Hidradenitis Suppurativa (HS) is a debilitating chronic inflammatory disease of the apocrine gland-bearing skin, for which effective medical treatment remains elusive. Several immunological derangements are now identified in the disease.1 Adalimumab is so far the sole European Medicines Agency approved drug. It shows encouraging, albeit suboptimal results.2 More immunosuppressants are in the pipeline. Despite this shift towards anti-inflammatory therapies in HS, evidence is scarce regarding the use of systemic steroids (SS), with only a limited number of case reports3–5 and series6,7 available. We aimed to evaluate SS as adjuncts to other medical therapies in HS.
A retrospective cohort study was conducted. The setting was an Adnexal Skin Diseases Clinic in a tertiary Dermatology department in Lisbon. Data was captured by searching the electronic and written medical records of the clinic. Patients were eligible if they had moderate or severe HS, as defined by the International HS Severity Score (IHS4), treated with SS at least in one occasion. Primary endpoint was a clinical response as defined by the HS Clinical Response Score (HiSCR).8 Changes in patient-reported outcomes (Dermatology Life Quality index – DLQI and pain Numeric Rating Scale - NRS) were also evaluated. For statistical analysis, a Wilcoxon signed-rank test at a level of significance of 0.05 was used with STATA/IC 15.1 (STATA Corp., Texas, USA).
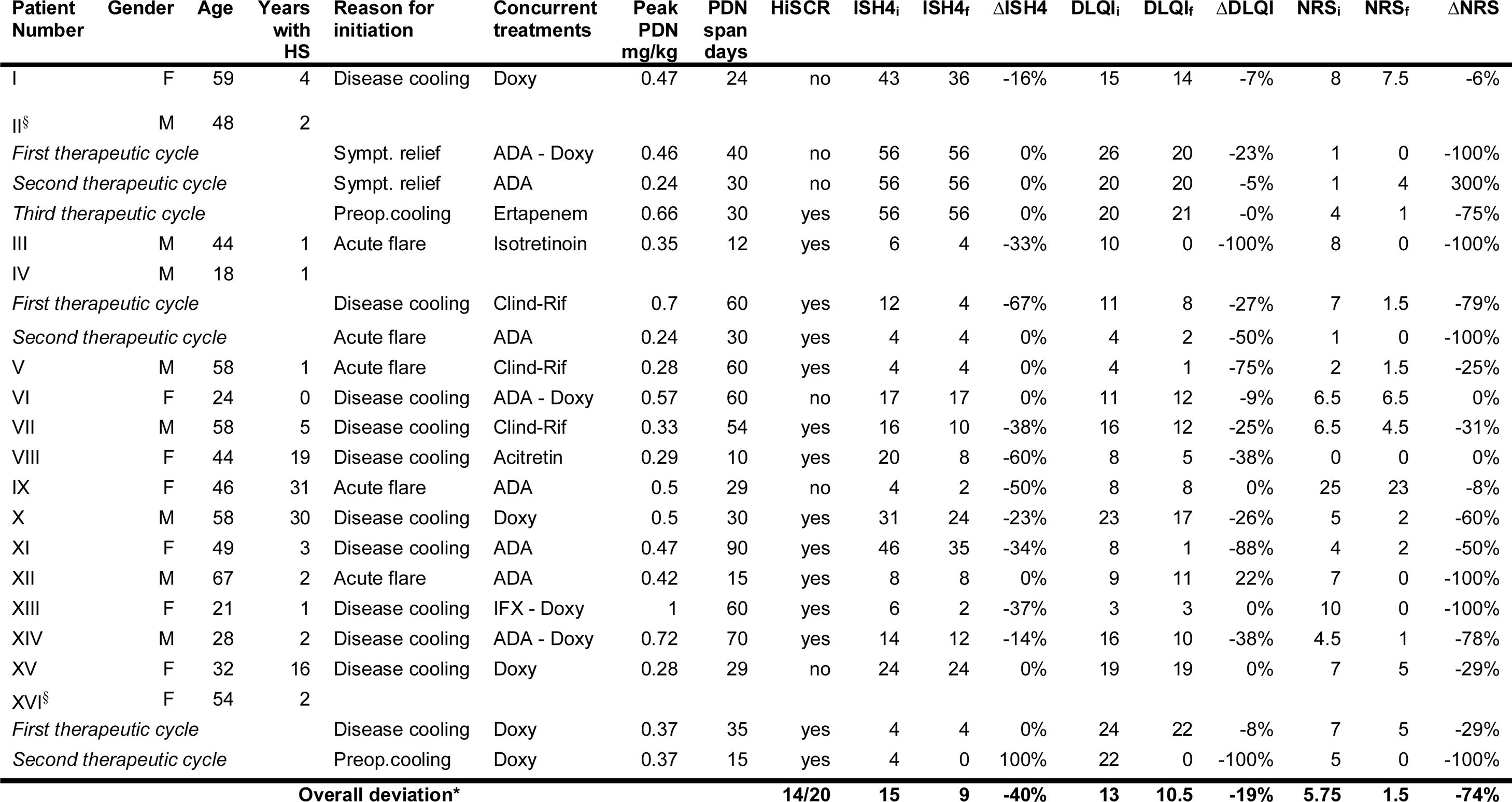
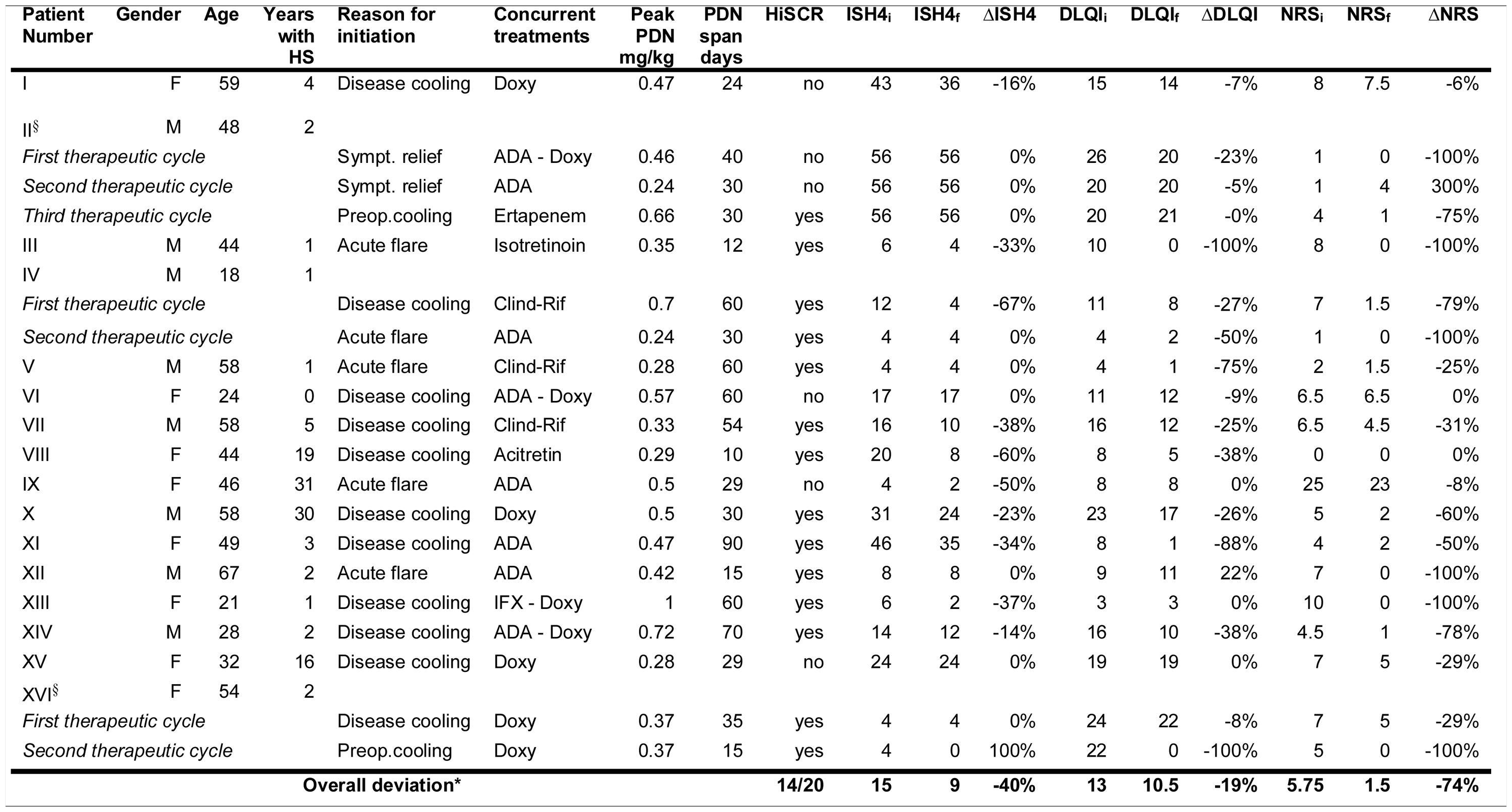
Among 121 HS patients followed at the clinic, 20 (16.5%) met eligibility criteria and 16 were analysed (Table 1). Four excluded due to poor compliance or lost to follow-up). Most patients were women (9/16), Caucasian (15/16), with a mean age of 45 years (18-67) and a mean duration of disease of 7.5 years (1-31). Most had severe disease (10/16; median IHS4 of 15).
Patients general data and treatment outcomes with adjunct systemic steroids. All scores present a pretreatment, a posttreatment and a delta value which is represented, respectively, by the letters i (initial), f (final) and Δ (change with treatment).
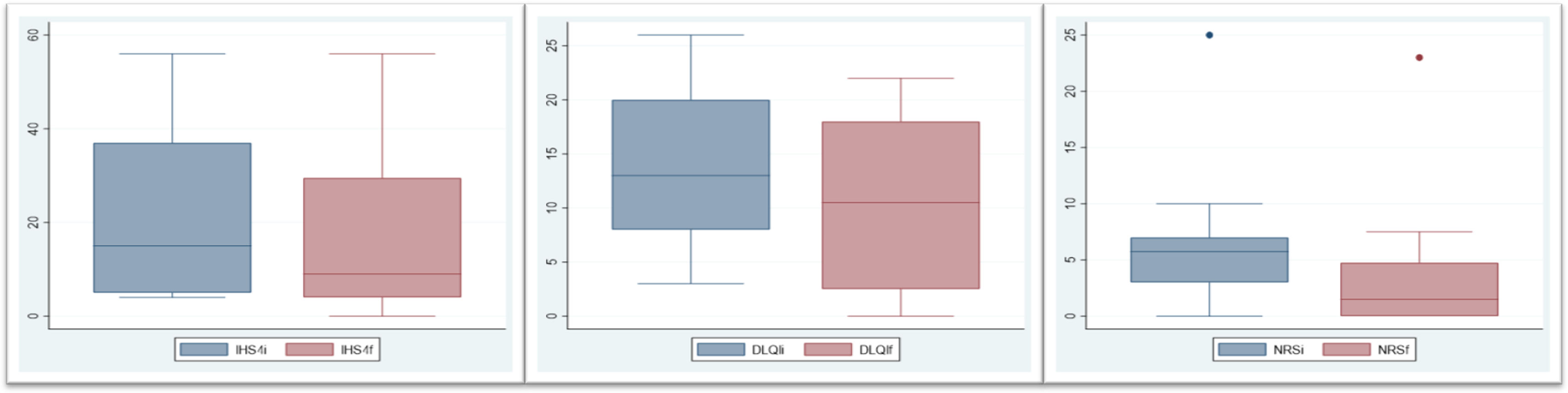
*All scores (IHS4, DLQI and NRS) and their respective Δ are represented by median values. All changes were statistically significant (Wilcoxon signed-rank test, p<0.05).
§Patient II and XV had only draining-fistulae. HiSCR was considered to be met despite absence of changes in IHS4, as treatment with PDN led to a clinically meaningful reduction in suppuration, inflammatory burden and overall disease severity.
HS – Hidradenitis Suppurativa; PDN – Prednisolone; Preop – Preoperative; Sympt – Symptomatic; ADA – Adalimumab; Doxy – Doxycycline; Clind-Rif – Clindamycin – Rifampicin; IFX – Infliximab.
Twenty cycles of adjunct systemic steroids were performed (three patients underwent >1 cycle). Therapy was initiated for disease cooling (11), acute flares (5), symptomatic relief (2) or preoperative cooling (2). The median maximum prednisolone dose was 0.44mg/kg (0.28-1) and the median duration was 30 days (10-90). SS were mostly used in combination with doxycycline (9 cycles) followed by adalimumab (8 cycles). Most cycles (14/20, 70%) met the HiSCR. Those who did not, were performed in patients with higher disease activity (median IHS4 of 33.3 in non-responders vs 10 in responders). Median IHS4 reduced 40% (p=0.0012) (Fig. 1). A significant improvement was observed in all patient-reported outcomes: median pain NRS and DLQIs reduced, respectively, 74% (p=0.0007) and 19% (p=0.003). Three patients had remarkable disease worsening shortly after steroid withdrawal. No treatment discontinuation or remarkable adverse events were noted.
There’s a paucity of research regarding the use of systemic steroids in HS, albeit they are prescribed in more than 1% of patients’ visits.9 To the best of our knowledge, this is the largest series to date evaluating SS as adjunct therapy in HS. Our results suggest that a short-to-medium term taper can be beneficial to rapidly control the painful hyperinflammatory flares while conventional HS treatments achieve proper disease control. Previously, long-term low-dose SS have showed advantages,7 but the risk of cumulative steroids’ detrimental effects must be considered. Additionally, uncontrolled inflammation is thought to increase the risk of postsurgical complications.10 In this series, 2 patients were treated preoperatively with favourable results and SS may thus be helpful to include in preoperative cooling strategies.
In conclusion, moderate-to-severe HS patients are likely to benefit from addition of SS to other medical therapies for both disease control and preoperative care. Due to limitations in the present study, regarding its retrospective design and sample size, a placebo-controlled trial is warranted to further clarify the role of SS in the anti-inflammatory strategies of HS.
Please cite this article as: Duarte B, Cunha N, Lencastre A, Cabete J. Uso de los corticoides sistémicos en el tratamiento de la hidradenitis supurativa moderada-grave. Actas Dermosifiliogr. 2020;111:879–883.