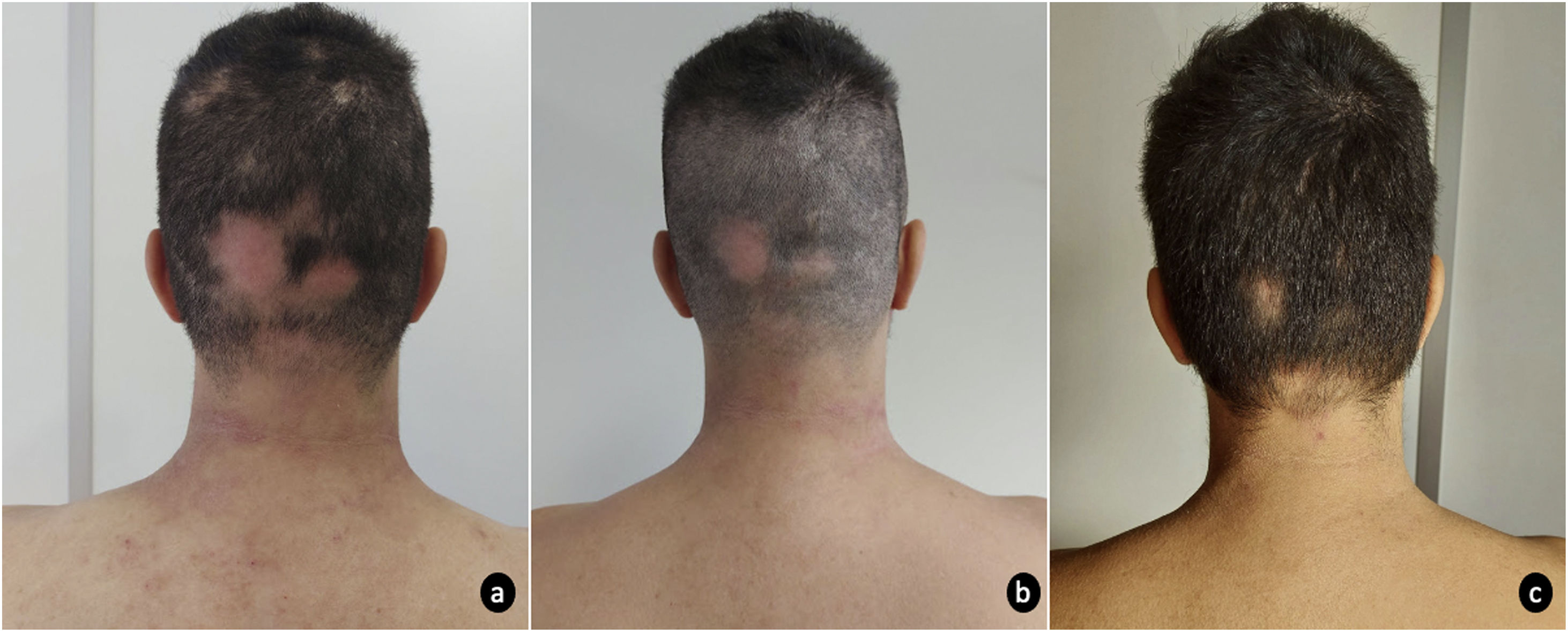
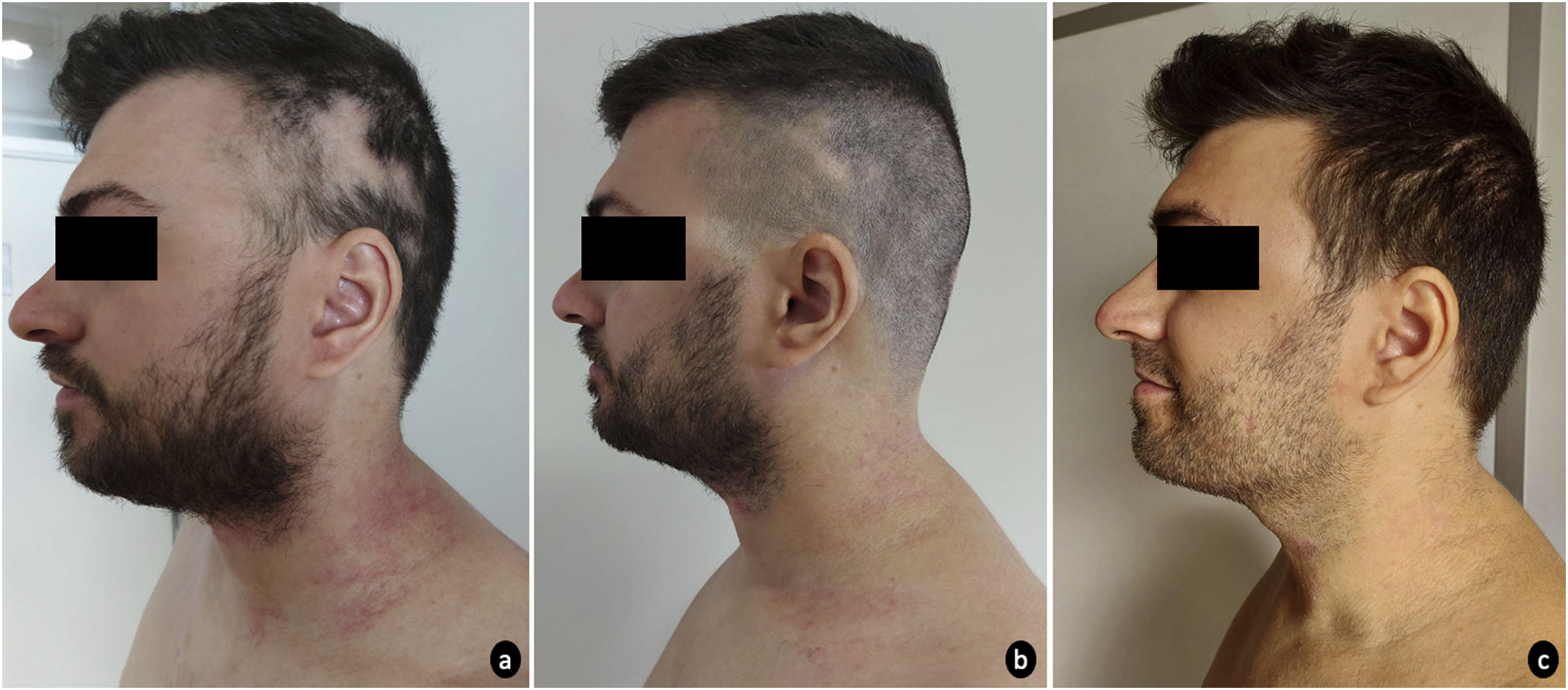
A 31-year-old man, with a personal history of asthma and allergic rhinitis was referred to our dermatology department due to severe atopic dermatitis (AD) refractory to conventional systemic therapies. The patient had mild AD since early childhood until the age of 27, when the dermatosis progressively worsened. Prior systemic treatment with oral steroids, cyclosporine (4mg/kg/day) and methotrexate (17.5mg/week) has proven ineffective. He presented with disseminated erythema and scaling, with predominant cervicofacial and flexural involvement. Treatment with dupilumab 300mg fortnightly was initiated, with primary failure of response at the 16th week of treatment and development of extensive multilocular alopecia areata (AA) at week 24. For this reason, dupilumab was switched to baricitinib 4mg/day. At baseline, skin lesions markedly involved the cervical region and limbs, with an eczema area and severity index (EASI) of 16 and SCORing Atopic Dermatitis (SCORAD) of 48.8, accompanied by a debilitating pruritus (Numeric Rating Scale itch (NRS itch) 8/10) that severely interfered with sleep (NRS sleep 8/10). Aside the AD lesions, the patient presented an extensive alopecia of the occipital and bi-parietotemporal regions (Severity of Alopecia Tool (SALT) 34%). At 4 weeks of therapy, the AD was improved (EASI 2.2; SCORAD 29.3) with significant reduction of associated pruritus (NRS itch 2) and no interference with sleep quality (NRS sleep 0). Moreover, AA was also responding with widespread hair regrowth of multiple regions (SALT 10%) (Figs. 1 and 2). Sustained improvement was reported at 12th week, with only scarce AD lesions mainly on cervical region (EASI 1.8; SCORAD 28.5) and hair regrowth in most AA areas (SALT 6%). Baricitinib was well tolerated, with only mild seborrhea as a side effect.
AD is an immunologically complex chronic inflammatory skin disease characterized by excessive activation of T lymphocytes and participation of several mediators such as interleukin (IL)-4, IL-13, IL-22 and interferon (INF)-γ.1 These mediators activate intracellular signaling pathways dependent on Janus kinase (JAK)1 and JAK2.2 AA, in its turn, is a non-scarring alopecia characterized by infiltration of hair follicles by TCD8+ cells and secretion of INF-γ, IL-15, and others γ-chain cytokines, whose intracellular signaling is dependent on JAK1-3.3,4 Up to one third of patients with AA have concomitant AD and recent studies suggest that AA is not only a type 1 inflammatory disease but is also involved in the Th2 immune pathway.5,6 Dupilumab is a monoclonal antibody that targets the IL-4 receptor, downregulating the Th2 response by blocking the signaling mechanisms of IL-4 and IL-13. Its role in AA has been conflicting, with both hair regrowth and loss seen in patients on dupilumab for AD.7 Baricitinib is an oral JAK inhibitor, blocking signaling pathways activated by multiple cytokines. It is currently approved for treatment of adults with moderate to severe AD who are candidates for systemic therapy. Although there is no approved drug for treating AA, two recent phase 3 clinical trials, BRAVE-AA1 and BRAVE-AA2, demonstrated efficacy of baricitinib in these patients.8 In our case, baricitinib resulted in a remarkable improvement of AD, whereas dupilumab failed to benefit, probably related to the pleiotropic inhibition of cytokines by the first. Despite the heterogeneity of cytokines involved in the pathophysiology of AD and AA, baricitinib demonstrated a rebust efficacy in both conditions, as this upstream immunological diversity converges to shared downstream effectors.
With this report, we praise the efficacy of baricitinib for treatment of AD and AA, both frequent, debilitating and potentially difficult to treat dermatological conditions. We also value the success of JAK inhibitors in the treatment of pathophysiological distinct entities, illustrating its promising versatility for the management of a wide number of immunomediated chronic diseases.
Conflict of interestMafalda Pestana has no conflicts of interest to declare.
Margarida Brito Caldeira has no conflicts of interest to declare.
Bruno Duarte is a speaker and/ or provides consultancy for Sanofi, Abbvie and Leo Pharma.