Chronic leg ulcers (CLUs) are a common condition in elderly patients. They require long-term care with various topical treatments and dressings; therefore, allergic contact dermatitis (ADC) is a frequent complication (46.0–82.5%).1–3 Positive results for various allergens have been reported in 70–80% of patients with CLU.1,2
The primary objective of the present study was to describe the prevalence of sensitization and its clinical relevance in patients with CLU seen at a specialized unit. The secondary objective was to know the association between the positive results detected and the etiology or time since onset of the CLU. We performed a retrospective review of patients with CLU, with or without associated ADC, who underwent patch testing between May 2003 and April 2018. The standard series and the chronic ulcer series of the Spanish Contact Dermatitis and Skin Allergy Research Group (Grupo Español de Investigación en Dermatitis de Contacto y Alergia Cutánea) were applied.
We analyzed 93 patients (38 men [40.9%], 55 women [59.1%]; median [interquartile range] age, 72 [62–80] years]). The ulcers were mainly venous in origin (77.4%), with a median time since onset of 37 weeks (interquartile range, 14.5–96). The clinical diagnosis of perilesional ADC was made in 69 patients (74.2%). At least 1 positive patch test result was recorded in 83 patients (91.4%). The median number of positive results per patient was 2 (interquartile range, 1–4). Polysensitization (≥3 allergens) was detected in 36.6%. The presence of ADC was associated with a greater number of positive results (P<.05, t test). Analysis of the allergens individually revealed that balsam of Peru was the only one associated with ADC (P<.05, χ2 test). No statistically significant association was detected between the number of positive results, etiology, and time since onset.
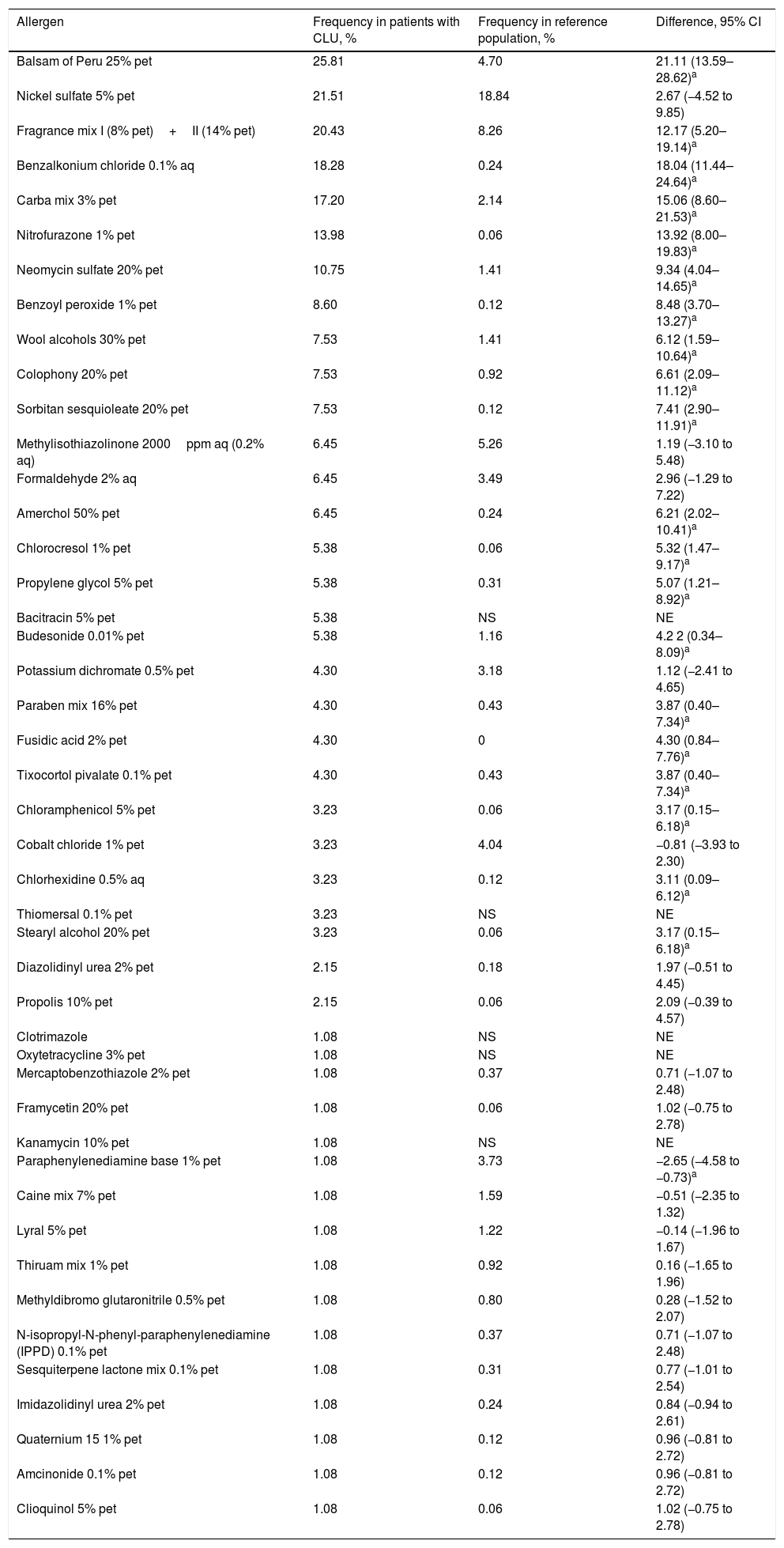
The frequency of sensitization among patients with CLU was generally higher than that reported in the reference population. Table 1 shows the frequency of sensitization and compares it with that of the reference population.
Comparison of the Frequency of Sensitization Compared with the Total Number of Positive Patch Tests for the Most Common Allergens in Our Case Series (>1%) and from Another Study Performed in Our Reference Population.
| Allergen | Frequency in patients with CLU, % | Frequency in reference population, % | Difference, 95% CI |
|---|---|---|---|
| Balsam of Peru 25% pet | 25.81 | 4.70 | 21.11 (13.59–28.62)a |
| Nickel sulfate 5% pet | 21.51 | 18.84 | 2.67 (−4.52 to 9.85) |
| Fragrance mix I (8% pet)+II (14% pet) | 20.43 | 8.26 | 12.17 (5.20–19.14)a |
| Benzalkonium chloride 0.1% aq | 18.28 | 0.24 | 18.04 (11.44–24.64)a |
| Carba mix 3% pet | 17.20 | 2.14 | 15.06 (8.60–21.53)a |
| Nitrofurazone 1% pet | 13.98 | 0.06 | 13.92 (8.00–19.83)a |
| Neomycin sulfate 20% pet | 10.75 | 1.41 | 9.34 (4.04–14.65)a |
| Benzoyl peroxide 1% pet | 8.60 | 0.12 | 8.48 (3.70–13.27)a |
| Wool alcohols 30% pet | 7.53 | 1.41 | 6.12 (1.59–10.64)a |
| Colophony 20% pet | 7.53 | 0.92 | 6.61 (2.09–11.12)a |
| Sorbitan sesquioleate 20% pet | 7.53 | 0.12 | 7.41 (2.90–11.91)a |
| Methylisothiazolinone 2000ppm aq (0.2% aq) | 6.45 | 5.26 | 1.19 (−3.10 to 5.48) |
| Formaldehyde 2% aq | 6.45 | 3.49 | 2.96 (−1.29 to 7.22) |
| Amerchol 50% pet | 6.45 | 0.24 | 6.21 (2.02–10.41)a |
| Chlorocresol 1% pet | 5.38 | 0.06 | 5.32 (1.47–9.17)a |
| Propylene glycol 5% pet | 5.38 | 0.31 | 5.07 (1.21–8.92)a |
| Bacitracin 5% pet | 5.38 | NS | NE |
| Budesonide 0.01% pet | 5.38 | 1.16 | 4.2 2 (0.34–8.09)a |
| Potassium dichromate 0.5% pet | 4.30 | 3.18 | 1.12 (−2.41 to 4.65) |
| Paraben mix 16% pet | 4.30 | 0.43 | 3.87 (0.40–7.34)a |
| Fusidic acid 2% pet | 4.30 | 0 | 4.30 (0.84–7.76)a |
| Tixocortol pivalate 0.1% pet | 4.30 | 0.43 | 3.87 (0.40–7.34)a |
| Chloramphenicol 5% pet | 3.23 | 0.06 | 3.17 (0.15–6.18)a |
| Cobalt chloride 1% pet | 3.23 | 4.04 | −0.81 (−3.93 to 2.30) |
| Chlorhexidine 0.5% aq | 3.23 | 0.12 | 3.11 (0.09–6.12)a |
| Thiomersal 0.1% pet | 3.23 | NS | NE |
| Stearyl alcohol 20% pet | 3.23 | 0.06 | 3.17 (0.15–6.18)a |
| Diazolidinyl urea 2% pet | 2.15 | 0.18 | 1.97 (−0.51 to 4.45) |
| Propolis 10% pet | 2.15 | 0.06 | 2.09 (−0.39 to 4.57) |
| Clotrimazole | 1.08 | NS | NE |
| Oxytetracycline 3% pet | 1.08 | NS | NE |
| Mercaptobenzothiazole 2% pet | 1.08 | 0.37 | 0.71 (−1.07 to 2.48) |
| Framycetin 20% pet | 1.08 | 0.06 | 1.02 (−0.75 to 2.78) |
| Kanamycin 10% pet | 1.08 | NS | NE |
| Paraphenylenediamine base 1% pet | 1.08 | 3.73 | −2.65 (−4.58 to −0.73)a |
| Caine mix 7% pet | 1.08 | 1.59 | −0.51 (−2.35 to 1.32) |
| Lyral 5% pet | 1.08 | 1.22 | −0.14 (−1.96 to 1.67) |
| Thiruam mix 1% pet | 1.08 | 0.92 | 0.16 (−1.65 to 1.96) |
| Methyldibromo glutaronitrile 0.5% pet | 1.08 | 0.80 | 0.28 (−1.52 to 2.07) |
| N-isopropyl-N-phenyl-paraphenylenediamine (IPPD) 0.1% pet | 1.08 | 0.37 | 0.71 (−1.07 to 2.48) |
| Sesquiterpene lactone mix 0.1% pet | 1.08 | 0.31 | 0.77 (−1.01 to 2.54) |
| Imidazolidinyl urea 2% pet | 1.08 | 0.24 | 0.84 (−0.94 to 2.61) |
| Quaternium 15 1% pet | 1.08 | 0.12 | 0.96 (−0.81 to 2.72) |
| Amcinonide 0.1% pet | 1.08 | 0.12 | 0.96 (−0.81 to 2.72) |
| Clioquinol 5% pet | 1.08 | 0.06 | 1.02 (−0.75 to 2.78) |
Abbreviations: aq, aqueous solution; CLU, chronic leg ulcer; NS, not studied; pet, petrolatum.
In patients with CLU, sensitization is caused by the application of multiple allergens under occlusion for long periods over tissue where venous circulation and the skin barrier are altered and undergoing repair, with abundant inflammatory infiltrate of lymphocytes and Langerhans cells.4,5
It has also been shown that sensitization to any of the products used to treat CLU followed by ADC points to a poorer course of the lesions.1
The prevalence of positive patch test results in patients with CLU is high.1,2 However, our study revealed the prevalence to be higher than reported (91.4%), possibly because some of the patients had positive results without symptoms of ADC. This finding was also reported by Barbaud et al.6 in a series of patients with CLU in which 57% of 108 patients without ADC had positive patch test results.
Polysensitization is common, has mainly been observed long after onset, and has been associated with delayed scarring.6–8 Nevertheless, we did not find this association, possibly because of the sample size.
The presence of ADC was associated with sensitization to a greater number of allergens and, on an individual basis, to positive results with balsam of Peru (Myroxylon pereirae resin). This substance can be found in our setting in dressings such as Linitul and Tulgrasum and is the most commonly positive allergen in patch tests.1
Nickel sulfate generated the second highest number of positive results, although this may be of little relevance, since the frequency recorded does not differ from that of the general population. In our series, nickel sulfate was followed in prevalence by fragrance mix I and II, benzalkonium chloride (disinfectant marketed as cutaneous solutions), carba mix (rubber products, especially gloves), nitrofurazone (antibiotic marketed under the name Furacin or Dertrase), and neomycin (antibiotic in some ointments such as Menaderm, Tisuderma, Iruxol Neo, and Blastoestimulina).9
The presence of some topical corticosteroids as positive results in a small percentage of patch tests is noteworthy, since this pharmacologic group is the basis of treatment of ACD. Positive results are found between classes A (tixocortol) and B (budesonide, amcinonide). Therefore, if such a situation is suspected, class D corticosteroids (hydrocortisone butyrate, prednicarbate, betamethasone valerate or dipropionate, or clobetasol dipropionate) should be used, and triamcinolone (class B) should be avoided.
In conclusion, our study confirms the high frequency of sensitization in patients with CLU. This is probably induced by topical treatment continuously applied on damaged skin. Therefore, it is advisable to evaluate the possibility of ADC in patients with CLU and perilesional dermatitis. Patch testing would make it possible to remove the causative allergens and optimize treatment regimens.
Conflicts of InterestThe authors declare that they have no conflicts of interest.