The efficacy of omalizumab in the treatment of chronic spontaneous urticaria has been demonstrated in phase iii clinical trials, but limited information is available regarding real-life effectiveness using the weekly Urticarial Activity Score (UAS7). The aim of the study was to assess clinical response (UAS7≤6) and complete response (UAS7=0) rates at weeks 12 and 24 in a real-life cohort and to identify possible predictors of response to omalizumab.
MethodsClinical records of consecutive patients with moderate-to-severe chronic spontaneous urticaria (UAS≥16) treated with omalizumab at a university-affiliated reference dermatology department in Barcelona, Spain, from February 2014 to September 2017 were retrospectively reviewed. UAS7 values and patients’ evolution were assessed according to a predefined protocol. Statistical analysis of data was done using SPSS 18 statistical package (SPSS 18 Inc., Chicago, IL, USA) software.
ResultsForty-eight patients were included in the study. All of them completed at least 24-weeks of treatment and follow-up. At week 12, clinical response rates (UAS7<6) were 70.8% and complete response rates (UAS7=0) were 47.9%. At week 24, clinical response rates were 64.6% and complete response rates were 52.1%.
Patientswith long-term urticaria (≥18 months’ duration) were less likely to achieve a clinical response at week 12 (odds ratio: 0.25; 95% confidence interval 0.06-0.96). Previous immunosuppressive treatment tended to be associated with a lower probability of complete response at week 12 (odds ratio: 0.27 95% confidence interval: 0.07-1.02). H1-antihistamine treatment was associated with lower probability of response at week 24 (odds ratio: 0.1 95% 95% confidence interval: 0.01-0.88)
ConclusionsThe effectiveness and safety of omalizumab in real life are similar to efficacy and safety in clinical trials. Duration of disease, previous immunosuppressive therapy and requirement for concomitant H1-antihistamine treatment may be helpful in predicting response to omalizumab treatment.
Aunque la eficacia del omalizumab en el tratamiento de la urticaria crónica espontánea está demostrada en ensayos clínicos de fase iii, la información disponible sobre la efectividad en la vida real utilizando la puntuación de actividad urticarial semanal (UAS7) es escasa. El objetivo del estudio fue evaluar la respuesta clínica (UAS7≤6) y la respuesta completa (UAS7=0) en las semanas 12 y 24 en una cohorte de la vida real e identificar posibles predictores de respuesta al omalizumab.
MétodosSe llevó a cabo una revisión retrospectiva de los registros clínicos de pacientes consecutivos con urticaria crónica espontánea de moderada a grave (UAS≥16) tratados con omalizumab en el Servicio de Dermatología de un hospital universitario en Barcelona, España, desde febrero de 2014 a septiembre de 2017. Se evaluaron los valores de UAS7 y la evolución siguiendo un protocolo predefinido. El análisis estadístico de los datos se realizó utilizando el paquete estadístico SPSS 18 (SPSS 18 Inc., Chicago, IL, EE. UU.).
ResultadosSe incluyeron en el estudio 48 pacientes, que completaron 24 semanas de seguimiento. Las tasas de respuesta clínica (UAS7<6) y completa (UAS7=0) fueron del 70,8% y del 47,9% a la semana 12 y del 64,6% y del 52,1% a la semana 24.
Los pacientes con urticaria de larga duración (≥18 meses) tuvieron menor probabilidad de lograr una respuesta clínica en la semana 12 (odds ratio: 0,25; intervalo de confianza del 95%: 0,06-0,96). El tratamiento inmunosupresor previo tendía a asociarse con una menor probabilidad de respuesta completa en la semana 12 (odds ratio: 0,27; intervalo de confianza del 95%: 0,07-1,02). El tratamiento con antihistamínicos H1 se asoció con una menor probabilidad de respuesta a la semana 24 (odds ratio: 0,1; intervalo de confianza del 95%: 0,01-0,88).
ConclusionesLa eficiencia y la seguridad del omalizumab en la vida real son similares a la eficacia y la seguridad en los ensayos clínicos. La duración de la enfermedad, la terapia inmunosupresora previa y el requerimiento de tratamiento concomitante con antihistamínicos H1 pueden ayudar a predecir la respuesta al tratamiento con omalizumab.
Chronic spontaneous urticaria (CSU) is a common disease characterized by the spontaneous occurrence of itchy wheals, angioedema, or both, for a period of more than 6 weeks. The condition usually lasts approximately one year in 70% of patients, but it can persist for more than 5 years in up to 15%,1 with a detrimental effect on patient's quality of life.
Two drugs are currently approved for the treatment of CSU in Europe and North America: non-sedating H1-antihistamines (anti-H1) and the monoclonal antibody omalizumab. Anti-H1 are safe and cheap and are recommended in the current American and European guidelines for the initial treatment of patients with CSU.2,3 However, with standard doses, up to 50% of patients1 are unable to achieve a good control of the disease.
Omalizumab, a recombinant humanized monoclonal antibody, has shown high efficacy in phase III clinical trials, and is currently approved for the treatment of moderate-to-severe CSU unresponsive to anti-H1. However, there is limited information on the use of omalizumab in real life using the same doses, response evaluation parameters and endpoints that were used in phase III clinical trials.2,4,5
Disease activity in CSU can be assessed in real practice and in clinical trials with the weekly Urticaria Activity Score (UAS7), a simple scoring system that was proposed in the 2017 version6 of the EAACI/ GA2LEN/ EDF/ WAO guidelines. The patient quantifies the daily number of wheals (range: 0-none to 3-severe) and daily severity of pruritus (range: 0-none to 3-severe) for 7 consecutive days, with a maximum sum score of 42. UAS7 allows comparison of the results from different studies and centers, and is a useful tool for both clinical trials and real life practice.7
Few studies have assessed UAS7 response at weeks 12 and 24 in real life using omalizumab 300mg every 4 weeks.7–18 Real world and randomized clinical trials outcomes need to be compared because patient populations in both settings may differ; for instance, patients with inducible urticaria or CSU with severe comorbidities (history of cancer, parasitic infection, history of anaphylaxis, pregnancy, previous treatment last 30 days of cyclosporine, etc.) were excluded from clinical trials.2,4,5
The aim of this study was to assess the effectiveness and safety of omalizumab in real life and compare them with the efficacy and safety reported in clinical trials. We also wanted to identify possible predictors of response to omalizumab.
MethodsWe conducted a retrospective review of all patients with moderate- to-severe CSU (UAS≥16) treated with omalizumab from February 2014 to September 2017 at the Department of Dermatology of the Hospital de la Santa Creu i Sant Pau, a University-affiliated referral hospital in Barcelona, Spain. Data were retrieved from the medical records and from a prospective registry started in 2009, which received the approval of our local Ethics Committee (16/049 (OBS)), enrolling all patients starting omalizumab for CSU in our center. Patients’ informed consent was obtained prior to inclusion in the study.
In agreement with the summary of product characteristics (SmPC), and the European Guidelines,6 only patients with CSU who did not respond to high doses of anti-H1 (up to 4-fold licensed dose) and received the approved dose of omalizumab (300mg every four weeks) for at least 12 weeks (three doses) were included in this study. Omalizumab was administered by experienced medical and nursing personnel at the day-care hospital facilities.
Collected data included patients’ demographics (age, weight, height and body mass index [BMI]), duration of CSU prior to the start of omalizumab treatment, UAS7 value at baseline, week 12 and week 24, use of previous or concomitant immunosuppressants (cyclosporine A, azathioprine, mycophenolate mofetil and/or methotrexate) and other treatments (montelukast), adverse events, concomitant angioedema, concomitant inducible urticaria, and use of corticosteroids. Analytical parameters studied included blood counts and leukocyte counts, standard serum biochemistry determinations, serum C-reactive protein (CRP), total immunoglobulin E and complement (C3 and C4) levels, plasma D dimer levels, antinuclear antibody titres, and anti-thyroid antibody titres. Long-term urticaria was arbitrarily defined at 18 months after statistical analysis and consistent with the median duration of disease in a recently published cohort from Barcelona.19
UAS7 values were recorded twice daily during a week for every patient at baseline, before the start of treatment, and prior to visits corresponding to week 12 and week 24 of treatment. The outcome of treatment was defined according to UAS7 values as “clinical response” (UAS7 ≤6) and “complete response” (UAS7=0), as in published clinical trials.2,4,5
All adverse events (AEs) were recorded and classified into mild, moderate and severe. All cutaneous reactions that occurred on the injection site without systemic symptoms were considered to be mild AEs; anaphylaxis or death were considered as severe AEs, and all the other AEs related to omalizumab administration were considered to be moderate in severity.
Statistical analysisStatistical analysis of data was done using the SPSS 18 statistical package (SPSS Inc., Chicago, IL, USA) software. Chi-squared test with Yates correction was used to compare proportions. Mann-Whitney test was used to compare parametric variables. Linear regression was used to evaluate the correlation of UAS at week 12 and 24 with BMI and age. All tests were two-tailed and a value of p<0.05 was considered statistically significant.
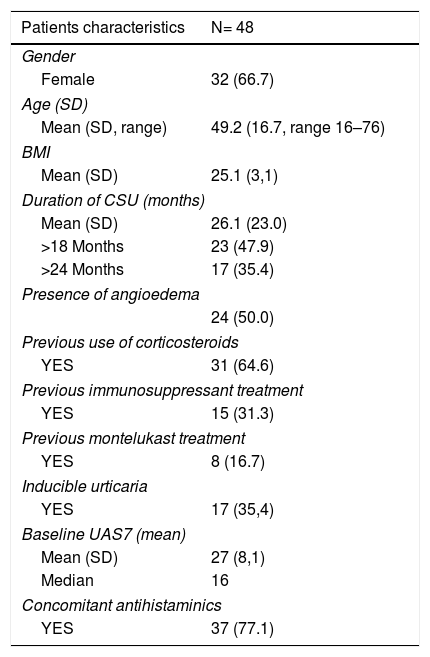
ResultsPatients and treatmentsForty-eight patients were included in the study. The baseline characteristics of patients are summarized in Table 1. Mean duration of CSU prior to the start of omalizumab treatment was 26.1 months (SD 23.0, median 16, range 2–84). Twenty-four patients (50%) had angioedema, and seventeen patients (35.4%) had inducible chronic urticaria (ten had delayed pressure urticaria, six cholinergic urticaria, four symptomatic dermographism, and unknown physical urticaria in one).
Baseline characteristics of patients.
| Patients characteristics | N= 48 |
|---|---|
| Gender | |
| Female | 32 (66.7) |
| Age (SD) | |
| Mean (SD, range) | 49.2 (16.7, range 16–76) |
| BMI | |
| Mean (SD) | 25.1 (3,1) |
| Duration of CSU (months) | |
| Mean (SD) | 26.1 (23.0) |
| >18 Months | 23 (47.9) |
| >24 Months | 17 (35.4) |
| Presence of angioedema | |
| 24 (50.0) | |
| Previous use of corticosteroids | |
| YES | 31 (64.6) |
| Previous immunosuppressant treatment | |
| YES | 15 (31.3) |
| Previous montelukast treatment | |
| YES | 8 (16.7) |
| Inducible urticaria | |
| YES | 17 (35,4) |
| Baseline UAS7 (mean) | |
| Mean (SD) | 27 (8,1) |
| Median | 16 |
| Concomitant antihistaminics | |
| YES | 37 (77.1) |
DE: desviación estándar; IMC: índice de masa corporal; UAS7: puntuación de actividad urticarial semanal; UCE: urticaria crónica espontánea.
Thirty-one patients (64.6%) received oral corticosteroids, eight (16.7%) montelukast and fifteen (31.3%) an immunosuppressive treatment prior to the start of omalizumab treatment. The use of concomitant corticosteroid and/or immunosuppressive treatment was not dependent on the duration of the disease. Prior immunosuppressive treatments included cyclosporine alone in nine patients, methotrexate alone in one patient, concomitant cyclosporine and methotrexate treatment in two patients, azathioprine in one patient and non-concomitant mycophenolate mofetil and cyclosporine in one patient. All immunosuppressive treatments were stopped at least two weeks prior to the start of omalizumab.
During omalizumab treatment patients were asked to continue treatment with anti-H1 if required, but it was stopped due to good control of the disease in 11 (22.9%) of patients, with no evidence of relapse upon discontinuation.
EffectivenessAt week 12, 70.8% (34/48) of patients had clinical response (UAS7 ≤6) and 47.9% (23/48) complete response (UAS7=0). Five patients discontinued treatment at week 12 because of therapeutic success (complete response), and their complete response status was maintained by week 24. Two patients stopped treatment due to lack of response at week 12. Fifteen patients who achieved complete response by week 12 maintained their complete response status by week 24, six lost response and two maintained clinical response.. From eleven patients who had “clinical response” but not “complete response” at week 12, six became complete responders at week 24, four lost response and one maintained clinical response. In five patients who did not achieve clinical response at week 12, the dose of omalizumab was increased to 450mg monthly, starting at week 12, and three of them obtained clinical response at week 24. Four of the remaining 9 patients who had no response at week 12 achieved complete response, two achieved clinical response and three remained in the non-responder group. At week 24, 64.6% of patients (31/48) achieved clinical response (58.3% excluding those patients who were up-dosed) and 52.1% (25/48) had complete response.
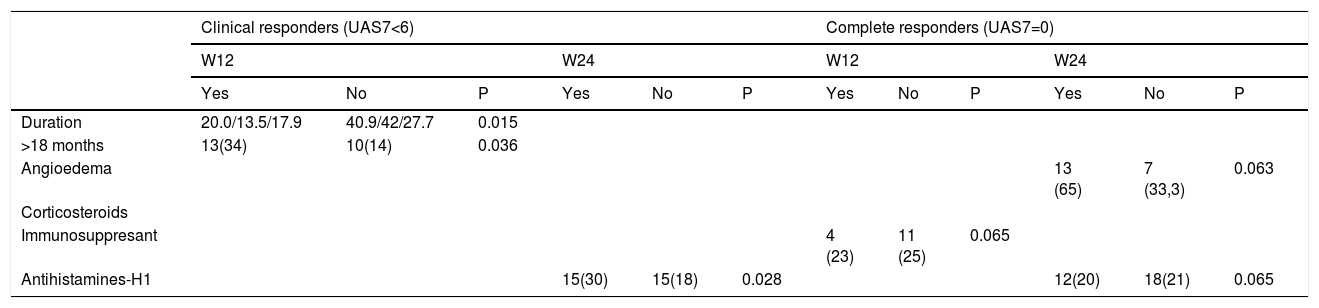
None of the clinical variables and laboratory parameters included in the analysis were associated with the achievement of clinical response or complete response at weeks 12 or 24, with the exception of disease duration, presence of angioedema, previous immunosuppressive treatment and concomitant anti-H1 treatment, as detailed in Table 2.
Results.
| Clinical responders (UAS7<6) | Complete responders (UAS7=0) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W12 | W24 | W12 | W24 | |||||||||
| Yes | No | P | Yes | No | P | Yes | No | P | Yes | No | P | |
| Duration | 20.0/13.5/17.9 | 40.9/42/27.7 | 0.015 | |||||||||
| >18 months | 13(34) | 10(14) | 0.036 | |||||||||
| Angioedema | 13 (65) | 7 (33,3) | 0.063 | |||||||||
| Corticosteroids | ||||||||||||
| Immunosuppresant | 4 (23) | 11 (25) | 0.065 | |||||||||
| Antihistamines-H1 | 15(30) | 15(18) | 0.028 | 12(20) | 18(21) | 0.065 | ||||||
S12/24: semana 12 y 24; UAS7: puntuación de actividad urticarial semanal; UCE: urticaria crónica espontánea.
Mean duration of disease was shorter in patients who achieved clinical response (UAS7 ≤6) at week 12 (13.5 months vs 42, P<0.015). Patients with long-term urticaria (≥18 months’ duration) were less likely to achieve clinical response (UAS7 ≤6) at week 12 (odds ratio [OR] 0.25, 95% confidence interval [CI] 0.06-0.96 for those with ≥18 months’ duration), but the duration of disease was not predictive of clinical response at week 24 or complete response at either week 12 or week 24.
A history of angioedema was associated with a higher probability of complete response only at week 24 (OR 3.33, 95% CI 1.02-10.90). Previous immunosuppressive treatment (cyclosporin in 13 out of 15 patients) was associated with a lower probability of complete response at week 12 (OR 0.27, 95% CI 0.07-1.02). The proportion of patients who continued using anti-H1 treatment was lower among patients who achieved response (OR 0.1 95% CI 0.01-0.88) or complete response (OR 0.25 95% CI 0.06-1.14) at week 24, but this association was not observed at week 12.
Safety profileNo serious adverse events (AEs) were reported, and no treatment course had to be discontinued because of AEs. Mild AEs (erythema in the administration site) were reported in two cases and moderate AE (headache and asthenia) was reported by one patient.
DiscussionOmalizumab is a monoclonal antibody approved for the treatment of moderate-to-severe CSU. Licensed dosage according to the European Medicines Agency-approved SmPC is 300mg every 4 weeks and 150 and 300mg every 4 weeks according to the Food and Drug Administration label. Patients treated with 300mg show better responses compared to those treated with 75mg and 150mg, and the higher dose is associated with better control of angioedema.5
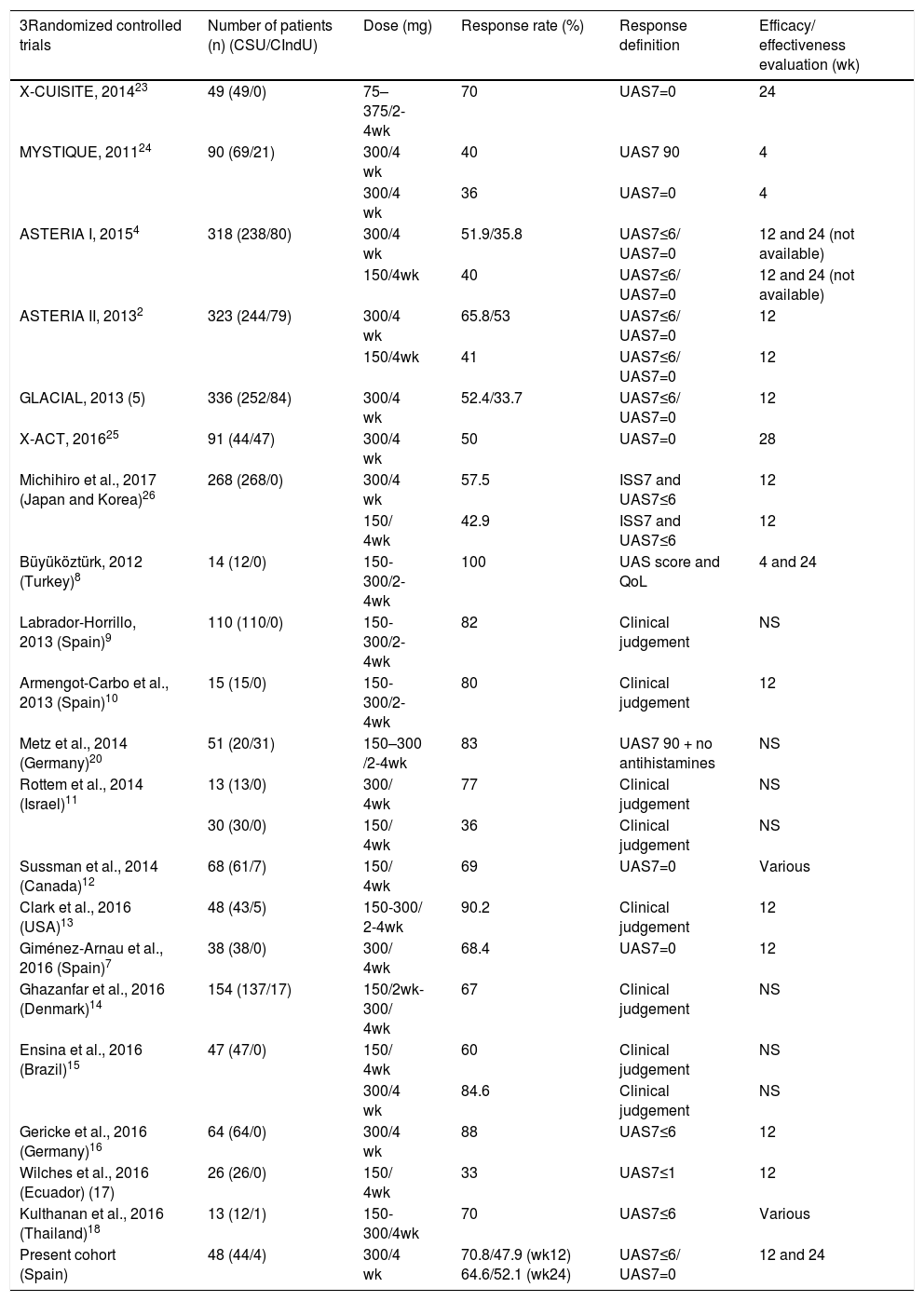
Previously published real-life studies show marked heterogeneity in comparing the percentage of omalizumab responders. Differences in dosage, definition of response and time point of evaluation are seen compared to clinical trials, as shown in Table 3. Many real-life studies define response as clinical improvement, a subjective criterion that makes comparisons difficult. UAS7 was first used in real-life clinical practice by Metz et al.,20 and other authors8,13–15,17,20 have subsequently used UAS7=0 (complete response) or UAS7≤6 (response). We agree with the EAACI/GA2LEN/EDF/WAO urticaria guideline6 recommendation that UAS7 should be the standard method of measurement and UAS7=0/UAS7≤6 the criteria of efficacy, as in clinical trials.
Omalizumab clinical trials and real-life studies.
| 3Randomized controlled trials | Number of patients (n) (CSU/CIndU) | Dose (mg) | Response rate (%) | Response definition | Efficacy/ effectiveness evaluation (wk) |
|---|---|---|---|---|---|
| X-CUISITE, 201423 | 49 (49/0) | 75–375/2-4wk | 70 | UAS7=0 | 24 |
| MYSTIQUE, 201124 | 90 (69/21) | 300/4 wk | 40 | UAS7 90 | 4 |
| 300/4 wk | 36 | UAS7=0 | 4 | ||
| ASTERIA I, 20154 | 318 (238/80) | 300/4 wk | 51.9/35.8 | UAS7≤6/ UAS7=0 | 12 and 24 (not available) |
| 150/4wk | 40 | UAS7≤6/ UAS7=0 | 12 and 24 (not available) | ||
| ASTERIA II, 20132 | 323 (244/79) | 300/4 wk | 65.8/53 | UAS7≤6/ UAS7=0 | 12 |
| 150/4wk | 41 | UAS7≤6/ UAS7=0 | 12 | ||
| GLACIAL, 2013 (5) | 336 (252/84) | 300/4 wk | 52.4/33.7 | UAS7≤6/ UAS7=0 | 12 |
| X-ACT, 201625 | 91 (44/47) | 300/4 wk | 50 | UAS7=0 | 28 |
| Michihiro et al., 2017 (Japan and Korea)26 | 268 (268/0) | 300/4 wk | 57.5 | ISS7 and UAS7≤6 | 12 |
| 150/ 4wk | 42.9 | ISS7 and UAS7≤6 | 12 | ||
| Büyüköztürk, 2012 (Turkey)8 | 14 (12/0) | 150-300/2-4wk | 100 | UAS score and QoL | 4 and 24 |
| Labrador-Horrillo, 2013 (Spain)9 | 110 (110/0) | 150-300/2-4wk | 82 | Clinical judgement | NS |
| Armengot-Carbo et al., 2013 (Spain)10 | 15 (15/0) | 150-300/2-4wk | 80 | Clinical judgement | 12 |
| Metz et al., 2014 (Germany)20 | 51 (20/31) | 150–300 /2-4wk | 83 | UAS7 90 + no antihistamines | NS |
| Rottem et al., 2014 (Israel)11 | 13 (13/0) | 300/ 4wk | 77 | Clinical judgement | NS |
| 30 (30/0) | 150/ 4wk | 36 | Clinical judgement | NS | |
| Sussman et al., 2014 (Canada)12 | 68 (61/7) | 150/ 4wk | 69 | UAS7=0 | Various |
| Clark et al., 2016 (USA)13 | 48 (43/5) | 150-300/ 2-4wk | 90.2 | Clinical judgement | 12 |
| Giménez-Arnau et al., 2016 (Spain)7 | 38 (38/0) | 300/ 4wk | 68.4 | UAS7=0 | 12 |
| Ghazanfar et al., 2016 (Denmark)14 | 154 (137/17) | 150/2wk-300/ 4wk | 67 | Clinical judgement | NS |
| Ensina et al., 2016 (Brazil)15 | 47 (47/0) | 150/ 4wk | 60 | Clinical judgement | NS |
| 300/4 wk | 84.6 | Clinical judgement | NS | ||
| Gericke et al., 2016 (Germany)16 | 64 (64/0) | 300/4 wk | 88 | UAS7≤6 | 12 |
| Wilches et al., 2016 (Ecuador) (17) | 26 (26/0) | 150/ 4wk | 33 | UAS7≤1 | 12 |
| Kulthanan et al., 2016 (Thailand)18 | 13 (12/1) | 150-300/4wk | 70 | UAS7≤6 | Various |
| Present cohort (Spain) | 48 (44/4) | 300/4 wk | 70.8/47.9 (wk12) 64.6/52.1 (wk24) | UAS7≤6/ UAS7=0 | 12 and 24 |
wk: week; NS: not stated; UAS7 90: ≥90% reduction of UAS7 with respect to baseline; mg: milligrams; CSU/CindU: Chronic spontaneous urticaria/chronic induced urticaria
Moreover, the evaluation time points in real-life studies have not been specified8,9,11,12,14,15,18,20 or have differed (i.e. week 4)8 with respect to those used in the main clinical trials,2,4,5 namely week 12 and week 24. Late response can only be assessed by reporting results at week 24, as in the ASTERIA I and GLACIAL clinical trials4,5,8 and the real-life study by Büyüköztürk et al..8 However, in none of these studies was any information provided in terms of complete or clinical response, and Büyüköztürk et al. only reported the absolute reduction in UAS7.
The response to omalizumab treatment in real-life studies has been shown to be better than in clinical trials,2,4,5,7–18,20 as detailed in Table 3. The clinical response (UAS7 ≤6) and complete response (UAS7=0) rates at week 12 in our cohort were also numerically higher than in the ASTERIA I and II and the GLACIAL clinical trials, but the ‘response’ rates in earlier real-life studies are higher than in our cohort, probably due to the choice of clinical judgement, not UAS7, as outcome measure.8,13,15 Comparison of response rates at week 24 cannot be made, since both GLACIAL and ASTERIA I only include safety and Itch Severity Scale (ISS) data and ASTERIA II did not include an evaluation at week 24.
The mean duration of CSU was much longer in pivotal trials (approximately 7 years (range 6.1 to 7.4) compared to approximately 2 years in our series. This might provide an explanation for differences in response, as suggested by lower response rates at week 12 in patients with longer duration of symptoms in our series.
Mean age, baseline UAS7, presence of angioedema, and prior use of corticosteroids do not differ in our cohort with respect to pivotal studies. Patients’ mean body mass index in our cohort was 25.1 (IC 95% 24, 2-26), compared to 29.4, 29.3 and 29.5 in GLACIAL and ASTERIA I and II, respectively. Curto-Barredo et al21 recently identified high BMI as a predictor of partial response to 300mg dose, but good response with updosing. However, we did not find any statistically significant association in our series.
According to Maurer et al.,1 inducible urticaria is a negative predictor of response, and it was an exclusion criteria in pivotal trials. Seventeen (35.4%) of our patients had inducible urticaria at some point during the course of their disease, but this was not found to be a prognostic factor regarding response to omalizumab at any time point in our cohort. High baseline UAS7 values were not a negative prognostic factor regarding response to omalizumab, again in contrast with the findings of Maurer et al..1
In our cohort, complete response rates increased with time whereas clinical response rates decreased from week 12 to week 24. Some patients with clinical response at week 12 lost this status by week 24. On the other hand, complete response status tended to be consistent over time; 25 out of 48 patients maintained their complete response status from week 12 to week 24, including 5 patients who stopped treatment at week 12 because of complete response.
Presence of angioedema has also been associated with a decreased therapeutic response to omalizumab.1 In our real-life cohort, a history of angioedema was associated with a higher probability of complete response at week 24; no significant associations were found at other time points and with clinical response. However, the limitations of UAS7 to assess angioedema may influence this result. Otherwise, our data are consistent with those of Tonacci et al,22 who found clinical response to omalizumab treatment in patients with CSU to be independent of disease severity or IgE levels at baseline.
Regarding prior treatment of CSU, ASTERIA studies included only approved doses of anti-H1 as background therapy, whereas the GLACIAL study permitted higher doses of anti-H1 as well as other types of background therapy. In a post-hoc analysis, the intensity of prior treatment has not been found to influence the response to omalizumab,22 in agreement with our cohort data. On the other hand, the odds of achieving complete response at week 12 were close to 4:1 for patients who had not been previously treated with immunosuppressive agents. Previous treatment with immunosuppressive agents was common in our population (31.3% versus 9.5% in the GLACIAL study), and a trend has been previously reported for immunosuppressant-naïve patients to present a better response to omalizumab,14 which is consistent with our results.
Limitations of this study are its observational nature, without a control group, measurement scale limitations and the small sample size compared to pivotal trials.
In conclusion, this retrospective review of a cohort of 48 consecutive patients treated with omalizumab 300mg every 4 weeks for at least 12 weeks in real life practice shows numerically better response rates than randomized clinical trials at week 12.2,4,5 At week 24, more than 50% of patients had complete response as defined by UAS 7. Long term (≥18 months) duration of CSU was associated with a lower probability of response to omalizumab at different time points. A similar tendency was observed for previous immunosuppressive treatment. The safety profile of omalizumab in our cohort was consistent with randomized clinical trials, with minimal, tolerable and infrequent side effects.
Further studies will be needed to confirm our results and their applicability to other patient populations.
Conflicts of interestX. Cubiró and E. Rozas-Muñoz declare no conflicts of interest. L. Puig, E. Serra-Baldrich and J Spertino have received speaker's honoraria and have participated in clinical trials sponsored by Novartis
The authors thank Dr Ignasi Gich Saladich, Department of Clinical Epidemiology, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain, for his assistance in the statistical analysis, and Dr Oriol Yélamos for his assistance in reviewing the manuscript.
Please cite this article as: Cubiró X, Spertino J, Rozas-Muñoz E, Serra-Baldrich E, Puig L. The Effectiveness of Omalizumab Treatment in Real-Life is Lower in Patients with Chronic Urticaria Longer than 18 Months’ Evolution and Prior Immunosuppressive Treatment. Actas Dermosifiliogr. 2019;110:289–296.