Apalutamide is an androgen receptor inhibitor approved by the US Food and Drug Administration in 2018 for use against certain prostate cancers. It was approved for use in Europe in March 2021.1 Skin rashes, hypothyroidism, and bone fractures are among apalutamide's common adverse effects.1
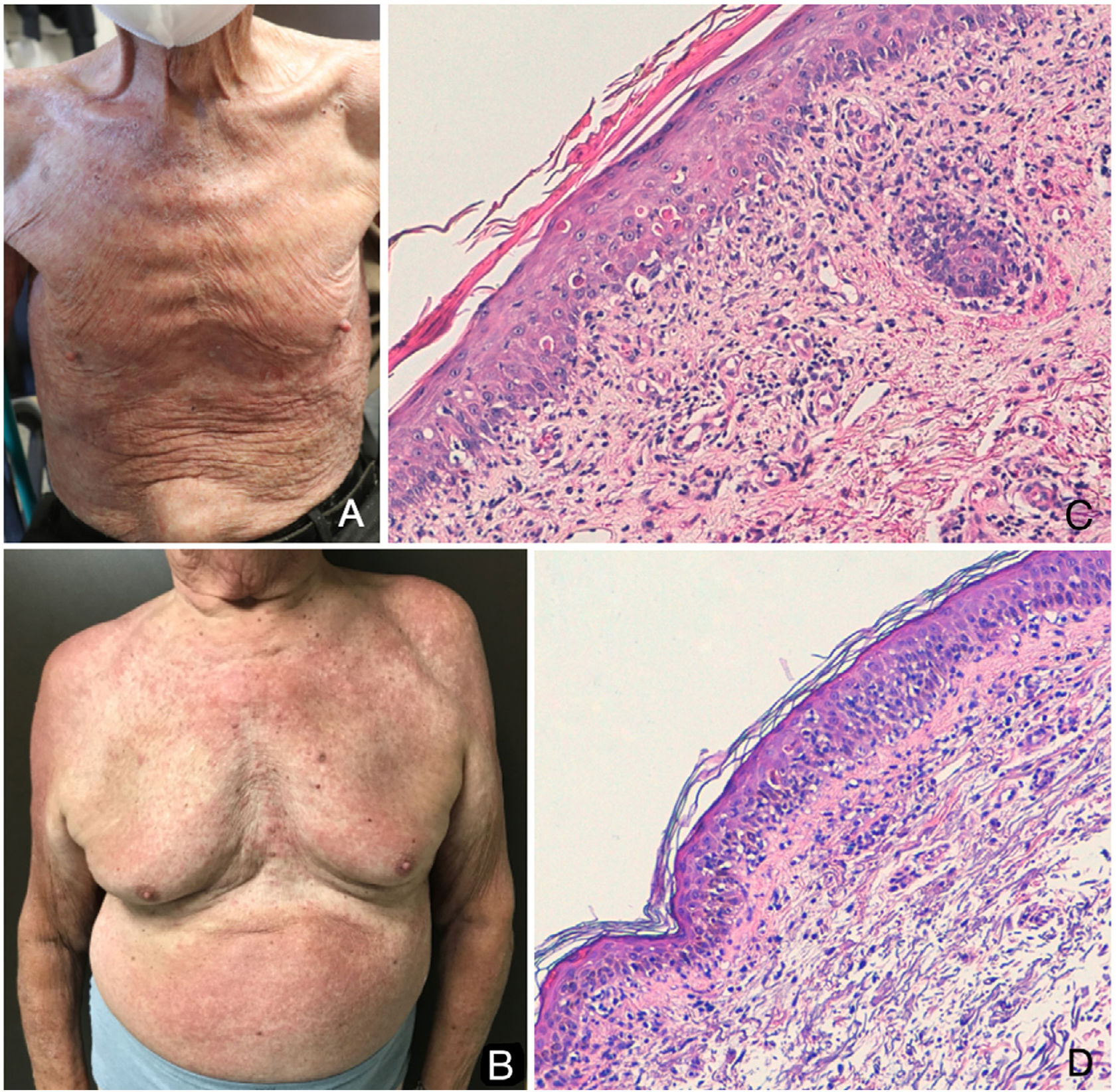
Case DescriptionsAn 89-year-old man with a history of prostate cancer in treatment with apalutamide for 2 months complained of a skin rash that had first appeared 30 days earlier. The macules and intense peeling started on his chest and spread to involve 80% of his body (Fig. 1A). Another 89-year-old man with a history of prostate cancer also consulted us for erythematous papules over large areas of his trunk and limbs. This patient had been in treatment with apalutamide for 1 month and the lesions had appeared 7 days before he consulted us. The rash started on his chest and arms and spread upward and downward, sparing areas not exposed to the sun (Fig. 1B). Both patients reported intense itching and general discomfort. The first man was admitted to hospital because his overall condition had declined. In both cases, the biopsies demonstrated a lymphocytic infiltrate in the superficial dermis with significant damage at the dermal-epidermal junction and an abundance of apoptotic keratinocytes, suggesting an adverse drug reaction (Fig. 1C and 1D). The rashes resolved after apalutamide was stopped and a short course of systemic corticosteroids was prescribed.
A, Erythroderma on the anterior surface of the trunk of the first patient. B, Erythematous maculopapular rash on the front of the trunk and sides of the shoulders and arms of the second patient. C and D, Skin biopsies of the chest of each patient showed a predominantly lymphocytic infiltrate in the superficial dermis, associated with significant damage at the dermal–epidermal interface and an abundance of apoptotic keratinocytes (hematoxylin–eosin, magnification ×200).
Reports of adverse skin reactions to apalutamide have been scarce in the literature to date. Such reactions are described in the summary of product characteristics for apalutamide and in a report of a series of 68 Japanese patients in clinical trials prior to approval; the adverse skin effects were described ambiguously as rashes, maculopapular rashes, and generalized rashes.1,2 Such skin lesions developed at one time or another in 51.7% of the patients on apalutamide, and there was a significant correlation between the presence of rashes and drug plasma concentration. In contrast, no correlation was detected between exposure indicated by plasma concentrations and symptom severity, nor between the incidence of lesions and any patient characteristics at baseline.2 The frequency and severity of rashes in the Japanese series was greater than has been observed in patients in other geographic areas. A case of epidermal necrolysis due to apalutamide has been reported.3
Other androgen receptor inhibitors, however, have more often been reported to cause adverse skin reactions. Noteworthy are photosensitivity reactions associated with flutamide and bicalutamide, and case reports of a maculopapular rash and acute generalized exanthematous pustulosis related to enzalutamide.4,5
ConclusionsWe have presented 2 cases of toxic skin eruptions related to apalutamide, a drug that was recently introduced into our clinical practice; our report shows correlations between histology and symptoms. This drug is expected to be more widely used in the future, and dermatologists should therefore become aware of the characteristics of adverse reactions to it in order to be able to manage both diagnosis and treatment.
Conflicts of InterestThe authors declare that they have no conflicts of interest.