A fixed drug eruption (FDE) may be caused by different drugs, especially NSAIDs, paracetamol, and antibiotics. In recent years, cases due to the quinine in tonic water have been reported. We report a case of FDE caused by quinine after drinking a gin and tonic and we review the cases published to date. We analyze the current legislation on the amount of quinine allowed in drinks and the differences between the different brands sold in Spain.
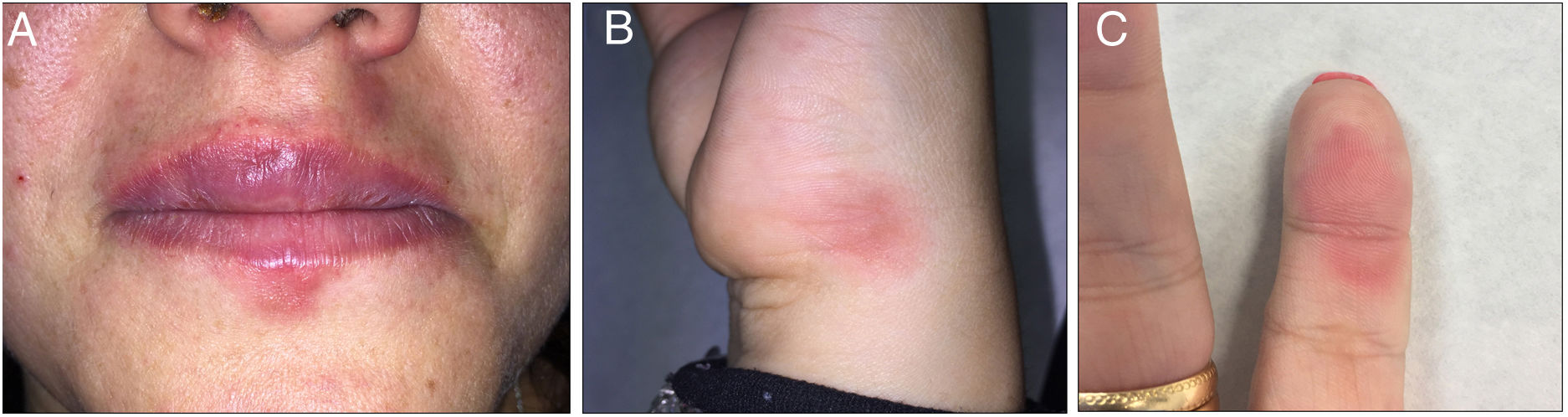
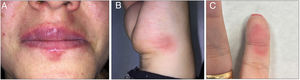
A 32-year-old woman visited our department with repeated outbreaks of erythematous-violaceous lesions with irregular, edematous margins in the perioral region, the 5th finger of the left hand, and the lateral surface of the right hand, compatible with FDE (Fig. 1). The patient occasionally took diclofenac and metamizole but did not improve. On examining the patient’s full medical history, we discovered that the lesions coincided with the consumption of gin and tonic; quinine was therefore suspected.
A skin-patch test performed with Schweppes® tonic water was negative, and epicutaneous tests with the tonic, with quinine in petrolatum at 20%, and in aqueous solution at 1%, were also negative after 48 and 96 h. An oral provocation test was positive, with appearance of the lesions a few hours after consumption. The patient has remained asymptomatic since removing tonic water from her diet.
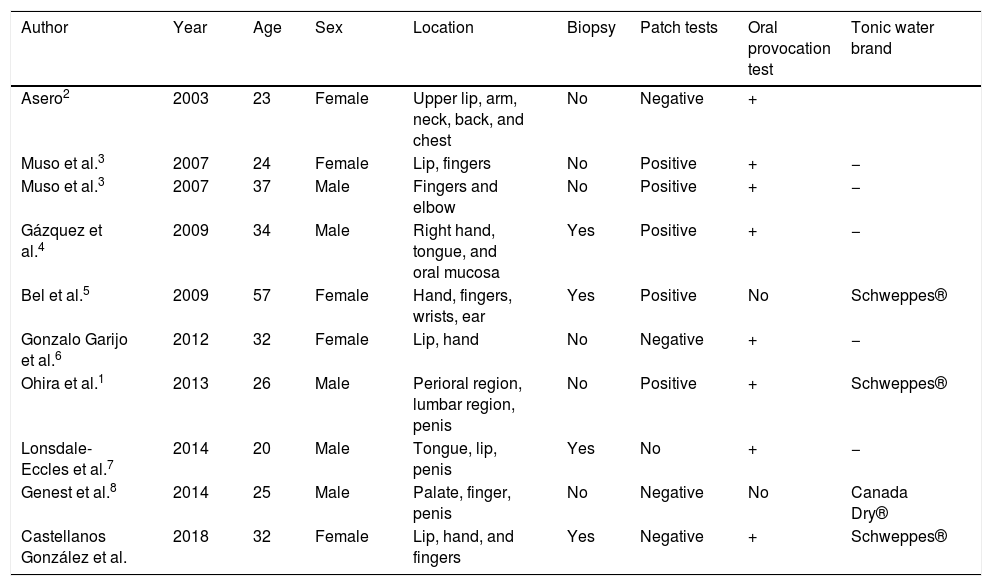
FDE is characterized by the appearance of erythematous-violaceous, eczematous or bullous lesions that cause pruritus or a burning sensation, always in the same locations, after exposure to a specific agent in sensitized patients. It is considered to be a type of delayed hypersensitivity and is caused by many drugs. On rare occasions, foods may be involved, such as strawberries, green beans, asparagus, and cashews.1In these cases, it is called fixed food eruption. FDE due to quinine was first reported in 20032 and 11 cases have since been published, coinciding with the increased consumption of tonic water, particularly in combination with gin (Table 1).
Summary of cases of FDE published to date.a
| Author | Year | Age | Sex | Location | Biopsy | Patch tests | Oral provocation test | Tonic water brand |
|---|---|---|---|---|---|---|---|---|
| Asero2 | 2003 | 23 | Female | Upper lip, arm, neck, back, and chest | No | Negative | + | |
| Muso et al.3 | 2007 | 24 | Female | Lip, fingers | No | Positive | + | − |
| Muso et al.3 | 2007 | 37 | Male | Fingers and elbow | No | Positive | + | − |
| Gázquez et al.4 | 2009 | 34 | Male | Right hand, tongue, and oral mucosa | Yes | Positive | + | − |
| Bel et al.5 | 2009 | 57 | Female | Hand, fingers, wrists, ear | Yes | Positive | No | Schweppes® |
| Gonzalo Garijo et al.6 | 2012 | 32 | Female | Lip, hand | No | Negative | + | − |
| Ohira et al.1 | 2013 | 26 | Male | Perioral region, lumbar region, penis | No | Positive | + | Schweppes® |
| Lonsdale-Eccles et al.7 | 2014 | 20 | Male | Tongue, lip, penis | Yes | No | + | − |
| Genest et al.8 | 2014 | 25 | Male | Palate, finger, penis | No | Negative | No | Canada Dry® |
| Castellanos González et al. | 2018 | 32 | Female | Lip, hand, and fingers | Yes | Negative | + | Schweppes® |
Quinine is a natural alkaloid obtained from the bark of the Cinchona officinalis tree, of the Rubiaceae family. It was used traditionally to treat malaria, but its current principal use is as an aroma in tonic water, due to its bitter flavor; this is now considered to be the main source of quinine in the diet. It is considered toxic at doses of greater than 1 g per day and causes gastrointestinal, visual, and auditory problems. Nevertheless, the legislation governing its use as a food is heterogeneous at the national level. Thus, in the US, the FDA limits its concentration in tonic water to 83 mg/L, whereas in Japan, quinine is classed as a drug and its use as a food additive is not permitted. The European Union, in Regulation (CE) 872/2012, of 1 October 2012, establishes a maximum limit of quinine in soft drinks of 100 mg/L. Furthermore, it must be indicated on the list of ingredients.9
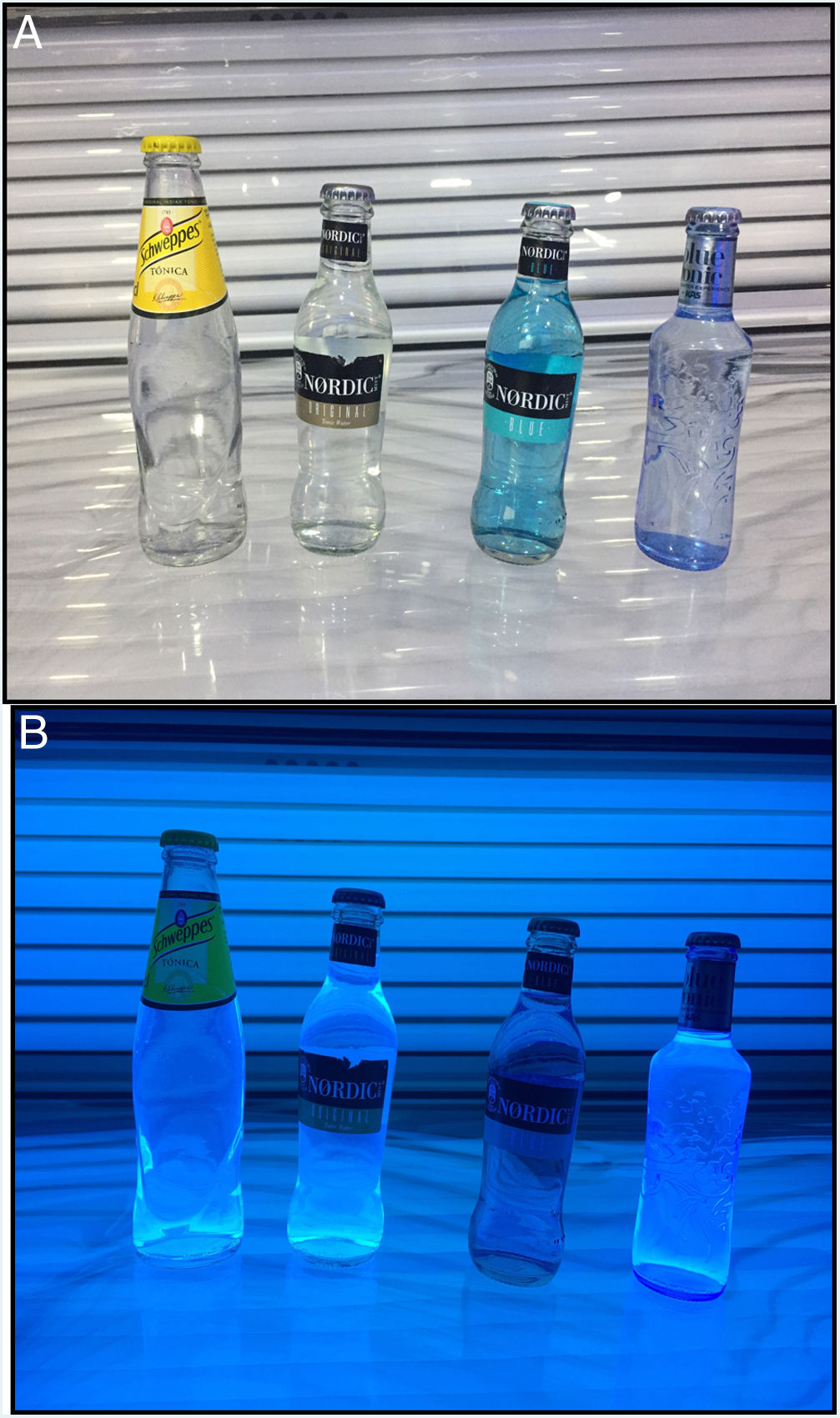
The lesions in our patient appeared on consuming Schweppes® tonic water but not Nordic Blue®. This fact led us to wonder whether differences exist in the amount of quinine present in different tonic waters.
Labeling does not mention quinine concentration in drinks sold in Spain. However, this has been studied by the scientific community and high-resolution liquid chromatography has shown that the quantity of quinine differs depending on the commercial brand. The concentration is higher in Schweppes® than in Nordic Mist® (60.3 mg/L vs 55.0 mg/L, respectively).10 Other authors found that some brands had twice as much quinine as others.11 Ohira et al.1 detected peaks in the chromatogram only in Schweppes® and Canada Dry®. Nevertheless, the levels of quinine in all the brands studied are below the maximum levels allowed by current technical health regulations, and, according to consumption data, estimated consumption is below the toxic dose. In our case, we exposed different brands to ultraviolet light and found that the only brand that did not fluoresce was precisely the brand that did not cause lesions in our patient (Fig. 2A and B). This is a drink without quinine and cannot therefore be considered a tonic water but rather a soft drink with extracts. It is bottled in a similar form and is sold in supermarkets alongside the tonic waters, which gives rise to confusion. It may, however, be an alternative to tonic water in cases of fixed food eruption due to quinine, as its flavor is similar.
We highlight the importance of taking a thorough medical history to find the agent responsible for an FDE, as it is not always a drug. We also note that not all tonic waters on the market contain the same amount of quinine. We propose an alternative to tonic water, with a similar flavor, for patients with FDE due to quinine.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Castellanos-González M, et al. Cuando tomarse un gin-tonic se convierte en una mala experiencia: exantema fijo medicamentoso por quinina. Actas Dermosifiliogr. 2020;111:178–180.