Upadacitinib is a Janus Kinase (JAK)-1 inhibitor approved for the treatment of moderate-to-severe atopic dermatitis (AD). Despite robust clinical trial data on its safety and efficacy profile, real-world evidence remains limited, especially in refractory forms.1–3 In Portugal, until very recently, access was restricted to patients with the most severe forms of AD. Eligibility required adult patients to have either failed treatment or contraindications to, at least, two conventional systemic immunosuppressants, dupilumab and baricitinib.
We conducted a 26-week prospective observational trial at a Portuguese AD referral center. Our goal was to assess the safety and efficacy profile of upadacitinib in adult patients with AD with use of, at least, two failed systemic immunosuppressants, baricitinib and dupilumab. Baseline demographic data were collected. Patient-reported outcome measures and objective outcomes were collected on weeks 0/6/16/26, with a 2-week error margin allowed for each period of time. Data were analyzed using descriptive statistics. Wilcoxon signed-rank test was used to compare scores between baseline and follow-up. Significance was determined at p<0.05.
The cohort included a total of 14 patients: 64.3% men; mean age, 29.9 (20–51) years; mean disease duration, 20.6 years; mean BMI, 27.1kg/m2. All patients received upadacitinib 30mg/day and had not responded to oral prednisolone, cyclosporine, methotrexate, dupilumab, and baricitinib. The mean duration of treatment was 10.6 (5–22) months for dupilumab (300mg subcutaneously every 2 weeks) and 8.1 (3–18) months for baricitinib (4mg/day).
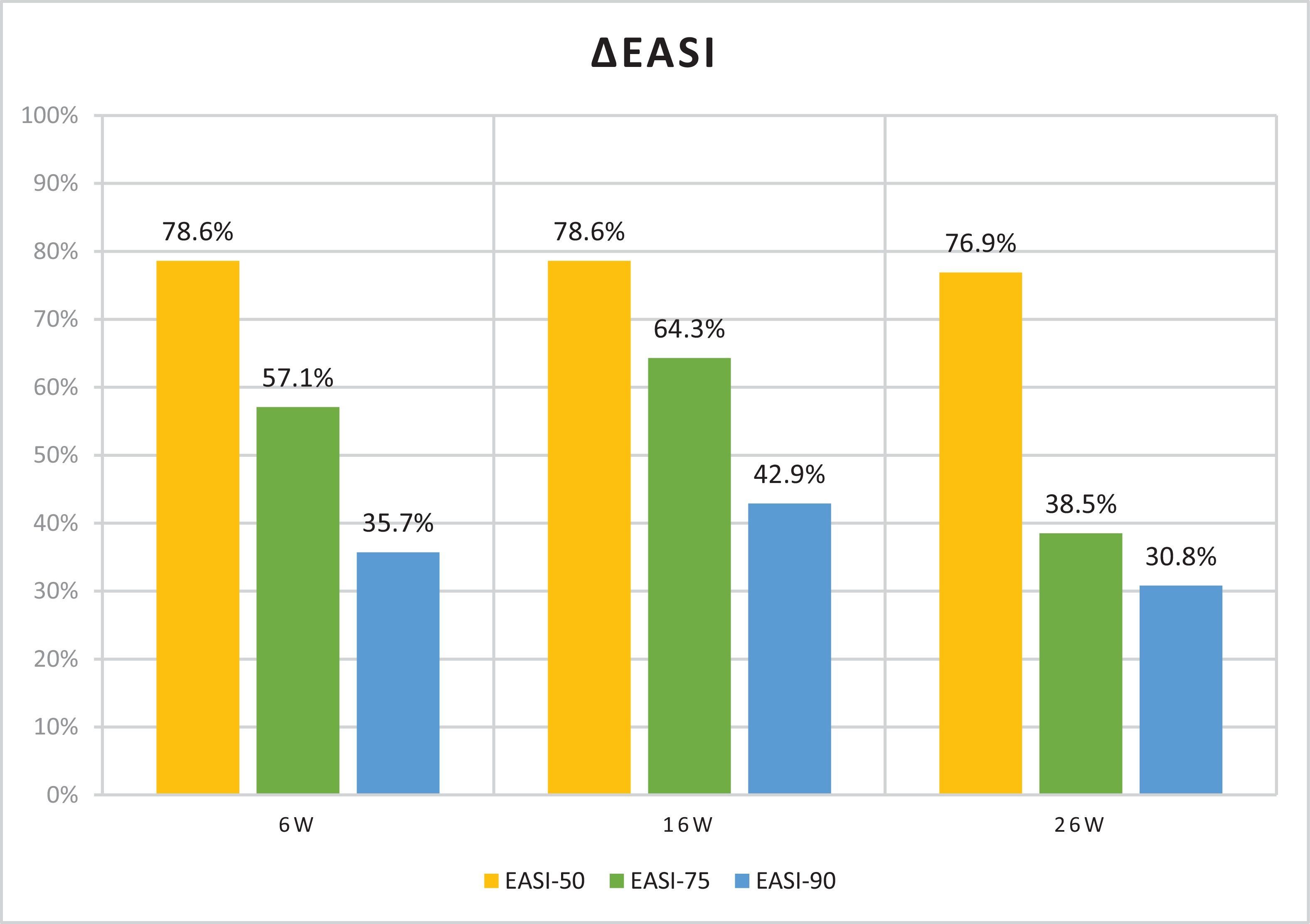
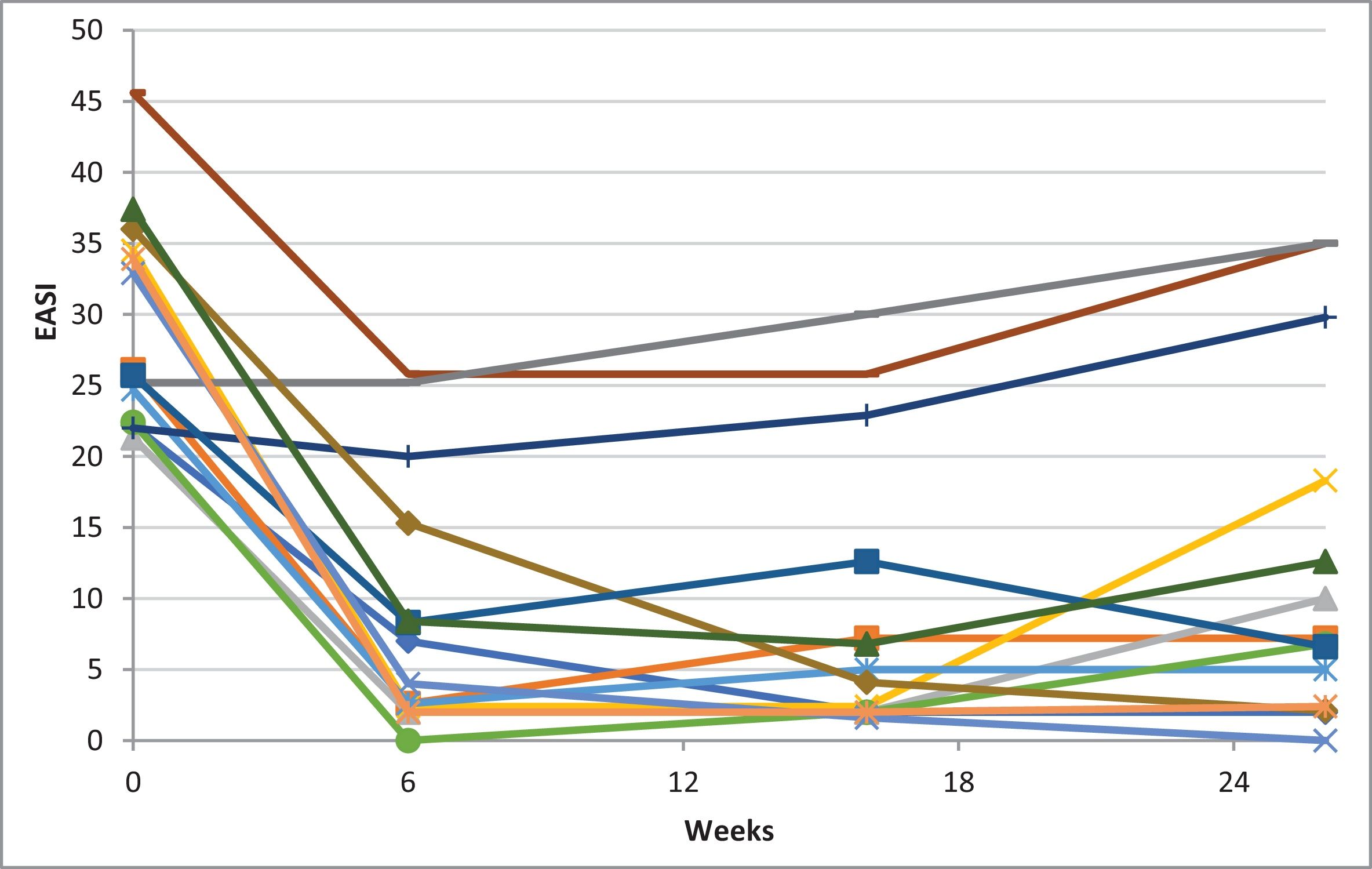
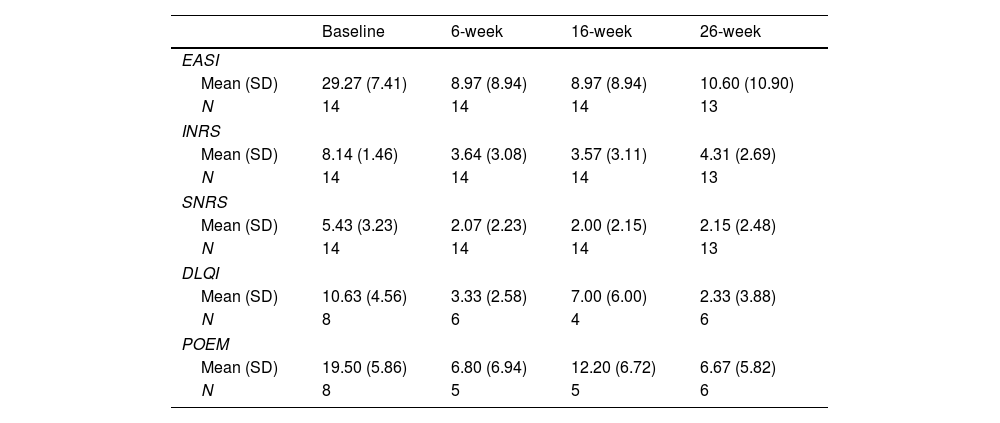
Table 1 illustrates the baseline scores and subsequent endpoint measurements. Significant improvements were seen across all subjective and objective scores in 6 weeks. Mean reductions of 69.2% in Eczema Area and Severity Index (EASI), 56.3% in itch-Numeric Rating Scale (NRS) and 52.0% in sleep-NRS were reported. Fig. 1 illustrates EASI-50 and EASI-75 responses. On week 6, 57.1% of patients achieved an EASI-75 response. On week 16, continued improvements are reported, with mean reductions of 67.7% in EASI, 57.2% in itch-NRS, and 49.7% in sleep-NRS; with 64.3% of patients achieving an EASI-75 response. Milder improvements continued on week 26, with mean reductions of 58.9% in EASI, 47.9% in itch-NRS, and 47.0% in sleep-NRS. At this point, 38.5% of patients had already achieved an EASI-75 response. All differences were statistically significant (p<0.001). Of note, as seen in Fig. 2, by week 26, some patients showed a slight increase in EASI scores vs week 16, nonetheless, with no statistical significance.
Objective outcomes measures and patient-reported outcomes measures variation with upadacitinib treatment.
| Baseline | 6-week | 16-week | 26-week | |
|---|---|---|---|---|
| EASI | ||||
| Mean (SD) | 29.27 (7.41) | 8.97 (8.94) | 8.97 (8.94) | 10.60 (10.90) |
| N | 14 | 14 | 14 | 13 |
| INRS | ||||
| Mean (SD) | 8.14 (1.46) | 3.64 (3.08) | 3.57 (3.11) | 4.31 (2.69) |
| N | 14 | 14 | 14 | 13 |
| SNRS | ||||
| Mean (SD) | 5.43 (3.23) | 2.07 (2.23) | 2.00 (2.15) | 2.15 (2.48) |
| N | 14 | 14 | 14 | 13 |
| DLQI | ||||
| Mean (SD) | 10.63 (4.56) | 3.33 (2.58) | 7.00 (6.00) | 2.33 (3.88) |
| N | 8 | 6 | 4 | 6 |
| POEM | ||||
| Mean (SD) | 19.50 (5.86) | 6.80 (6.94) | 12.20 (6.72) | 6.67 (5.82) |
| N | 8 | 5 | 5 | 6 |
DLQI: Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; INRS: Itch Numeric Rating Scale; SD: standard deviation; SNRS: Sleep Numeric Rating Scale; POEM: Patient Oriented Eczema Measure.
AE affected 50.0% of patients, including acne vulgaris (28.5%, N=4), dyslipidemia (14.2%, N=2), and herpes zoster (7.1%, N=1). Eczema herpeticum caused 1 patient to discontinue upadacitinib temporarily. Primary failures occurred in 2 patients, causing 1 patient to discontinue the drug on week 26 and another to start concurrent dupilumab on week 16.
As far as we know, we conducted a novel evaluation that focused exclusively on hard-to-treat AD patients who failed to respond cumulatively to three conventional systemic immunosuppressants and two innovative therapies (1 biologic drug and 1 JAK inhibitor).4–6 In this hard-to-treat cohort of 14 patients, upadacitinib 30mg/day demonstrated rapid and robust effectiveness, as documented on week 6. Nevertheless, a trend towards reduced effectiveness was observed on weeks 16 and 26, albeit the response remained clinically significant and superior to what was attained with prior therapies. Our EASI-75 and EASI-50 response rates were lower vs those reported in the literature, likely due to our highly refractory patient population.3,5,7
Although the treatment landscape for AD has changed dramatically over the past decade, many patients respond suboptimally to some, or most, novel therapies. For instance, 31–37% of dupilumab-exposed patients do not achieve an EASI-75 response by week 24.8 On the other hand, at the highest dose of 30mg/day upadacitinib has proven superior vs dupilumab in two different trials and, although no head-to-head data is available, meta-analysis have identified this JAK inhibitor as having the highest efficacy profile of all innovative drugs available, making it a potential best-in-class to address the most resistant AD patients.9,10 Our real-world finding verifies this data and highlights the suitability of upadacitinib for this patient cohort, even after failed biologics or other JAK inhibitors.
The safety profile was favorable, with no permanent AE-related discontinuations. Reported AEs were mostly mild and affected 50% of patients, which is consistent with other real-world data.5,7
Limitations include its single-center nature, small sample, missing data, and short follow-up.
In conclusion, high-dose upadacitinib significantly improves the most severe forms of AD as early as week 6, even in partial or non-responders to other biologics and JAK inhibitors. Upadacitinib could be considered a therapeutic option in this hard-to-treat cohort, particularly when other treatments have failed. However, a trend towards reduced effectiveness was observed by weeks 16 and 26, which has not been widely reported in the literature. Further studies, especially multicenter trials with longer follow-up periods, are needed to clarify this observation. No new safety signals were noted.
Conflicts of interestC. Valente, A. Ferreirinha, and P. Farinha declared no conflicts of interest whatsoever.
B. Duarte declared to have received speaker fees from Sanofi, Abbvie, Pfizer, Leo pharma, and Lilly.