43
In general, prognosis is favorable and the onset of paradoxical psoriasis does not always require discontinuation of the biologic agent, particularly when the underlying disease is under control and the skin lesions are mild and can be tolerated. In these cases, a specific treatment, usually topical, is applied.10,19,21 Despite maintaining the drug, total resolution (32.9%) or partial improvement (57.3%) of the lesions is common. Complete remissions are achieved most frequently by withdrawing the drug (47.7%).33 Switching to another anti-TNF-α agent is possible, but lesions may reoccur given this adverse reaction is a class effect.12,44 Up to 44.9% of patients might not improve with switching to another anti-TNF-α agent and 9% would only achieve a partial improvement.33 Alternatively, it has been suggested to use a drug with a different mechanism of action, such as ustekinumab, with good response.17,45 However, this latter drug has been associated with paradoxical worsening of psoriasis23 and even PsA in patients with plaque psoriasis.46
Joint InflammationThe onset of paradoxical joint inflammation has been reported above all in patients with IBD during anti-TNF-α therapy, with the appearance of arthritis in up to 11% of cases, essentially with peripheral involvement of the hands and carpi and, to a lesser degree, in form of spondyloarthropathy.47,48
There are also numerous cases of new-onset PsA in patients with psoriasis treated with ustekinumab and anti-TNF-α agents, especially etanercept (45.4%).49 Often, joint inflammation occurs in association with severe skin lesions and the simultaneous appearance of distal interphalangeal arthritis with dactylitis, pustular psoriasis, and nail involvement has been reported.50 Psoriasis and PsA have common pathogenic pathways such as the participation of Th17 cells.51 It is therefore possible to demonstrate that, in PsA induced by ustekinumab and probably other biologic agents, there are cases of early-onset arthritis resulting from the cytokine imbalance caused by the agent and cases of late onset disease. Subclinical PsA becomes manifest due to the lower efficacy of the biologic agent in the joints, making clinical expression unavoidable.46,52
Inflammatory Bowel DiseaseBoth paradoxical worsening of IBD and new-onset cases induced by anti-TNF-α agents have been reported in patients with different inflammatory diseases, including psoriasis and PsA.53,54 There is a clear predominance of development of CD (50%) or similar forms (43.7%) over ulcerative colitis (UC) (6.25%). The drug responsible for more than 80% of cases is etanercept53 which, unlike infliximab and adalimumab, has not clearly demonstrated efficacy in IBD.27,55 In most cases, there is complete response on switching biologic agent.53,55
Unlike the situation with infliximab or adalimumab, the development of IBD with anti-IL-17 agents is considered a nonparadoxical adverse effect. IL-17 inhibition is not effective in IBD, probably because of the protective function of IL-17 on the intestinal epithelium.56,57 Secukinumab58 and ixekizumab59 (IL-17A antagonists), as well as brodalumab60 (IL-17RA antagonist), have been associated with exacerbations and, to a lesser extent, with induction of IBD. In general terms, the adverse reaction has a low incidence similar to the association between IBD and psoriasis; however, brodalumab is formally contraindicated in active CD and precaution is recommended when using other aforementioned drugs in patients with IBD,56 with preference for alternatives that act on other pathways, such as ustekinumab.
Hidradenitis SuppurativaAnti-TNF-α agents can be effective in the treatment of hidradenitis suppurativa (HS).61 At least 30 cases attributed to this therapy have been published in patients with inflammatory diseases, including psoriasis.62,63 Although HS can be associated with other diseases such as CD,64 onset soon after starting the drug in different inflammatory diseases points to a likely link with the biologic agent.62,63 Moreover, at the same time, 44% of patients developed other paradoxical reactions such as psoriasis, alopecia areata (AA), CD, and HS. The epidemiology of paradoxical HS is similar to spontaneous HS, with a predominance in women, smokers, and overweight or obese individuals. Adalimumab is the drug associated most frequently with these reactions (48%).63 In most cases, partial or complete improvement can be achieved by withdrawal or switching to another drug and specific treatment for HS.62,63
Debatable Paradoxical ReactionsSeveral immune-mediated diseases occur with use of biologic agents. In many of these diseases, the efficacy of these agents has not been formally confirmed although clinical and pathophysiological data support their use. Thus, at present, these cannot be considered true paradoxical reactions.
Alopecia AreataThe onset of patchy AA (79%) has been reported in the scalp and chin of patients with psoriasis, RA, and IBD treated with anti-TNF-α agents.65 Although AA can appear as an associated autoimmune manifestation in predisposed patients,66 the male predominance and simultaneous development (25%) of vitiligo or psoriasis suggests that the condition may be drug induced.65 In parallel, psoriatic alopecia/AA-like reactions to anti-TNF-α are considered a disease in which erythematous lesions develop in the dermis in association with plaques of alopecia, which histologically show psoriasiform epidermal features and AA changes in the dermis.43,67,68 In most cases, it is possible to continue with the drug and administration of topical or intralesional corticosteroids produces satisfactory outcomes.65,67,68 Recently, janus kinase (JAK) inhibitors have been shown to be effective in AA, whether administered topically or systemically, Topical use could represent an alternative in AA induced by biologic agents.69
Three cases of AA have also been reported in patients with psoriasis treated with ustekinumab,42 although given the implication of IL-12 in the pathogenesis of AA, this agent would be expected to have a favorable effect in the disease.66,70 In addition to shared pathogenic pathways with other entities, the Renbök phenomenon, that is, normal hair growth in psoriatic lesions in patients who also present AA42 could explain why the immunoregulation that resolves psoriasis unmasks latent AA due to the psoriatic inflammation itself.70,71
VitiligoElevated TNF-α in serum of patients with vitiligo provides a rationale for treatment of this dermatosis with anti-TNF-α agents without achieving, in general terms, good outcomes.41–72 In contrast, there have been 18 reports of de novo cases and 18 of worsening of preexisting vitiligo in patients with chronic inflammatory diseases, the most frequent being psoriasis, treated with anti-TNF-α agents, ustekinumab, and secukinumab.73 Despite the association, at least in epidemiological terms, of psoriasis and vitiligo,74 the temporal relationship and absence of predisposing factors in most patients points to a pharmacologically induced origin.73,75 In 72.2% of reactions, the trigger has been infliximab and adalimumab whereas 22.2% have been attributed to ustekinumab and secukinumab. No cases have been reported with etanercept. In most cases, stabilization or improvement of vitiligo was achieved even though the biologic agent was maintained,73 and so the decision to withdraw it should be made on an individual basis.
Acneiform ReactionsTo a lesser extent, infliximab, adalimumab,76 and etanercept77 have been associated with the onset of cystic and comedonal acne. Latency is approximately 2 months from starting treatment and there are no predictive factors for its development. In many cases, additional treatment with isotretinoin could be sufficient.76
Lichen Planus and Lichenoid ReactionsThere are more than 20 case reports in the literature of lichen planus (LP), LP pilaris (LPP), and oral LP, as well as lichenoid reactions, caused by TNF-α inhibitors.78,79 Although anti-IL-17 therapy can be of use in the treatment of LP,80 cases have been reported of lichenoid mucositis in relation to secukinumab.81
Interstitial Granulomatous DermatisInterstitial granulomatous dermatis occurs spontaneously in association with diseases such as RA, systemic lupus erythematosus (SLE), and hematological neoplasms.82 The onset of these lesions in patients with RA or PsA treated with anti-TNF-α agents has been described, with complete resolution of lesions after withdrawal.83,84
LupusAnti-TNF-α drugs, in particular etanercept and infliximab, can cause lupus and lupus-like reactions.30,85 Unlike classic drug-induced lupus (DIL), lupus caused by anti-TNF-α agents occurs predominantly in women, with a female:male ratio of 5:1. This type of lupus is characterized by elevated anti-DNA-ds titers (70%-90%), greater complement consumption (59%), and a weaker link with anti-histone antibodies (57%) present in 95% of classic DILs.10,30 Anti-TNF-α induced lupus has manifestations similar to the classic form, although with greater skin involvement, particularly with etanercept, and a marked predominance of photosensitivity, malar erythema, and features of subacute or chronic cutaneous lupus.30 There have also been reports of onset of lupus panniculitis with adalinumab.86
On the other hand, it is worth mentioning the difficult distinction, based almost on the timing of onset, between a rhupus syndrome—that is, combination of SLE and RA—and SLE that presents in a patient with RA treated with an anti-TNF-α agent.29
In all cases, the decision to withdraw the drug is optional, although this does not necessarily preclude use of another agent of the same therapeutic class.10,29,30,47
VasculitisVasculitis can be caused by use of biologic agents such as rituximab and anti-TNF-α agents; in fact, the latter drugs have been become the most common cause of drug-induced vasculitis, superseding others such as propylthiouracil or hidralazine.87 More than 200 cases have been reported of these reactions to anti-TNF-α agents in patients with RA, CD, PsA and psoriasis, among others.41,87,88 The characteristics are similar to those caused by other therapies and onset tends to occur during the first year in middle-aged women.87,88 In general, the cutaneous forms are the most common and manifest as purpura and, less frequently, as nodules or ulcerative lesions.88 There have also been reports of systemic vasculitis, such as Takayasu arteritis,89 and Henoch-Schonlein purpura.90 In 90% of cases, withdrawal of the anti-TNF agent is effective for resolution of vasculitis.41,87,88
SarcoidosisThere are numerous reports of sarcoidosis induced by anti-TNF-α agents in patients with IBD,91,92 cutaneous psoriasis,93 RA, ankylosing spondylitis (AS), and PsA.25,26,94,95 More than half the published cases involved middle-aged women in treatment with etanercept, whereas the least frequently involved agents were infliximab and adalimumab.26,94 Drug-induced sarcoidosis behaves similarly to spontaneous forms. The most common clinical manifestations are pulmonary and cutaneous ones in the form of nodular erythema and cutaneous nodules. In 89% of patients, remission is observed after withdrawal of the drug.10,26,94 The onset of sarcoidosis has also been reported in a female patient with PsA treated with ustekinumab with good response to withdrawal,96 although this drug, unlike infliximab and adalimumab, does not appear to be useful in spontaneous sarcoidosis.97
ConclusionsBiologic agents used in psoriasis target specific molecules such as TNF-α, IL-12/23, and IL-17 (Table 2). When part of these immune pathways is blocked, a cytokine imbalance may arise in predisposed individuals leading to an inflammatory process. A single organ, often the skin, may be involved or the process can be systemic. When the drug that induces this inflammatory disease is an agent usually used to treat that disease, a true paradoxical reaction is considered to have occurred. In general, these events have a low incidence; however, with the increased use of new biologic agents, we might expect an increase in the number of cases as well as in the range of clinical expression of these entities. It is thus recommended to closely monitor patients receiving biologic treatment to detect such reactions. It is also desirable to assess degree of causal association between the paradoxical reaction and the biologic agent using standard methods. This would extend clinical knowledge and increase understanding of the immune mechanisms underlying these effects. Finally, the development of new biologic agents will increase the number of alternatives for controlling the underlying disease in those patients who develop a clinically relevant paradoxical reaction to a given agent.
Key Points.
| • Paradoxical reactions comprise a de novo or worsening condition that would normally respond to the same agent that induces it. |
| • They have been reported essentially with use of anti-TNF-α agents, where they are considered a class effect; however, cases caused by other biologics such as ustekinumab and anti-IL-17 agents are increasingly frequent. |
| • Direct or indirect blockade of TNF-α results in an excess of IFN-α, which stimulates T cell response and migration, thus inducing the inflammatory reaction. |
| • Paradoxical psoriasis is the most common type described. The most frequent forms are pustular psoriasis and plaque psoriasis, and palmoplantar involvement predominates. |
| • Other skin diseases caused by use of biologic agents are hidradenitis suppurativa, alopecia areata, vitiligo, interstitial granulomatous dermatitis, and acneiform reactions. |
| • The appearance of a paradoxical reaction does not always require withdrawal of the agent; at times, it may be sufficient to administer topical or systemic treatment for the reaction. If the reaction is severe or extensive, switching to another biologic agent should be considered. |
Jose Manuel Carrascosa has received speaker fees, and/or participated in clinical trials and/or advisory boards for Abbvie, Pfizer, Janssen, Novartis, Lilly, and Amgen.
Please cite this article as: Munera-Campos M, Ballesca F, Carrascosa JM. Reacciones paradójicas de los tratamientos biológicos utilizados en psoriasis: revisión de la literatura. Actas Dermosifiliogr. 2018;109:791–800.