Toxic epidermal necrolysis (TEN) is a life-threatening blistering skin disease induced by a medication. The incidence and main features of TEN in patients with hematopoietic stem cell transplantation (HSCT) have been poorly evaluated and there are only a few documented cases in the literature.
The extensive blistering and desquamation presentation of the cutaneous acute graft versus host disease (GVHD) is the most severe variant of this condition and it mimics a TEN.
Both skin diseases have exhibit clinical and histological similarities which makes it virtually impossible to differentiate between them in the context of HSCT.
Clinical casesPatient one: A 47-year-old man with myelodysplastic syndrome undergoing allogeneic HSCT. On day +84, the patient referred the development of a pruritic generalized acute erythema, associating fever and lichenoid erosions in the oral mucosa. A severe GVHD was suspected and intensification of treatment with sirolimus, ruxolitinib and extracorporeal photopheresis was decided. However, the eruption progressed with blistering and epidermolysis. Given the lack of response, TEN was suspected and all not strictly necessary drugs were withdrawn, while immunosuppressive treatment is reduced. From this moment on, the patient improved and epithelization of all the detached surface was achieved in some weeks. After numerous complications, on the day +229, the patient was released from the hospital with cutaneous, hepatic and ocular chronic GVHD, that was treated with immunosuppressive therapy until day +322, when he died from respiratory infection.
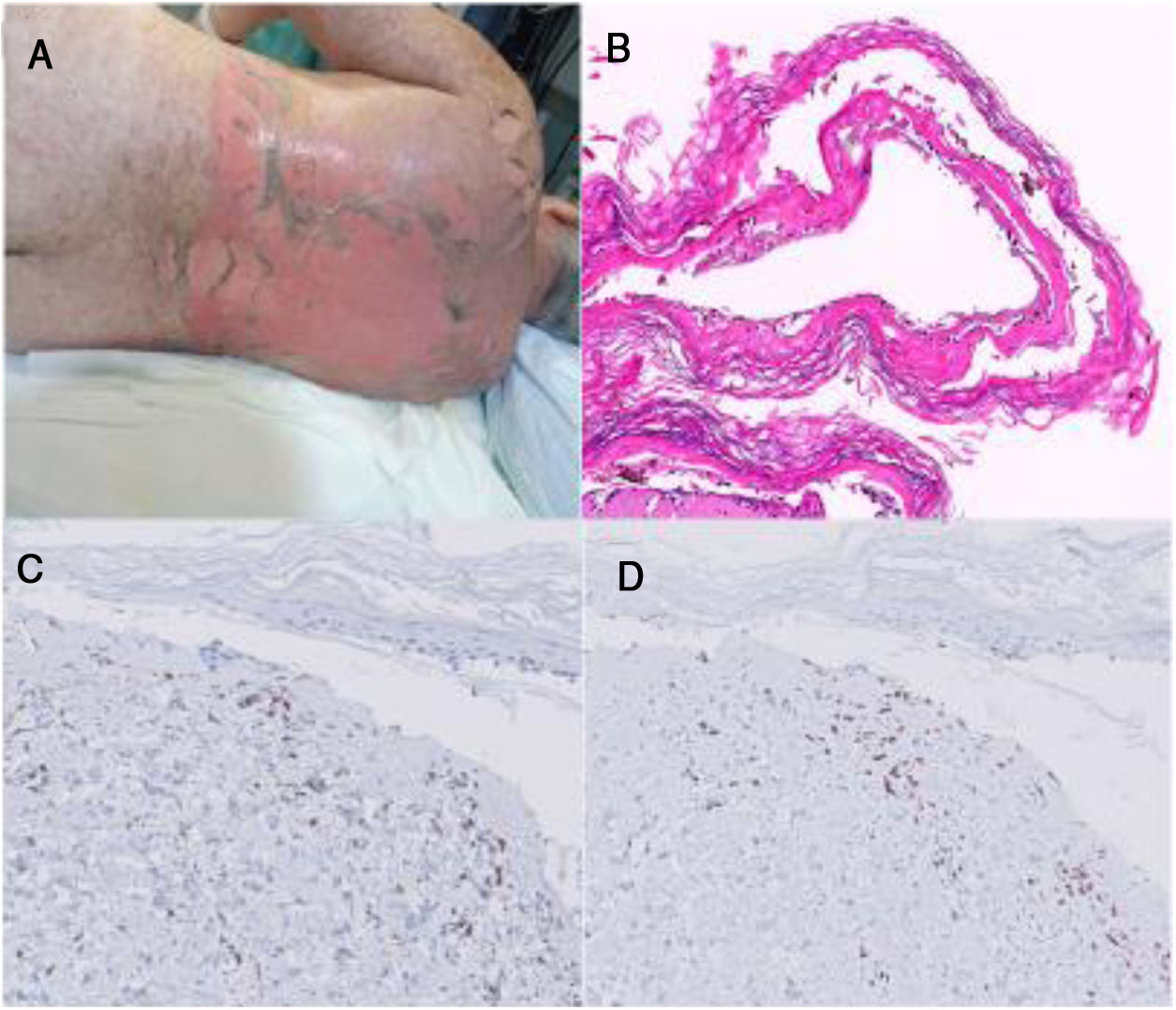
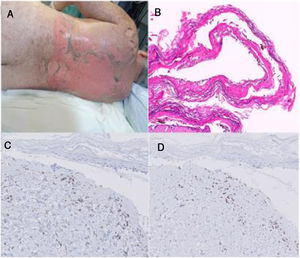
Patient two: An allogeneic HSCT was performed in a 61-year-old woman with chronic lymphatic leukemia. The day +16 she developed a gastric GVHD treated with corticosteroid and cyclosporine. On day +38, the patient reported a worsening of her digestive symptoms, associating fever and mild skin rash. Sirolimus was initiated and it coincided with the appearance of a more significant, painful and confluent rash, with serous blisters, epidermal denudation and fibrinoid exulcerations in oral mucosa. Sirolimus was interrupted, along with most of the prophylactic treatments of HSCT, but the rest of the immunosuppressive therapy, with high doses of corticosteroids, cyclosporine, ruxolitinib and photoapheresis, was prolonged. Progressive re-epithelialization and correct clinical-analytical stability are achieved. The patient was discharged from hospital on day +80, and later she showed typical lesions of chronic cutaneous GVHD (Fig. 1).
A. Clinical image of patient two. B. Hematoxylin-Eosin on a biopsy of patient two (20x). C. CD4+ lymphocytes on a skin biopsy of patient two (40x). D. CD8+ lymphocytes on a skin biopsy of patient two (40x). Note that in the dermal lymphocytic infiltrate, a CD8 / CD4 ratio of 2.5, less than 4 and more suggestive in this case of GHVD.
In the common scenario of an allogenic HSCT, it is very difficult to clearly separate a TEN from a severe acute cutaneous GVHD.
Acute cutaneous GVHD usually debuts in the first 4–6 weeks after allogeneic hematopoietic stem cell transplantation (HSCT), with common association with digestive and hepatic symptoms. The severe forms involve more than 50% of the body surface area (stage III) with even blistering and epidermolysis (stage IV). It corresponds to interface dermatitis with keratinocyte necrosis and dermal lymphocytic infiltrate predominantly T CD8, adding formation of a subepidermal cleavage in grade III and dermo-epidermal separation in grade IV.1 Regarding the treatment, the use of glucocorticoids, usually topical and systemic, is accepted as the first line. The second line is not clearly defined, being valid different combinations of immunosuppressants as well as extracorporeal photopheresis.1,2
Given that clinically and histologically TEN and severe acute cutaneous GVHD can be identical, dual moderate management is usually chosen. However, it is important to promote studies that define criteria for differential diagnosis, since optimal management of each entity runs in some differences.
The following points may be useful for the differential diagnosis:
- -
Appearance of NET in the 4 weeks following the incorporation of a new medication, with discontinuity of denudation once the suspect drug has been removed. On the contrary, in GVHD it is possible that skin reactions progress or recur, even in a changing manner.3
- -
The coexistence of gastrointestinal or hepatic GVHD, supports the diagnosis of severe acute cutaneous GVHD.
- -
Although the histology may be similar, and immunofluorescence lacks of value because it is negative or non-specific, the immunohistochemistry in both reflects a clear predominance of T CD8 lymphocytes in the dermal infiltrate. Recent studies advocate for a CD8 / CD4 ratio higher in the NET, where at least it will be > 4.4
- -
The determination, during the first hours of the suspicious eruption, of certain elevated serum biomarkers like elafin, regenerating islet-derived 3-α (REG3α) or soluble interleukin-2 receptor-α (sIL-2Rα), is a non-invasive procedure that can guide both for the diagnosis of the onset of GVHD, as to predict the evolution towards severe GVHD. In particular, the three biomarkers mentioned, measured jointly just when the symptomatology appears, constitute a composite panel that has shown with statistical significance, to be able to differentiate non-GVHD versus any degree GVHD. The analysis in recent literature of the ROC curve of this panel to diagnose GVHD at the time of onset of symptoms, describes a specificity of 100% and a sensitivity of 55.6%.5,6
In conclusion, we present two clinical cases in which the diagnostic difficulty between these two entities and the importance of looking for clinical, analytical and anatomopathological data that allow to focus therapeutic management, is reflected.
Please cite this article as: Rodríguez Tejero A, Badiola González J, Roldán Mateo L, Molina Leyva A. Necrólisis epidérmica tóxica versus enfermedad injerto contra huésped cutánea aguda después de un trasplante de células madre hematopoyéticas: un desafío diagnóstico y terapéutico. Actas Dermosifiliogr. 2021;112:81–82.