Keratoacanthoma is a fast-growing crateriform skin tumor. Approximately 25% of such tumors undergo malignant transformation and develop areas of squamous cell carcinoma (SCC). The presence of laminin-322 has been associated with progression to invasive forms of SCC. The aim of this study was to determine whether or not immunohistochemical staining for laminin-322 would be of value in distinguishing between keratoacanthomas, keratoacanthomas with areas of squamous cell carcinoma, and SCCs.
Material and methodsSeventy-four lesions were selected from the pathology archives of our hospital and divided into 4 groups: 20 keratoacanthomas without SCC, 20 keratoacanthomas with areas of squamous cell carcinoma, 20 invasive SCCs (8 with crateriform morphology) unrelated to keratoacanthoma, and 14 problem lesions (keratoacanthomas with areas suggestive of SCC). All 74 lesions were stained for laminin-322.
ResultsLaminin-322 staining was strongly positive both in areas of SCC in keratoacanthomas with malignant transformation and in invasive SCCs (mostly at the invasive front of the SCC). However, in benign keratoacanthomas, it was only weakly positive and furthermore it was confined to isolated cells or small groups of cells. The 14 problem lesions were reexamined after laminin-322 staining and 8 were diagnosed as keratoacanthomas with incipient SCC and 6 as keratoacanthomas without SCC.
ConclusionsLaminin-322 staining is different in keratoacanthomas and SCCs and would thus be a useful test for differentiating keratoacanthomas from both invasive SCCs and keratoacanthomas with areas of squamous cell carcinoma. It would also be of value in diagnosing keratoacanthomas with areas suggestive of SCC or with incipient SCC.
El queratoacantoma (QA) es un tumor cutáneo crateriforme, de crecimiento rápido; aproximadamente el 25% de los QA presentan transformación maligna (QAm), observándose áreas de carcinoma epidermoide (CE). La laminina-332 se ha relacionado con progresión a fases invasoras en diversos CE. El objetivo de este estudio es evaluar si la tinción con laminina-332 es útil para distinguir QA, QAm y CE.
Material y métodosSeleccionamos 74 casos del archivo de Anatomía Patológica. Se analizaron 4 grupos: 20 QA sin CE, 20 QAm con áreas evidentes de CE, 20 CE invasores sin relación con QA (8 con morfología crateriforme) y 14 casos «problema» (QA con «dudosas» áreas de CE). Posteriormente se realizó tinción inmunohistoquímica para laminina-332 a todas estas lesiones.
ResultadosEn las áreas de CE asociado a QAm y en los CE invasores, la tinción con laminina fue positiva de forma intensa, habitualmente en el frente invasor del CE, a diferencia de los QA, en que la tinción fue positiva solo de forma débil y focal, en células aisladas o en pequeños «grupos» celulares. Los casos «problema» se reexaminaron tras valorar la tinción con laminina-332 (8 se diagnosticaron de QA con CE incipiente, 6 de QA sin CE).
ConclusionesLa tinción con laminina-332 es diferente en los QA respecto a los CE, por lo que ayudaría a diferenciar los QA de los CE invasores y de las áreas de CE en QAm, así como en el diagnóstico de QA con «dudosas» áreas de CE y QA con CE incipientes.
Keratoacanthoma is a rapidly growing, crateriform, exophytic or endophytic skin tumor that regresses spontaneously after a few months. The most frequent clinical presentation is as a solitary nodule, usually located on sun-exposed areas, with a characteristic development that can be divided into a proliferative phase, a mature or stable phase, and regression.1,2
Differential diagnosis with squamous cell carcinoma (SCC) can be difficult, both in clinical and histologic terms. Some authors consider keratoacanthoma to be a well-differentiated squamous cell carcinoma.3,4 We, like Sánchez Yus et al.1,5 and other authors,2,6,7 think that only a subgroup, accounting for approximately 25% of all keratoacanthomas, undergoes malignant transformation to SCC.5 This malignant transformation is most common in older patients and in lesions on sun-exposed areas, and can occur during any phase of keratoacanthoma, including regression.5
Laminin-332 (formerly laminin-5) is a basement membrane glycoprotein that may play an important part in cell migration and mobility and in tumor progression and invasion in SCC.8-11 Laminin-332 interacts with integrin α6β4 and epidermal growth factor receptor, thereby activating phosphatidylinositol-3-kinase (p13K). p13K regulates several cell processes such as proliferation, growth, and apoptosis, and its activation has been directly associated with tumor invasion.11-13 Laminin-332 therefore promotes tumor invasion in SCC through activation of p13K and is associated with more advanced TNM stages and worse prognosis,11,14 although lymph node metastases are not more common.15 Laminin-332 staining has been used to monitor progression of SCC confined to the oral mucosa to invasive SCC,12-14 as well as to distinguish between verrucous carcinoma and well-differentiated SCC of the oral mucosa.16,17
As part of a study of the expression of laminin-332 in SCC of the oral mucosa, 2 keratoacanthomas with areas of malignant transformation to SCC were stained for laminin-332. After observing strong staining in the area corresponding to SCC in these 2 samples, we decided to study a greater number of samples with the aim of evaluating whether staining with laminin-332 could be useful for distinguishing between keratoacanthoma and areas of SCC in malignant keratoacanthoma.
Materials and MethodsAll histology samples were obtained from the tissue bank of the Hospital Universitario Virgen de la Arrixaca, Murcia, Spain; all samples were reassessed independently by 2 pathologists experienced in dermatopathology who were blinded to the clinical characteristics of the lesion (rapid lesion growth, for example).
In this descriptive, observational study of immunohistochemical staining for laminin-332, 74 lesions were divided into the following groups:
Group A: 20 keratoacanthomas in different phases of development without evidence of malignant transformation. Eight were in the proliferative phase, 4 in the stable phase, and 8 in regression.
Group B: 20 keratoacanthomas with areas showing clear cytologic and histologic evidence of malignant transformation to SCC (malignant keratoacanthoma). Nine of these malignant keratoacanthomas were in the proliferative phase, 5 in the stable phase, and 6 in regression.
Group C: 20 invasive SCC unrelated to keratoacanthoma, 8 of which had a crateriform morphology.
Group D: 14 histologically ambiguous or problem cases: Keratoacanthoma with areas possibly corresponding to incipient SCC.
We defined keratoacanthoma and SCC as follows:
Keratoacanthoma: a well-delimited, hyperplastic, crateriform, endophytic or exophytic epithelial tumor, with a central keratin-containing crater and epidermal hyperplasia at the edges of the lesion (epidermal lips), which protrudes beyond the central crater giving it a symmetrical appearance. The cells are large, with prominent nucleoli and eosinophilic ground-glass cytoplasm.1,2,6
Squamous cell carcinoma: a malignant tumor composed of nests or cords of atypical epithelial cells, with a variable degree of keratinization and anaplasia. Central keratinization occurs to a variable degree, with formation of corneal pearls according to the extent of differentiation.18
These 74 lesions were stained immunohistochemically for laminin-332 using the immunoperoxidase technique (anti-human laminin-5, γ-2 chain antibodies, Dako laboratories, Denmark, Clone 4G1), with appropriate external controls. Laminin-332 staining was considered positive when cytoplasmic granular staining was present. The staining was classified as weak or strong.
Statistical AnalysisThe data were processed using the SPSS 12.0 statistical package for Windows.
The results obtained were studied by generating contingency tables with the Pearson χ2 test, considering a probability of error less than 5% as significant (P<.05).
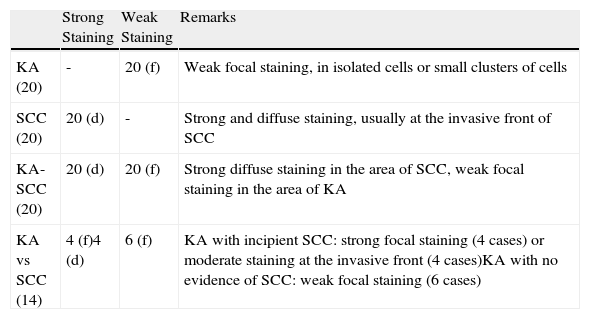
ResultsThe main results are presented in Table 1.
Laminin-332 Staining in Keratoacanthomas (KA) and Squamous Cell Carcinoma (SCC).
| Strong Staining | Weak Staining | Remarks | |
| KA (20) | - | 20 (f) | Weak focal staining, in isolated cells or small clusters of cells |
| SCC (20) | 20 (d) | - | Strong and diffuse staining, usually at the invasive front of SCC |
| KA-SCC (20) | 20 (d) | 20 (f) | Strong diffuse staining in the area of SCC, weak focal staining in the area of KA |
| KA vs SCC (14) | 4 (f)4 (d) | 6 (f) | KA with incipient SCC: strong focal staining (4 cases) or moderate staining at the invasive front (4 cases)KA with no evidence of SCC: weak focal staining (6 cases) |
Abbreviations: (d), diffuse positive staining at the invasive front; (f), focal positive staining; KA, keratoacanthoma with no evidence of squamous cell carcinoma (number of cases studied in parentheses); KA-SCC, keratoacanthoma with areas of malignant transformation to squamous cell carcinoma; KA vs SCC, histologically ambiguous cases or KA with possible areas of incipient SCC.
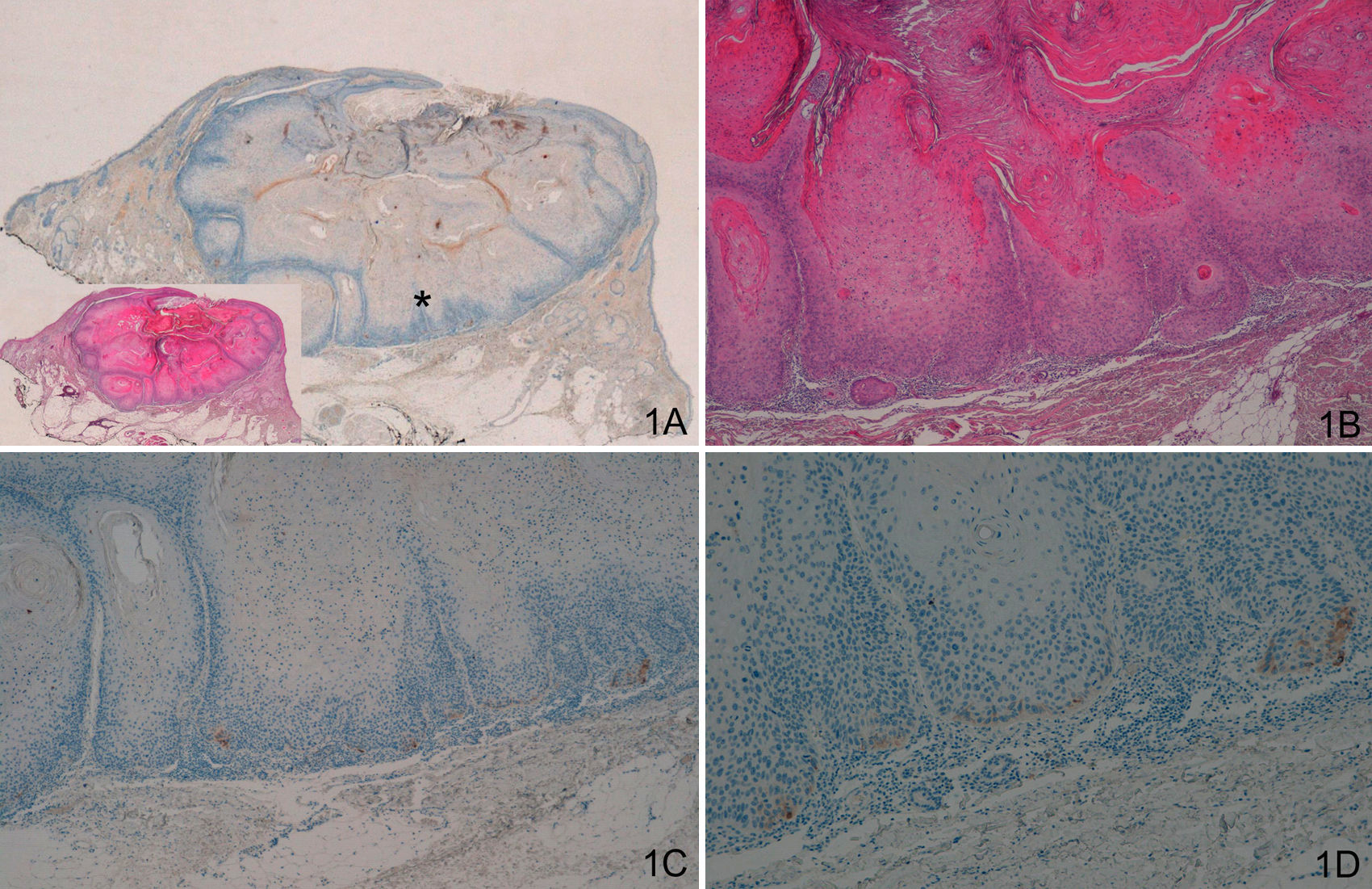
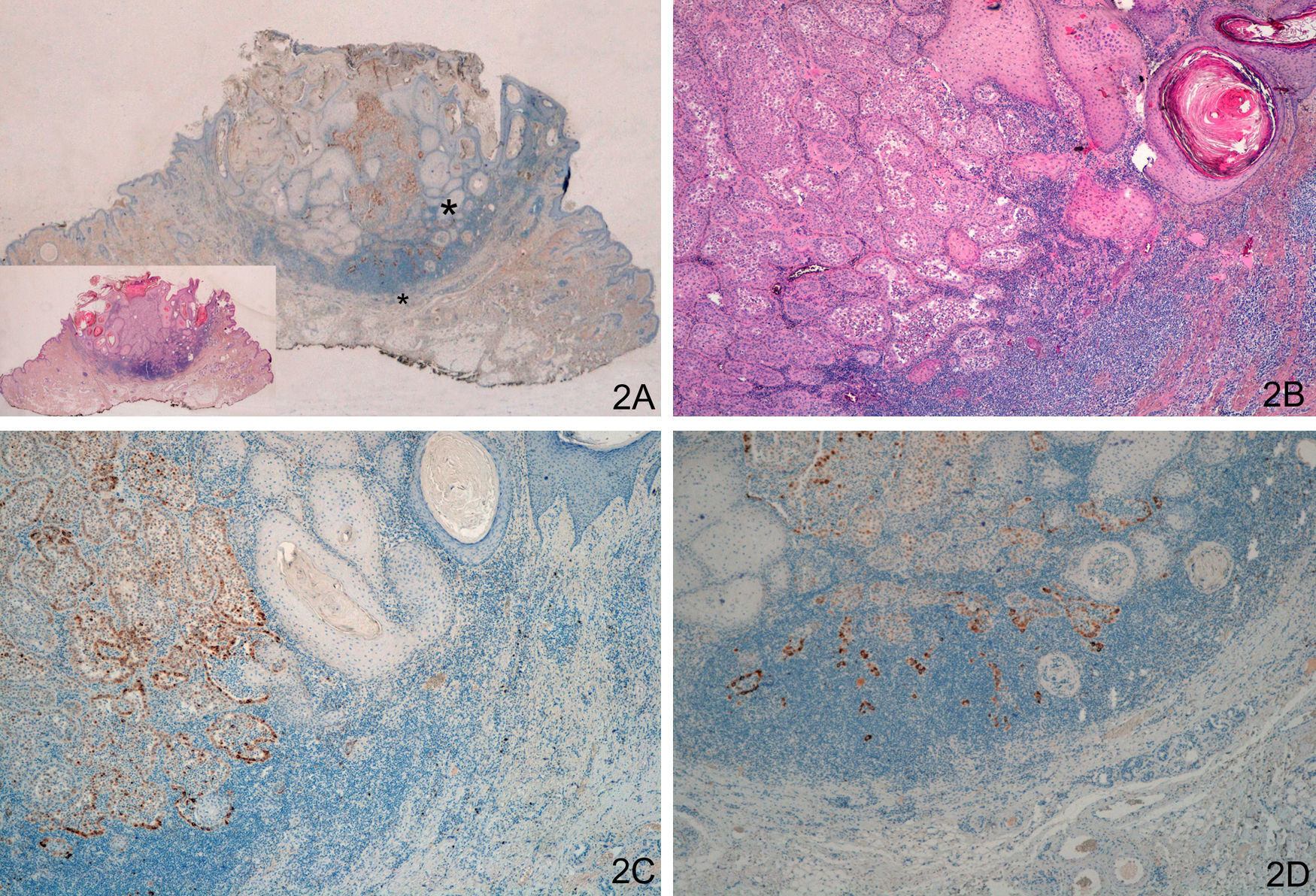
Staining with laminin-332 was negative in the normal epidermis and skin appendages. In the keratoacanthoma group, group A, we observed weak focal staining in isolated cells or small clusters of cells (Fig. 1). In all cases of malignant keratoacanthoma (group B), laminin-332 staining was strong and continuous in the area of malignant transformation to SCC, usually at the invasive front of the area of SCC (Fig. 2). Staining with laminin-332 was independent of the phase of development of both keratoacanthoma and malignant keratoacanthoma.
A and B. Keratoacanthoma in proliferative phase. C and D. Weak focal positive laminin-332 staining in isolated cells. (A. Lower-magnification view, inset shows lower-magnification view of hematoxylin/eosin [H/E] staining. B. H/E x40. C. Laminin x40. D. Laminin x100.) The asterisk indicates the amplified area.
A and B. Keratoacanthoma with malignant transformation to squamous cell carcinoma (SCC) at the center of the lesion and in deep layers. C. Strong staining at the invasive front of SCC (left), absence of staining in the keratoacanthoma area (right). D. Strong staining in the SCC area in deep layers of the keratoacanthoma. (A. Lower-magnification view of laminin-332 staining, inset shows lower magnification view of hematoxylin-eosin [H/E] staining. B. H/E x40. C. Laminin x40. D: Laminin x100.) The asterisks indicate amplified areas.
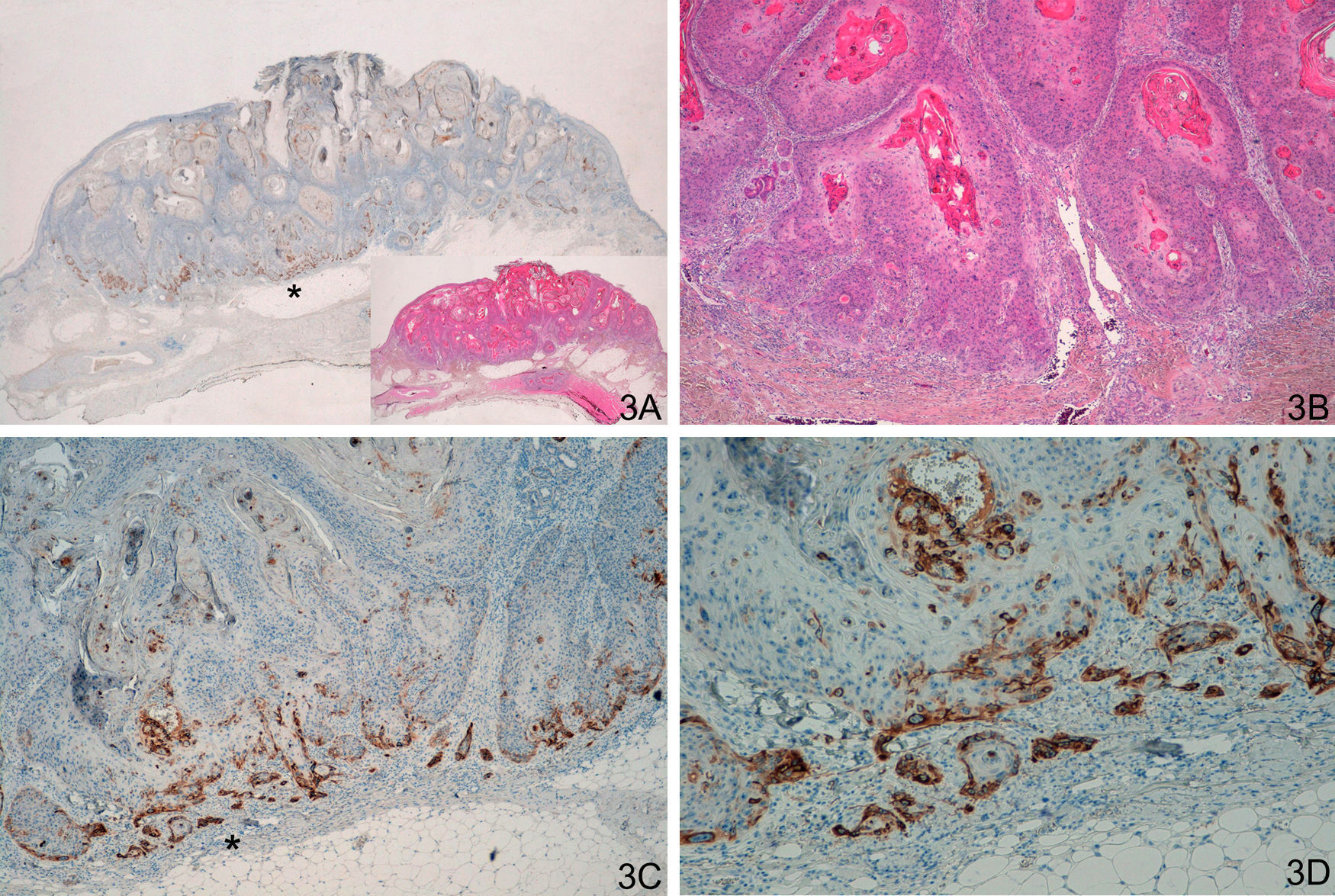
We observed similar staining in the 20 cases in the group of invasive SCC, including the 8 SCC with crateriform morphology, with strong continuous staining forming a strip, usually at the invasive front of the SCC (Fig. 3). In some cases, it was necessary to distinguish this staining from cases of keratoacanthoma with positive staining in the proliferative layer, although staining was weak and focal in the case of keratoacanthoma. The pattern of staining was different for the comparison both of keratoacanthoma with malignant keratoacanthoma (P<.0001) and of keratoacanthoma with invasive SCC (P<.0001).
A and B. Crateriform lesions of squamous cell carcinoma C and D. Strong laminin-332 staining at the invasive front of the squamous cell carcinoma. (A. Lower-magnification view of laminin staining, inset shows lower-magnification view of hematoxylin/eosin [H/E] staining. B. H/E x40. C. Laminin x40. D. Laminin x100.) The asterisks indicate amplified areas.
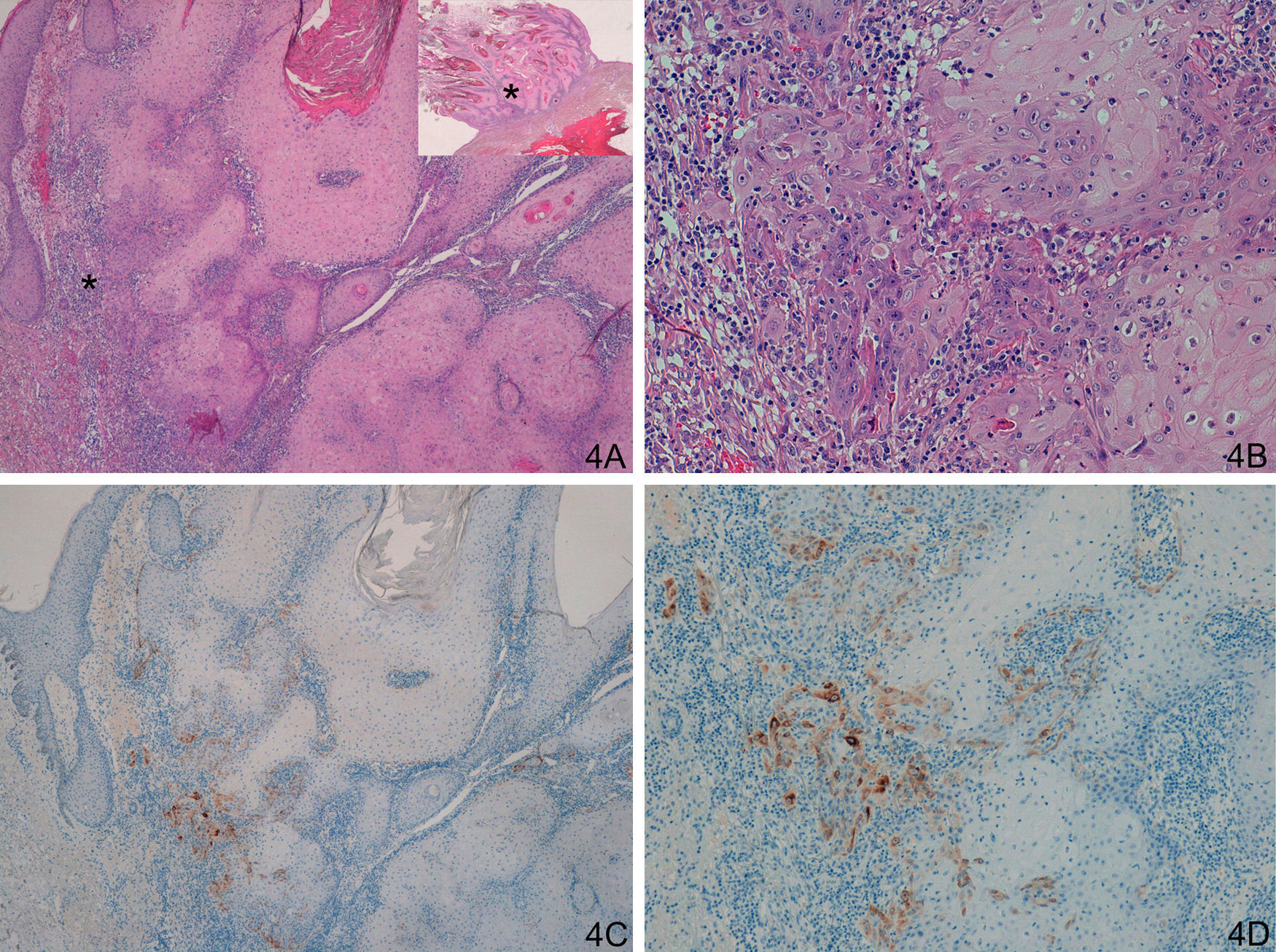
In the case of group D (problem or histologically ambiguous cases), the 14 histologic samples were carefully reassessed by 2 dermatopathology experts to assess the results of staining with laminin-332. This reassessment identified 4 cases of keratoacanthoma with a small region of malignant transformation to SCC (intense focal staining for laminin-332 confined to a small area, corresponding to the area of malignant transformation to SCC [Fig. 4]). Six cases showed weak staining for laminin in isolated cells or small clusters of cells; after reassessment of hematoxylin-eosin staining, it was concluded that the staining corresponded to benign proliferative areas of keratoacanthoma or areas of keratoacanthoma starting to regress, but without evidence of SCC (Fig. 5). In 4 cases, we observed an SCC-like staining pattern (positive staining at the invasive front), although with a much weaker intensity than in the other cases of SCC (Fig. 6). These were finally diagnosed as keratoacanthoma with incipient SCC.
A and B. Keratoacanthoma with an area of incipient squamous cell carcinoma (SCC). C and D. Focal but strong staining in the region of incipient SCC. (A: Hematoxylin/eosin [H/E] x40, inset shows lower-magnification view of H/E. B. H/E x200. C. Laminin x40. D. Laminin x100.) The asterisks indicate amplified areas.
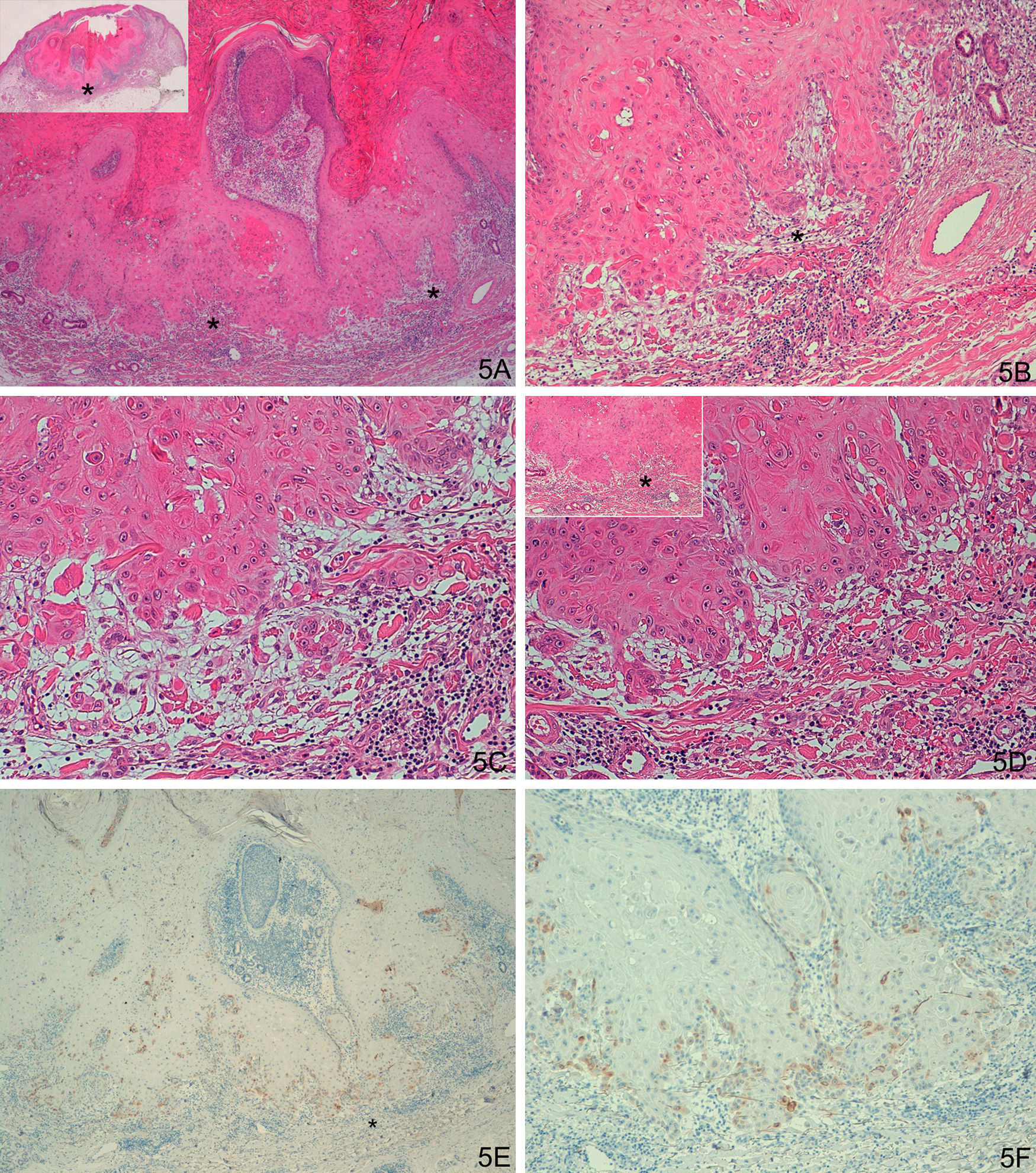
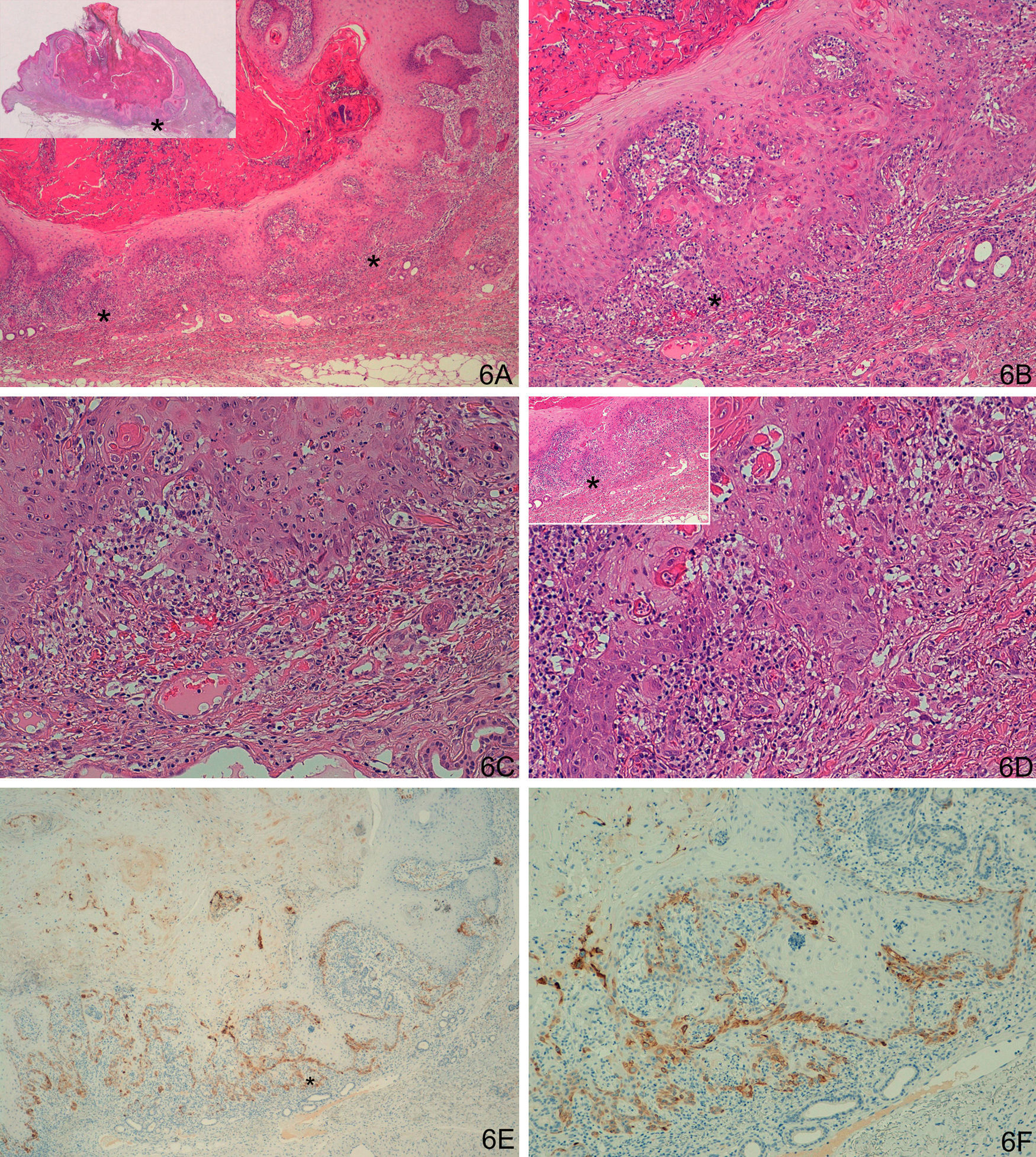
A-D. Histologically ambiguous lesion; keratoacanthoma in active regression with possible incipient squamous cell carcinoma in some areas. E-F. Weak focal staining in isolated cells, similar to the keratoacanthomas. (A. Hematoxylin/eosin [H/E] x40, inset shows lower-magnification view of H/E staining. B and C. H/E x100 and H/E x200. D. H/E x200, H/E x100 in inset. E. Laminin x40. F: Laminin x100). The asterisks indicate amplified areas.
A-D. Possible incipient squamous cell carcinoma in the deep layers of a keratoacanthoma in active regression. E and F. Staining for laminin-332 at the invasive front, with squamous type staining pattern, although weaker than the other cases of SCC. (A: Hematoxylin/eosin staining [H/E] x40, inset shows lower-magnification view of H/E staining. B and C. H/E x100 and H/E x200. D. H/E x200, H/E x100 in inset. E. Laminin x40. F. Laminin x100.) Asterisks show amplified areas.
The clinical outcome of these 74 lesions was then studied. Only 2 lesions (an SCC in group C and a keratoacanthoma with malignant transformation to SCC in group B) recurred (after 5 and 7 months, respectively) and repeat excision was required. We did not detect lymph node or visceral metastasis in any case. As this was a histology-based retrospective study, the duration of clinical follow-up was less than 1 year in 16 lesions and less than 2 years in a further 19 lesions.
DiscussionNumerous studies have attempted to differentiate between keratoacanthoma and SCC using histologic criteria,5,6,19 chromosomal abnormalities,20 or different markers such as p16, p53, Ki-67, vascular cell adhesion molecule 1 (CD106), intercellular adhesion molecule 1 (CD54), telomerase, cyclooxygenase-2, and angiotensin type 1 receptors.21–28 None of these approaches managed to clearly distinguish between keratoacanthoma and SCC.
According to the results of our study, staining with laminin-332 was useful for distinguishing between these 2 tumors. Thus, laminin-332 staining is completely different in keratoacanthoma (weak and focal) compared to SCC and/or areas of SCC in malignant keratoacanthoma (intense and continuous at the invasive front of SCC). Hematoxylin-eosin staining should be sufficient for diagnosis of cases of SCC and keratoacanthoma with evident histologic and cytologic changes. However, laminin-332 staining could be a great help in cases of histologically ambiguous keratoacanthoma, where areas of incipient SCC may be present. In cases of malignant keratoacanthoma with a small area of SCC, laminin-332 staining was strongly positive, but staining was confined to this small area of malignant transformation to SCC. When staining was focal but weak, there was no evidence of SCC. The greatest diagnostic difficulty was in 4 histologically ambiguous lesions, with areas that could have been SCC and with a similar staining pattern to that of SCC (positive invasive front), although staining was weaker than in the other cases of SCC. After carefully reassessing hematoxylin-eosin staining, and given that we had not observed this staining pattern in any keratoacanthoma (not even in the proliferative layer of some keratoacanthomas), we concluded that these were very incipient SCC. The oncogenic process in keratoacanthoma, like in most tumors, can be considered as a continuum, ranging from very incipient SCC to fully developed SCC, as was the case in these 4 lesions.
We have only found a single previous study of staining for laminin-332 in keratoacanthoma. Kuivanen TT et al.28 studied 7 subtypes of metalloproteases, laminin-332 (referred to as laminin-5 by those authors), and p16 in 31 cases of keratoacanthoma and 15 cases of SCC. With regard to their results for staining for laminin, we do not understand why they concluded that laminin is not usually useful for differentiating between keratoacanthoma and SCC, even though they, like us, observed positive staining at the invasive front of the SCCs.
In general, the clinical outcome of most of these lesions is good, with some exceptions.3 Recurrences and metastases are rare.2,6 In our study, recurrence was only observed in 2 lesions, 1 case of SCC and 1 case of keratoacanthoma with malignant transformation to SCC. Repeat excision of these lesions was performed. We should remember, however, that as this was a retrospective, histologic study, clinical follow-up was less than 2 years in almost half the cases. More studies, ideally with a prospective design and a greater number of patients, are required to assess the clinical outcomes of SCC arising from malignant transformation of keratoacanthoma. Other variables such as age, site, immune status, size and depth of the tumor, and perineural invasion should also be taken into account.
Numerous studies have been published on the etiopathogenesis and possible mechanisms underlying the oncogenesis of KA,1,2,5–7,21–28 although detailed analysis of these mechanisms was not the objective of this study. We do however wish to highlight that the presence of laminin-332 could mark a point of inflection in the malignant transformation of some keratoacanthomas to SCC, although this would need to be confirmed in additional studies. According to our results, all keratoacanthomas that underwent malignant transformation to SCC stained strongly positive for laminin-332, even in those cases in which the area of malignant transformation was small or the SCC was very incipient. The clinical importance of this observation is that laminin-332 could be a potential therapeutic target.10,11 It has been shown that use of antibodies against a laminin-332 fraction induces tumor apoptosis and slows proliferation and tumor growth or disease progression in SCC.10 In the case of keratoacanthoma, if it could be shown that laminin-332 is actually needed for malignant transformation to SCC, these antibodies could be used to block malignant transformation to SCC in some cases of keratoacanthoma (we would almost be certain that these keratoacanthomas would not undergo malignant transformation; this could be very useful for example in multiple keratoacanthomas, giant keratoacanthomas, or keratoacanthomas that, given their site, could leave sequelae and/or ugly scars after excision).
The limitations of this study are the low number of cases, the fact that it was retrospective, and the lack of a gold standard for histologic diagnosis of keratoacanthoma.
In conclusion, staining for laminin-332 is different in SCC and in areas of SCC resulting from malignant transformation in some keratoacanthomas (strong and continuous staining, usually at the invasive front of SCC) compared to staining in keratoacanthoma with no evidence of SCC (weak and focal staining, in isolated cells or in small clusters of cells). Staining with laminin-332 would help distinguish between keratoacanthoma and areas of SCC, including incipient SCC. Such staining could also be very useful in diagnosis of histologically ambiguous lesions, that is, keratoacanthoma with areas that may correspond to SCC.
Laminin-332 could play a fundamental role in malignant transformation to SCC in some keratoacanthomas, although further studies would be needed for confirmation. Thus, laminin-332 could be a potential therapeutic target.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Corbalán-Vélez R, et al. Utilidad de la tinción con laminina-332 para diferenciar queratoacantoma, queratoacantoma con áreas de carcinoma epidermoide y carcinoma epidermoide crateriforme. Actas Dermosifiliogr.2012;103:308-16.

![A and B. Keratoacanthoma in proliferative phase. C and D. Weak focal positive laminin-332 staining in isolated cells. (A. Lower-magnification view, inset shows lower-magnification view of hematoxylin/eosin [H/E] staining. B. H/E x40. C. Laminin x40. D. Laminin x100.) The asterisk indicates the amplified area. A and B. Keratoacanthoma in proliferative phase. C and D. Weak focal positive laminin-332 staining in isolated cells. (A. Lower-magnification view, inset shows lower-magnification view of hematoxylin/eosin [H/E] staining. B. H/E x40. C. Laminin x40. D. Laminin x100.) The asterisk indicates the amplified area.](https://static.elsevier.es/multimedia/15782190/0000010300000004/v1_201304241303/S1578219012001497/v1_201304241303/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![A and B. Keratoacanthoma with malignant transformation to squamous cell carcinoma (SCC) at the center of the lesion and in deep layers. C. Strong staining at the invasive front of SCC (left), absence of staining in the keratoacanthoma area (right). D. Strong staining in the SCC area in deep layers of the keratoacanthoma. (A. Lower-magnification view of laminin-332 staining, inset shows lower magnification view of hematoxylin-eosin [H/E] staining. B. H/E x40. C. Laminin x40. D: Laminin x100.) The asterisks indicate amplified areas. A and B. Keratoacanthoma with malignant transformation to squamous cell carcinoma (SCC) at the center of the lesion and in deep layers. C. Strong staining at the invasive front of SCC (left), absence of staining in the keratoacanthoma area (right). D. Strong staining in the SCC area in deep layers of the keratoacanthoma. (A. Lower-magnification view of laminin-332 staining, inset shows lower magnification view of hematoxylin-eosin [H/E] staining. B. H/E x40. C. Laminin x40. D: Laminin x100.) The asterisks indicate amplified areas.](https://static.elsevier.es/multimedia/15782190/0000010300000004/v1_201304241303/S1578219012001497/v1_201304241303/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![A and B. Crateriform lesions of squamous cell carcinoma C and D. Strong laminin-332 staining at the invasive front of the squamous cell carcinoma. (A. Lower-magnification view of laminin staining, inset shows lower-magnification view of hematoxylin/eosin [H/E] staining. B. H/E x40. C. Laminin x40. D. Laminin x100.) The asterisks indicate amplified areas. A and B. Crateriform lesions of squamous cell carcinoma C and D. Strong laminin-332 staining at the invasive front of the squamous cell carcinoma. (A. Lower-magnification view of laminin staining, inset shows lower-magnification view of hematoxylin/eosin [H/E] staining. B. H/E x40. C. Laminin x40. D. Laminin x100.) The asterisks indicate amplified areas.](https://static.elsevier.es/multimedia/15782190/0000010300000004/v1_201304241303/S1578219012001497/v1_201304241303/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![A and B. Keratoacanthoma with an area of incipient squamous cell carcinoma (SCC). C and D. Focal but strong staining in the region of incipient SCC. (A: Hematoxylin/eosin [H/E] x40, inset shows lower-magnification view of H/E. B. H/E x200. C. Laminin x40. D. Laminin x100.) The asterisks indicate amplified areas. A and B. Keratoacanthoma with an area of incipient squamous cell carcinoma (SCC). C and D. Focal but strong staining in the region of incipient SCC. (A: Hematoxylin/eosin [H/E] x40, inset shows lower-magnification view of H/E. B. H/E x200. C. Laminin x40. D. Laminin x100.) The asterisks indicate amplified areas.](https://static.elsevier.es/multimedia/15782190/0000010300000004/v1_201304241303/S1578219012001497/v1_201304241303/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![A-D. Histologically ambiguous lesion; keratoacanthoma in active regression with possible incipient squamous cell carcinoma in some areas. E-F. Weak focal staining in isolated cells, similar to the keratoacanthomas. (A. Hematoxylin/eosin [H/E] x40, inset shows lower-magnification view of H/E staining. B and C. H/E x100 and H/E x200. D. H/E x200, H/E x100 in inset. E. Laminin x40. F: Laminin x100). The asterisks indicate amplified areas. A-D. Histologically ambiguous lesion; keratoacanthoma in active regression with possible incipient squamous cell carcinoma in some areas. E-F. Weak focal staining in isolated cells, similar to the keratoacanthomas. (A. Hematoxylin/eosin [H/E] x40, inset shows lower-magnification view of H/E staining. B and C. H/E x100 and H/E x200. D. H/E x200, H/E x100 in inset. E. Laminin x40. F: Laminin x100). The asterisks indicate amplified areas.](https://static.elsevier.es/multimedia/15782190/0000010300000004/v1_201304241303/S1578219012001497/v1_201304241303/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![A-D. Possible incipient squamous cell carcinoma in the deep layers of a keratoacanthoma in active regression. E and F. Staining for laminin-332 at the invasive front, with squamous type staining pattern, although weaker than the other cases of SCC. (A: Hematoxylin/eosin staining [H/E] x40, inset shows lower-magnification view of H/E staining. B and C. H/E x100 and H/E x200. D. H/E x200, H/E x100 in inset. E. Laminin x40. F. Laminin x100.) Asterisks show amplified areas. A-D. Possible incipient squamous cell carcinoma in the deep layers of a keratoacanthoma in active regression. E and F. Staining for laminin-332 at the invasive front, with squamous type staining pattern, although weaker than the other cases of SCC. (A: Hematoxylin/eosin staining [H/E] x40, inset shows lower-magnification view of H/E staining. B and C. H/E x100 and H/E x200. D. H/E x200, H/E x100 in inset. E. Laminin x40. F. Laminin x100.) Asterisks show amplified areas.](https://static.elsevier.es/multimedia/15782190/0000010300000004/v1_201304241303/S1578219012001497/v1_201304241303/en/main.assets/thumbnail/gr6.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)



