The landscape of treatment of atopic dermatitis (AD) has improved dramatically since dupilumab emerged. The efficacy of dupilumab has been widely studied, with favorable data even in special populations, such as the elderly, children and during pregnancy.1–3 More recently, an ambitious, rheumatology-inspired, target-driven strategy – treat-to-target (TT) for AD– was developed.4 Prospective, long-term real-world data is warranted to evaluate the efficacy of dupilumab effectiveness vs these guiding treatment goals.
We conducted a 1-year prospective observational study from January 2022 through December 2022 at an AD referral center to assess the safety and efficacy profile of dupilumab and compare it to TT established goals. Patients had moderate-to-severe AD (Eczema Area and Severity Index (EASI) ≥16) and were on 300mg dupilumab every other week after a loading dose of 600mg.
Baseline demographic data on age, gender, body mass index (BMI), family history, and allergic comorbidities were collected. Several measures were collected at baseline and then at 4/6/12 months; the number of patients analyzed at each time point depended on eligibility (sufficient follow-up or dropout). Specifically, we recorded patient-reported measures such as Itch Numeric Rating Scale (INRS), Sleep NRS (SNRS), Dermatology Life Quality Index (DLQI), Patient Oriented Eczema Measure (POEM), Patient Global Assessment (PtGA); SCORing Atopic Dermatitis (SCORAD); and objective outcomes such as EASI.
Statistical analysis was conducted with the Wilcoxon matched-pairs rank or paired T-tests to evaluate differences between baseline and follow-ups. Fisher's exact test was used to test the association between EASI-75 and demographic variables. P values <0.05 were considered statistically significant.
The cohort (N=79) had 51 (64.6%) men, a mean age of 32.3 years (15–70), and a mean BMI of 24.6kg/m2. A total of 63 (79.7%) patients had atopic comorbidities, and 30 (38.0%) a family history of atopy. Other comorbidities included 14 (17.7%) patients who were overweight, 11 (13.2%) were obese, 5 (6.3%) had hypertension, 5 (6.3%) had dyslipidemia (namely, hypercholesterolemia), and 20 (25.3%) had some kind of depressive disorder. Cyclosporin was used as a concomitant immunosuppressant within the first 3 months of dupilumab in 43 patients (54.4%). No other immunosuppressant was ever used in this cohort. All patients were on dupilumab monotherapy after that.
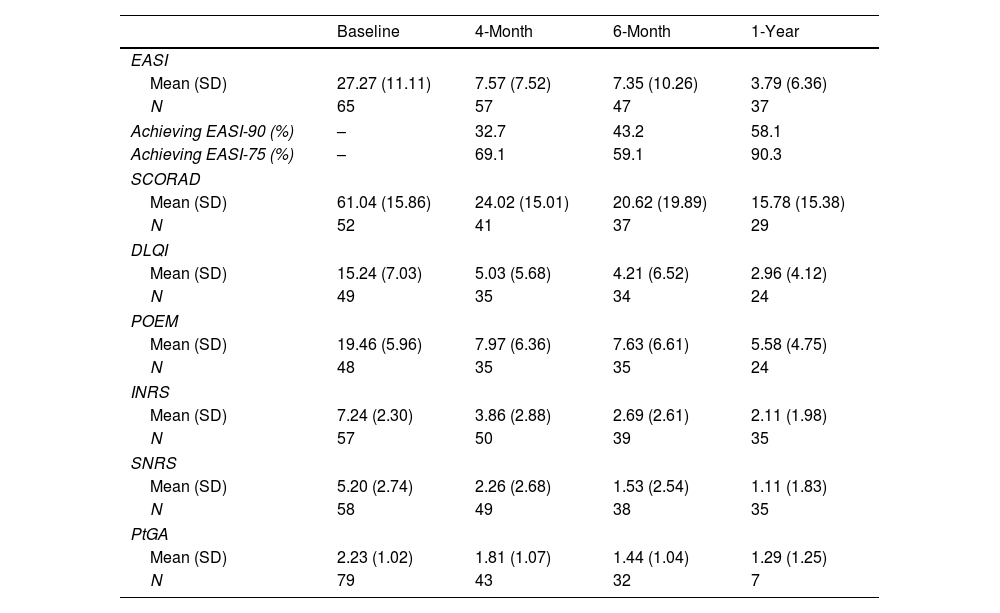
Baseline evaluations are listed in Table 1. Consecutive improvements in all subjective and objective scores were observed from baseline up to 1-year. By the 4-month endpoint, there was a mean reduction of 75.8% in EASI. By 1-year, there was a mean reduction of 88.4% in EASI compared to baseline. All differences were statistically significant (P<0.001). EASI-75 at months 4/6/12 was not impacted by sex, age, BMI, or number of allergies (P>0.05).
Objective outcomes measures and patient-reported outcomes measures variation with dupilumab treatment.
| Baseline | 4-Month | 6-Month | 1-Year | |
|---|---|---|---|---|
| EASI | ||||
| Mean (SD) | 27.27 (11.11) | 7.57 (7.52) | 7.35 (10.26) | 3.79 (6.36) |
| N | 65 | 57 | 47 | 37 |
| Achieving EASI-90 (%) | – | 32.7 | 43.2 | 58.1 |
| Achieving EASI-75 (%) | – | 69.1 | 59.1 | 90.3 |
| SCORAD | ||||
| Mean (SD) | 61.04 (15.86) | 24.02 (15.01) | 20.62 (19.89) | 15.78 (15.38) |
| N | 52 | 41 | 37 | 29 |
| DLQI | ||||
| Mean (SD) | 15.24 (7.03) | 5.03 (5.68) | 4.21 (6.52) | 2.96 (4.12) |
| N | 49 | 35 | 34 | 24 |
| POEM | ||||
| Mean (SD) | 19.46 (5.96) | 7.97 (6.36) | 7.63 (6.61) | 5.58 (4.75) |
| N | 48 | 35 | 35 | 24 |
| INRS | ||||
| Mean (SD) | 7.24 (2.30) | 3.86 (2.88) | 2.69 (2.61) | 2.11 (1.98) |
| N | 57 | 50 | 39 | 35 |
| SNRS | ||||
| Mean (SD) | 5.20 (2.74) | 2.26 (2.68) | 1.53 (2.54) | 1.11 (1.83) |
| N | 58 | 49 | 38 | 35 |
| PtGA | ||||
| Mean (SD) | 2.23 (1.02) | 1.81 (1.07) | 1.44 (1.04) | 1.29 (1.25) |
| N | 79 | 43 | 32 | 7 |
DLQI: Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; INRS: Itch Numeric Rating Scale; SCORAD: SCORing Atopic Dermatitis; SD: standard deviation; SNRS: Sleep Numeric Rating Scale; POEM: Patient Oriented Eczema Measure; PtGA: Patient Global Assessment.
Adverse events were reported in 29 (36.7%) patients, mainly ocular inflammation (N=17, 21.5%), eosinophilia (N=6, 7.6%), and paradoxical facial erythema (N=3, 3.8%). Isolated cases of new-onset inflammatory, psoriatic-like arthritis, orolabial herpes, and injection site reaction were also reported.
Dupilumab was discontinued in 9 (11.4%) patients: 7 (8.8%) were primary failures, and 5 stopped between months 6 and 12. No secondary failures were observed. One case of arthritis led to treatment withdrawal. Another patient stopped dupilumab between months 4 and 6 due to pregnancy.
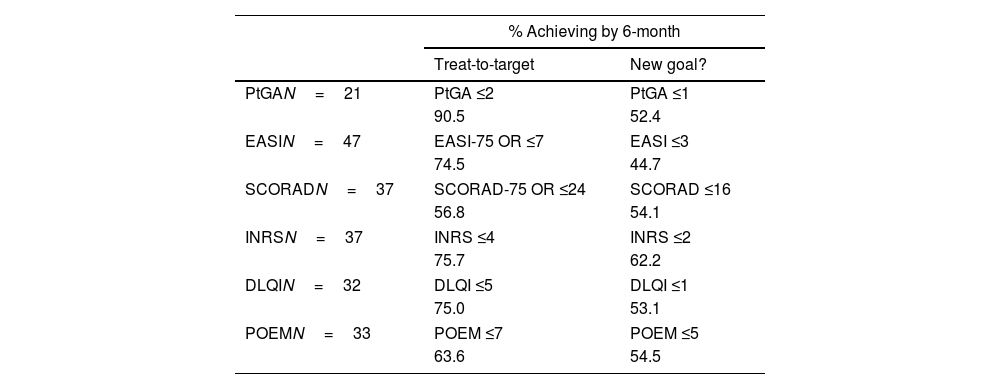
TT is a novel target-driven treatment strategy for AD. As far as we know, the efficacy of dupilumab has yet to be compared to these recommendations. We performed a TT analysis at 6 months. A total of 47 patients who reached this endpoint were eligible for this analysis, while 32 were excluded (3 patients stopped dupilumab before month 6, and 29 patients had an insufficient follow-up). Table 2 illustrates that most patients achieved or exceeded the proposed targets on month 6, with 90.5% reaching PtGA ≤2 and 74.5% the target EASI-75 or EASI ≤7. Given the robust effectiveness of dupilumab at this endpoint, we proposed a set of stricter criteria with favorable results. If PtGA ≤1 and EASI ≤3 were considered, 52.4% and 44.7% reached those milestones, respectively.
Treat-to-target analysis by 6-month assessment, and proposed new stringent goals.
| % Achieving by 6-month | ||
|---|---|---|
| Treat-to-target | New goal? | |
| PtGAN=21 | PtGA ≤2 | PtGA ≤1 |
| 90.5 | 52.4 | |
| EASIN=47 | EASI-75 OR ≤7 | EASI ≤3 |
| 74.5 | 44.7 | |
| SCORADN=37 | SCORAD-75 OR ≤24 | SCORAD ≤16 |
| 56.8 | 54.1 | |
| INRSN=37 | INRS ≤4 | INRS ≤2 |
| 75.7 | 62.2 | |
| DLQIN=32 | DLQI ≤5 | DLQI ≤1 |
| 75.0 | 53.1 | |
| POEMN=33 | POEM ≤7 | POEM ≤5 |
| 63.6 | 54.5 | |
Only patients who reached the 6-month endpoint are included in this evaluation.
DLQI: Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; INRS: Itch Numeric Rating Scale; SCORAD: SCORing Atopic Dermatitis; POEM: Patient Oriented Eczema Measure; PtGA: Patient Global Assessment.
The main limitations of our study are its single-center design, small study population, and missing information. Missing data can be attributed to the challenges of prospective data collection, such as patient non-adherence to follow-ups within the designated time frame. Although we only compared response with TT guidelines by month 6, the time marks used in our study are months 4 and 6, and TT strategy assessments by months 3 and 6. New target goals were proposed through a single-center panel consensus; however, we are confident that these goals translate meaningful clinical improvement for patients.
In conclusion, we report a remarkable response to dupilumab, similar to other real-life studies.5–7 Despite an initial partial response, the clinical response continues to improve past the 4-month mark. The rate of adverse events is slightly lower vs other real-world evidence.7,8 Similarly, the emergence of the above-mentioned events rarely resulted in the discontinuation of dupilumab. Moreover, we believe the current treatment revolution in AD allows for more ambitious treatment goals than those currently established at international recommendations.
Conflicts of interestC. Valente and B. Figueira Vilela declared no conflicts of interest whatsoever.
M.J. Paiva-Lopes received honoraria from AbbVie, Almirall, Boehringer Ingelheim, Janssen, Leo-Pharma, Eli Lilly, Novartis, Pfizer, Sanofi-Genzyme, and Viatris.
B. Duarte received honoraria as a speaker for Sanofi, Abbvie, Leo-Pharma and Lilly.