The second of this series describes the characteristics of 3 types of photobiologic studies: the light test, the photochallenge test, and the photopatch test. We explain how the tests are carried out, the expected results, and their clinical usefulness in various photodermatoses. These tests are needed before attempting to induce adaptation (skin hardening or light tolerance) in the most debilitating cases.
En esta segunda parte se describen las características de los 3 tipos de estudios fotobiológicos: el fototest, la fotoprovocación y la prueba del fotoparche. Se detalla la metodología, los resultados esperados y la utilidad clínica de estos métodos en las distintas fotodermatosis estudiadas. Estos estudios son esenciales para la inducción de fotoadaptación o fototolerancia que se emplea para los casos más invalidantes.
Photobiologic studies are skin tests performed to determine a person's degree of photosensitivity and assess abnormal cutaneous responses to light. In the second part of this review, we will look at the different types of tests performed in clinical practice and focus on the interpretation of results in idiopathic and exogenous photodermatoses.
Types of Photobiologic StudiesPhototestPhototesting involves exposing an area of skin to a known dose of UV or visible light and then observing, recording, and interpreting the response (erythema, whealing, or pigmentary changes) at the irradiated site after a pre-established time (generally 24 hours).
The threshold above which different biologic responses are triggered is established by exposing the individual to incremental doses of light radiation. Measures include minimal erythema dose (MED), minimal urticaria dose (MUD), immediate pigment darkening, and minimal tanning dose. These last 2 measures are not widely used in clinical practice, but they do have experimental value and are also used to determine the sun protection factor of sunscreens.
Phototesting has a range of clinical applications.
- -
Diagnosis. The phototest is used to confirm a diagnosis of solar urticaria, systemic phototoxicity, or photosensitivity (reduced sensitivity threshold to light) in certain idiopathic photodermatoses, such as chronic actinic dermatitis (CAD). It can also help to characterize certain secondary photodermatoses induced by endogenous agents, such as erythropoietic protoporphyria or photosensitive forms of lupus erythematosus (lupus tumidus and subacute cutaneous lupus erythematosus).
- -
Therapy. The phototest is used to calculate the starting dose (30%-70% of MED) for desensitization therapy in photodermatoses.
- -
Prognosis and response monitoring. The phototest can also be conducted at different points throughout the course of a disease to monitor response to treatment, for example, to assess changes in photosensitivity in patients with solar urticaria undergoing phototherapy or immunomodulator therapy.
Phototests should preferably be performed on parts of the body that are not usually exposed to the sun. The test site recommended by the Spanish Photobiology Group (GEF) is the lower back and buttock area, although some authors consider the abdomen or the inner aspect of the arm or forearm to be acceptable. Antihistamines and nonsteroidal anti-inflammatory drugs (NSAIDs) must be withdrawn at least 2 days before the test; corticosteroids, psoralens, chlorpromazine, and high-dose vitamins a week beforehand; and chloroquine and immunosuppressive medication at least a month beforehand.
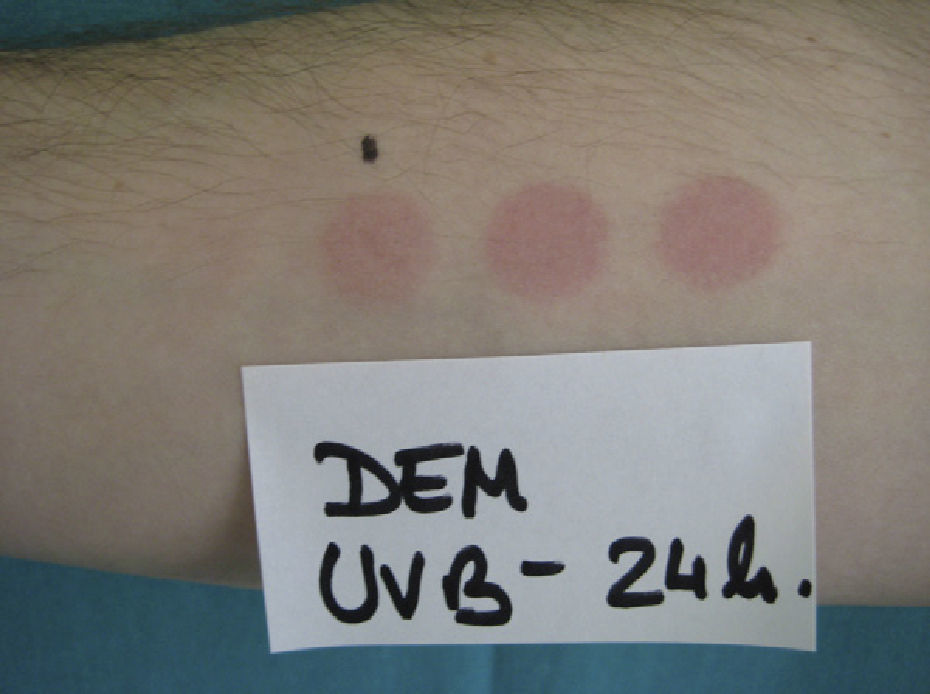
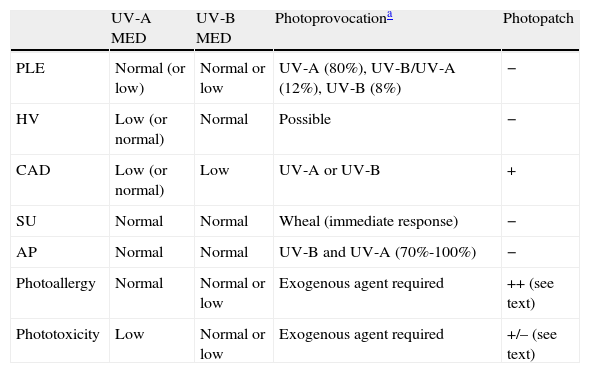
The MED for a given wavelength in the UV spectrum is the minimum dose of radiation (J/cm2) needed to induce erythema (Fig. 1). Because UV-B radiation provokes erythema, the MED always refers to the erythemal response to UV-B unless otherwise specified. Clinical skin abnormalities induced by UV-A radiation are considered to be abnormal responses to UV-A light. Erythematous reactions are generally evaluated by the naked eye, but more objective methods, such as laser Doppler perfusion imaging, may be needed in lesions with diffuse or indistinct borders.1
Because practically 100% of effective erythema-producing radiation is UV-B radiation, broadband fluorescent UV-B lamps are generally used for phototesting. However, while these lamps offer stable output, are simple to use, and are relatively cheap, their spectral region is far from that of natural sunlight. Solar simulators offer the best spectral match in this respect because, when fitted with appropriate interference filters, they closely approximate natural light. The erythemogenic effect of solar UV-A radiation can also be measured with filters that block wavelengths under 315 to 320 nm. Fluorescent light kits that emit UV-A light do not generally produce sufficient radiation to provoke erythema, unless the patient has a considerably reduced threshold to this portion of the spectrum, as occurs in certain phototoxic reactions. It is therefore advisable to use a monochromator, if available, to test sensitivity to UV-A light. Note that any skin reaction to visible light from any source is abnormal.
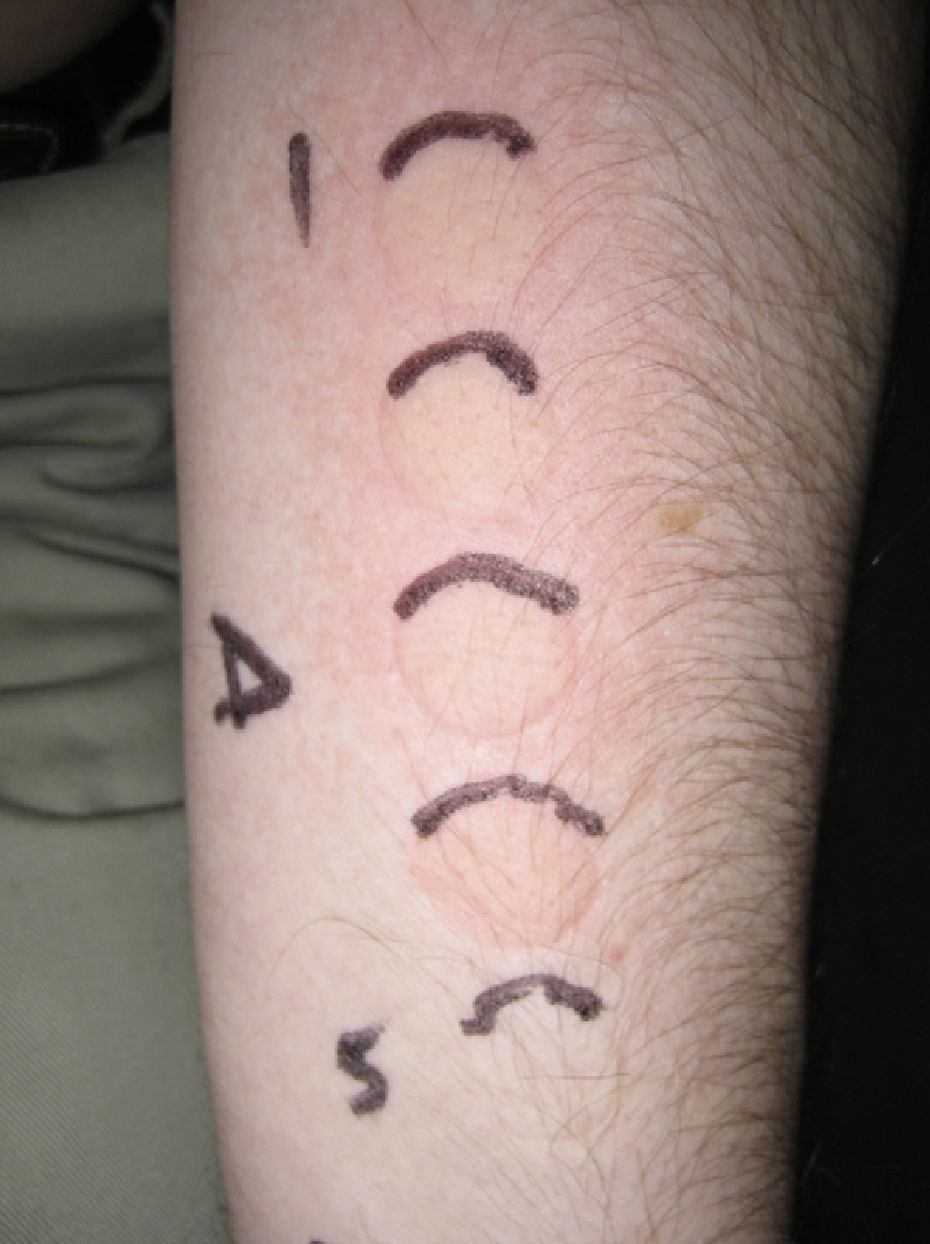
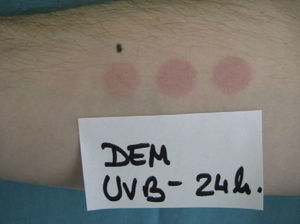
The MUD is the minimum dose of radiation needed to produce a wheal located exclusively or predominantly in the irradiated field. It must be accompanied by a reference to the action spectrum: UV-B, UV-A, visible, or infrared light, or a combination of these (Fig. 2). Irradiation doses from visible or infrared light sources cannot be measured in J/cm2. Unlike MED responses, MUD-provoked reactions generally appear several minutes after exposure and last for between 30 and 90 minutes. Inhibition spectra can result in delayed reactions, with wheals sometimes appearing after several hours.
Minimal urticaria dose (MUD) in a patient with solar urticaria.
Whealing response. MUD determined using a fluorescent lamp with 5 test fields and a filter to determine the exposure dose according to skin contact time (Gigatest UVB, Medisun). (Photograph courtesy of Dr Diego de Argila, Hospital La Princesa, Madrid, Spain).
The phototest in patients with solar urticaria is in the strictest sense a photoprovocation test. Standardization is difficult because of the variability of individual factors (phototype, tanning ability, test site, or food-related factors such as colorants). To determine the action spectrum, the European Dermatology Forum recommends using a solar simulator or a monochromator to irradiate 3 different sites in the buttock area with 6J/cm2 of UV-A light, 60 mJ/cm2 of UV-B light, and visible light from a slide projector for 10 minutes. The reaction is scored on a 6-point scale, where 0 indicates no response; (+), just perceptible erythema (corresponding to the MUD); +, erythema in the irradiated area; ++, erythema outside the irradiated area; +++, wheal in part of the irradiated area; and ++++, wheal in the entire irradiated area. The MUD is determined by irradiation with incremental doses of radiation.
PhotoprovocationPhotoprovocation consists of repeatedly exposing an area of skin to a pre-established dose of radiation of a shorter or longer wavelength with the aim of reproducing the clinical lesions being investigated. The test should be performed on a part of the body that is generally not exposed to sunlight. The test field should be larger than that used in phototesting, as photoprovocation sometimes induces separate papules and the response could be underestimated if smaller fields are used. The recommended size is 5×8cm.
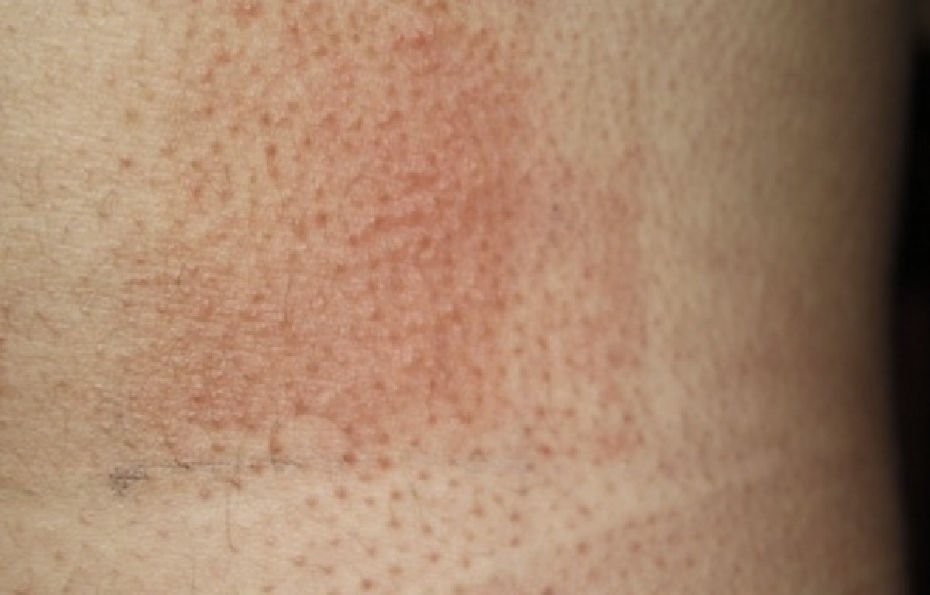
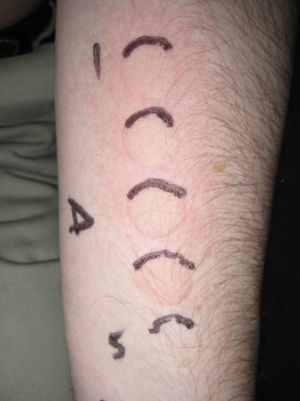
The following photoprovocation protocol has been proposed for polymorphic light eruption (PLE): irradiation of a previously exposed area of skin measuring 5×8cm for 3 consecutive days with polychromatic UV-A light at a dose of 60 to 100J/cm2 (Fig. 3) and polychromatic UV-B light at a dose of 1.5 times the UV-B MED, with reading of results at 24 and 72 hours.2 Other authors have successfully provoked lesions on the arms of patients with PLE using lower UV-A doses (10-20J/cm2 for 4 days) and narrowband (NB) UV-B therapy (0.4 and 0.8J/cm2 for 2-4 days). They used fluorescent UV lamps fitted in a cylindrical device into which the patients’ arms were placed.3 It is important to distinguish between PLE lesions and erythema when using UV-B radiation. The best time of the year for photoprovocation in PLE is early spring, before patients are exposed to the intense summer sun.
The recommended protocol for CAD is irradiation of an unexposed area of skin (5×8cm) for 3 consecutive days with UV-A light (doses of 0.5, 1, 5, 10, 20, and 30J/cm2) and UV-B light (doses of 0.5, 1, and 1.5 times the UV-B MED); results should be read at 24, 48, and 72 hours and after a week.2 Photoprovocation tests should preferably precede any photopatch tests. It should be noted, however, that photoprovocation is often not necessary in CAD, as phototests generally produce clear results: very low MEDs and abnormal UV-A responses.
Photoprovocation results vary greatly from one series to the next. This variability, combined with the fact that the test is laborious and time-consuming, means that photoprovocation is of limited clinical value. It can, however, be useful in cases of PLE with an unclear clinical history or in photosensitive forms of lupus erythematosus.4
Photopatch TestingPhotopatch testing is performed when the patient's history and the physical examination suggest a photosensitivity disorder in which various exogenous chemical substances may be involved. This test is indicated in the diagnosis of photoallergic contact dermatitis and the evaluation of certain idiopathic photodermatoses (e.g., CAD) or photodermatoses that cannot be explained by the patient's history.
Photopatch tests are generally used in photobiology and contact dermatology units.5 The methods employed vary considerably between centers, with variations in allergens, vehicles, concentration, occlusion time, dose and type of radiation, time of readings, and interpretation of results. In 2002, a panel of European dermatologists and photobiologists met with the aim of standardizing most of these variables to facilitate the comparison of results between centers.5 However, the allergens and allergen concentrations used in different photopatch series vary with the geographical area and their inclusion depends on the frequency of sensitization in the population. Numerous photopatch studies have been undertaken in recent years to identify and determine the frequency of photoallergens.6–12 In a publication reporting the results of a photoallergen study in Spain, the Spanish Photobiology Group agreed to add most NSAIDs and UV filters marketed in Spain to the next baseline photopatch series.13 A subsequent European multicenter study confirmed that the European baseline photopatch series should also include UV filters and NSAIDs.14
Currently, the phototest technique consists of the occlusive application of duplicate sets of allergens on the patient's back (normally the upper back, next to the spine) for 2 days. The allergens can be attached using a range of patch devices. On day 2 (48hours), the patches are removed and any skin reactions observed are noted. One of the allergen sets is then covered with an occlusive dressing and the other is irradiated with 5J/cm2 of UV-A radiation, measured using a radiometer. In patients with CAD, it may be necessary to irradiate the area with a suberythemal UV-A dose to avoid confusion with abnormal reactions to UV-A radiation. The results are read for a second time on day 4 (2 days after irradiation) and scored using the International Contact Dermatitis Research Group scoring system (Table 1). If the results are unclear on day 4, another reading is made on day 7.15
Intensity of Photopatch Skin Reactions According to the International Contact Dermatitis Research Group Scoring System.
| - | No reaction |
| +/- | Doubtful reaction (erythema only) |
| + | Weak positive reaction (erythema, infiltration, papules) |
| ++ | Strong positive reaction (erythema, infiltration, papules, vesicles) |
| +++ | Very strong positive reaction (erythema, infiltration, vesicles, blisters) |
Important factors in photopatch testing are irradiation dose, occlusion time, and type of lamp. This last factor has been shown to affect results.8 An ideal light source should have a continuous emission spectrum in the UV-A range (320-400nm) and sufficient irradiance to generate 5 to 10J/cm2 in a reasonable time (several minutes).16 One study that analyzed 3 light sources with different emission spectra used in photopatch testing showed that the broadband UV-A lamp had the highest sensitivity.17 A later study confirmed that UV-A irradiation results in more positive reactions.15
Photopatches have traditionally been irradiated 48 hours after occlusion in contact dermatitis units and 24 hours after occlusion in photobiology units. An occlusion time of 48 hours has been shown to be associated with a higher rate of detection of photoallergy cases.18
Most studies use an irradiation dose of between 1 and 10J/cm2, although some authors have recommended 5J/cm2 for photopatch testing.19,20 In 2004, following the recommendations of the European Task Force for Photopatch Testing, the Spanish Photobiology Unit established an irradiation dose of 5J/cm2.13 In a recent study, Kerr et al.14,59 obtained a much higher percentage of positive results with 5J/cm2 than with 2.5J/cm2. Accordingly, in the absence of contraindications, a dose of 5J/cm2 should be used.
The optimum concentration for UV filters in photopatch testing has been established in recent years. Most filters are tested at a concentration of 10% in petrolatum. Benzophenone-4 should not be used at a concentration of more than 2% in petrolatum because of the risk of false positives.21 However, studies of sunscreens have shown that most skin reactions are elicited by the irritant effects of filters and excipients.22,23
Proposed System for a Photobiology StudyThere are no established guidelines or protocols for performing a photobiology study. As mentioned above, it is difficult to guarantee reliability and reproducibility due to the difficulty of controlling for all possible individual and contextual factors.
One group of authors proposed the following system based on their experience24:
- -
Day 1: Conduct phototests with UV-B, UV-A, and visible light and read the results immediately to check for wheals. Apply a duplicate set of photopatches.
- -
Day 2: Determine the UV-B MED and irradiate one set of patches with a dose of 5 to 10 J/cm2 of UV-A or with 50% of the UV-A MED.
- -
Days 3 and 5: Read the results of the irradiated and nonirradiated photopatch sets.
Not all tests are appropriate for all patients. A thorough medical history should generally lead to a suspicion of a particular photodermatosis, thereby guiding the selection of appropriate tests.
Clinical Application of Photobiologic StudiesUtility and Results of Photobiology Tests in Different PhotodermatosesTable 2 shows the expected results of photobiologic tests for a range of idiopathic photodermatoses.
Expected Phototest and Photopatch Results in Idiopathic Photodermatoses.
| UV-A MED | UV-B MED | Photoprovocationa | Photopatch | |
| PLE | Normal (or low) | Normal or low | UV-A (80%), UV-B/UV-A (12%), UV-B (8%) | − |
| HV | Low (or normal) | Normal | Possible | − |
| CAD | Low (or normal) | Low | UV-A or UV-B | + |
| SU | Normal | Normal | Wheal (immediate response) | − |
| AP | Normal | Normal | UV-B and UV-A (70%-100%) | − |
| Photoallergy | Normal | Normal or low | Exogenous agent required | ++ (see text) |
| Phototoxicity | Low | Normal or low | Exogenous agent required | +/– (see text) |
Abbreviations: AP, actinic prurigo; CAD, chronic actinic dermatitis; HV, hydroa vacciniforme; PLE, polymorphic light eruption; SU, solar urticaria.
Adapted from Bylaite et al. 2009 (Br J Dermatol. 2009;161 Suppl 3: 61-8).
The best way of diagnosing PLE is through a detailed medical history, with attention to the relationship between the skin reaction, light exposure, and the course of the lesions. Evaluation becomes problematic, however, when the patient's history is inconclusive, and furthermore patients often visit the dermatologist weeks or even months after the eruption. In such cases, phototesting and photoprovocation can provide valuable information that will guide the diagnosis and help to predict the severity of the condition.3,25–27
Patients with PLE tend to have a normal photosensitivity threshold. In other words, they generally have normal sensitivity to UV-A light and a normal UV-B MED, although these may be lower than normal in more severe forms of the disease. In a retrospective study of 110 cases of PLE, the UV-B MED was reduced in a significantly higher proportion of men (43%) than women.25
The results of photoprovocation tests are highly variable across different series of patients with PLE.26 In some cases, tests failed to provoke PLE lesions in the majority of patients tested,26 while in others, they elicited positive reactions in up to 93% of patients.25,27 In most cases, the lesions were triggered by UV-A radiation or UV-A and UV-B radiation; few cases were triggered by UV-B radiation alone. In one series of 68 patients, PLE lesions were provoked by UV-A fluorescent lamps in 56% of cases, by NB–UV-B lamps in 50% of cases, by both types of lamps in 80.9% of cases, and by broadband UV-B lamps in just 18% of cases.3 The likelihood of a positive reaction with UV-A light was associated with young age and lower MEDs.3
The action spectrum and dose required to provoke lesions can vary over time in the same patient, probably due to seasonal factors.27 It has even been suggested that this dose can vary between different types of PLE lesions.28
The higher component of UV-A and UV-B radiation in the springtime in countries with a temperate climate could explain why PLE is more common in these countries than in tropical areas.29 Furthermore, the use of sunscreens that protect against erythema but not against the effects of UV-A radiation could lead to higher sun exposure, possibly explaining, at least partly, why these sunscreens fail to prevent PLE lesions.29 Nevertheless, one open, placebo-controlled, intraindividual photoprovocation trial showed that both UV-A and UV-B absorbing filters were effective at preventing PLE.30
Photoprovoked reactions are useful for evaluating the predominant type of lesion in PLE (papule, plaque, vesicle, or erythema) and for performing biopsies to facilitate the correlation of clinical and pathological findings.2 However, some authors have found that provocation with artificial light or with suberythemal doses of natural sunlight produces contradictory and difficult-to-interpret results due to the large number of variables (irradiation dose, exposure site, tolerance of the patient at the time of testing, etc.).24
According to one group of authors, the high proportion of positive photopatch test reactions to fragrances and UV filters in PLE patients might be due to higher exposure to these products.25 However, it is also possible that these reactions were actually contact photodermatitis reactions, as patients with this disorder do not generally attribute their lesions to the use of sun creams.
Hydroa VacciniformeThe action spectrum in most cases of hydroa vacciniforme lies in the UV-A range, with very few cases caused by UV-B light.31 Patients may or may not have a low sensitivity threshold to UV-A radiation.
Repetitive broadband UV-A irradiation with doses of 30, 50, and 75J/cm2 in affected and unaffected areas (back or arms) of patients with hydroa vacciniforme can cause erythematous vesicles that are histologically identical to those caused by sunlight; the healing process is also similar.24 Photoprovocation with doses of 10J/cm2 has also triggered lesions on the oral mucosa.2
Solar UrticariaPhototesting in patients with solar urticaria can identify the action spectrum (UV-B, UV-A, visible light, or a combination) and the degree of photosensitivity (via MUD determination). Immediate whealing following exposure to UV-B, UV-A, or visible light should be considered a positive reaction.
Most cases (up to 57%) of solar urticaria are caused by visible light, with or without a UV-A component.32 Up to 20% of cases have a wide action spectrum that includes UV-B, UV-A, and visible light, but few cases are caused by UV light alone.32 The action spectrum also varies between different geographical areas and races. European patients, for instance, appear to be more sensitive to UV or broadband light (UV-visible light) than Japanese patients, who appear to be sensitive to visible light alone.33 There have also been reports of solar urticaria with an action spectrum in the infrared range.31 The action spectrum tends to remain unchanged over the course of years, as the disease develops, although it can occasionally shift to longer wavelengths.34
Re-irradiation with longer wavelengths (500-630nm) immediately after exposure to the action spectrum has been seen to inhibit wheal formation in some patients.35 This inhibitory phenomenon—known as the inhibition spectrum—has been demonstrated in up to 68% of Japanese patients and is probably the result of light interfering with binding between the photoproduct and immunoglobulin (Ig) E or mast cells.36 The inhibition spectrum could explain why in some cases provocation tests with natural sunlight trigger lesions in patients who do not react to artificial light37,38 and it may also explain delayed responses (sometimes occurring hours after exposure). The inhibition spectrum may also have a role in the induction of light tolerance in patients receiving phototherapy.
Some patients with solar urticaria and an action spectrum in the UV-A range may also develop more intense and larger wheals when exposed to light with a longer wavelength (visible light) before, at the same time, or immediately afterwards.39,40 This phenomenon is known as the augmentation spectrum. It is augmentative rather than additive as the same effect is not achieved when the order is reversed. Preirradiation with visible light probably activates the chromophore, enabling it to absorb a wider range of light within the action spectrum. The augmentation spectrum (present in up to 29% of patients) could explain small—sometimes daily—changes in photosensitivity (measured by the MUD) observed in some patients, although these tend to have little clinical relevance.34 Interaction between the action spectrum and the augmentation spectrum might also explain why short exposures to sunlight cause solar urticaria in patients who only react to high doses of radiation from artificial sources.31
A negative phototest result for a single source of light, or even several sources, does not rule out a diagnosis of solar urticaria. In such cases, provocation with natural sunlight (exposure of the upper chest to midday sun for 15-30 minutes, preferably in the spring or autumn) is essential.
Chronic Actinic DermatitisThe phototest (performed with a solar simulator or an other source of broadband light) is nearly always positive in patients with CAD, except in very early phases of the disease when there are few symptoms.41 Most patients are sensitive to UV-B radiation, but there have been reports of cases triggered by broadband UV radiation and even UV-A radiation alone. Very few cases are induced exclusively by visible light (around 600nm), and while these patients do not meet the diagnostic criteria for CAD, some authors believe that they might be special cases with different chromophores.42
The UV-B MED is almost always reduced at 24 hours, but in addition to erythema, patients also develop papules or eczema and intense itching. The reaction peaks 7 to 24 hours after the phototest. In some patients, involvement of extensive areas of skin makes it difficult to choose a test site. In very severe cases, the patient may need to be admitted to a dark room for several days so that topical or systemic corticosteroids or immunosuppressive drugs can be suspended without causing an unwanted flare-up. In patients with a negative phototest, additional tests must be performed to rule out similar conditions such as atopic dermatitis, photoaggravated seborrheic dermatitis, and even cutaneous T-cell lymphoma.
Patch and photopatch tests should also be performed in patients with CAD because a range of allergens and photoallergens, many of which are airborne, can trigger and perpetuate the condition. Up to 80% of patients test positive to 1 or more allergens in these tests.43–48 A large panel of allergens should be tested, including the national baseline series, topical medications, UV filters, and plants. Based on reports in the literature, the most common contact allergens implicated in CAD are Compositae oleoresin extracts, metals, fragrances, colophony, rubber antioxidants, phosphorus sesquisulfide, and UV filters.43–48
It may not always be possible to irradiate the photopatch test site with a 5-J/cm2 dose of UV-A radiation, because this can trigger an abnormal erythematous response in some patients. In such cases, suberythemal doses—determined by the patient's UV-A MED—are necessary. It is important to perform the tests when the disease is controlled to avoid false positives encountered in patients with angry back syndrome.
CAD is a chronic condition. Based on the results of a cohort study at a tertiary referral centre, abnormal photosensitivity is resolved in 20% of patients over a 10-year period.49 Few cases of contact allergy have been resolved in the series studied to date,49,50 and in such cases, allergen avoidance and adequate sun protection are essential for achieving gradual clinical improvement.49 Prognosis is worse in patients with severe photosensitivity or patients sensitized to multiple unrelated allergens.49
Actinic PrurigoUV-A and UV-B MEDs are generally normal in patients with actinic prurigo.51
Abnormal sensitivity to monochromatic UV-B light at a dose of 50 to 60mJ/cm2 has been detected in up to two-thirds of Mexican patients with actinic prurigo.51 The administration of daily doses of 3 to 5mJ/cm2 of UV-B radiation for 15 days and of 2.5mJ/cm2 of UV-A radiation for 10 days has provoked actinic prurigo lesions.51 Photoprovoked lesions are similar to those seen in PLE.52
Exogenous Photosensitivity Induced by Drugs and Other Chemical Agents: Phototoxicity and PhotoallergyPhototoxic reactions can be diagnosed by phototesting both during and after the use of the causative agent. The normalization of a low MED on withdrawal of a suspected drug confirms a diagnosis of phototoxicity and enables identification of the cause in patients taking multiple drugs. The tests should be performed in a lesion-free (unexposed) area. Patients typically have very high sensitivity to UV-A radiation (and much less frequently to UV-B radiation) while taking the drug, but this normalizes several days after withdrawal.
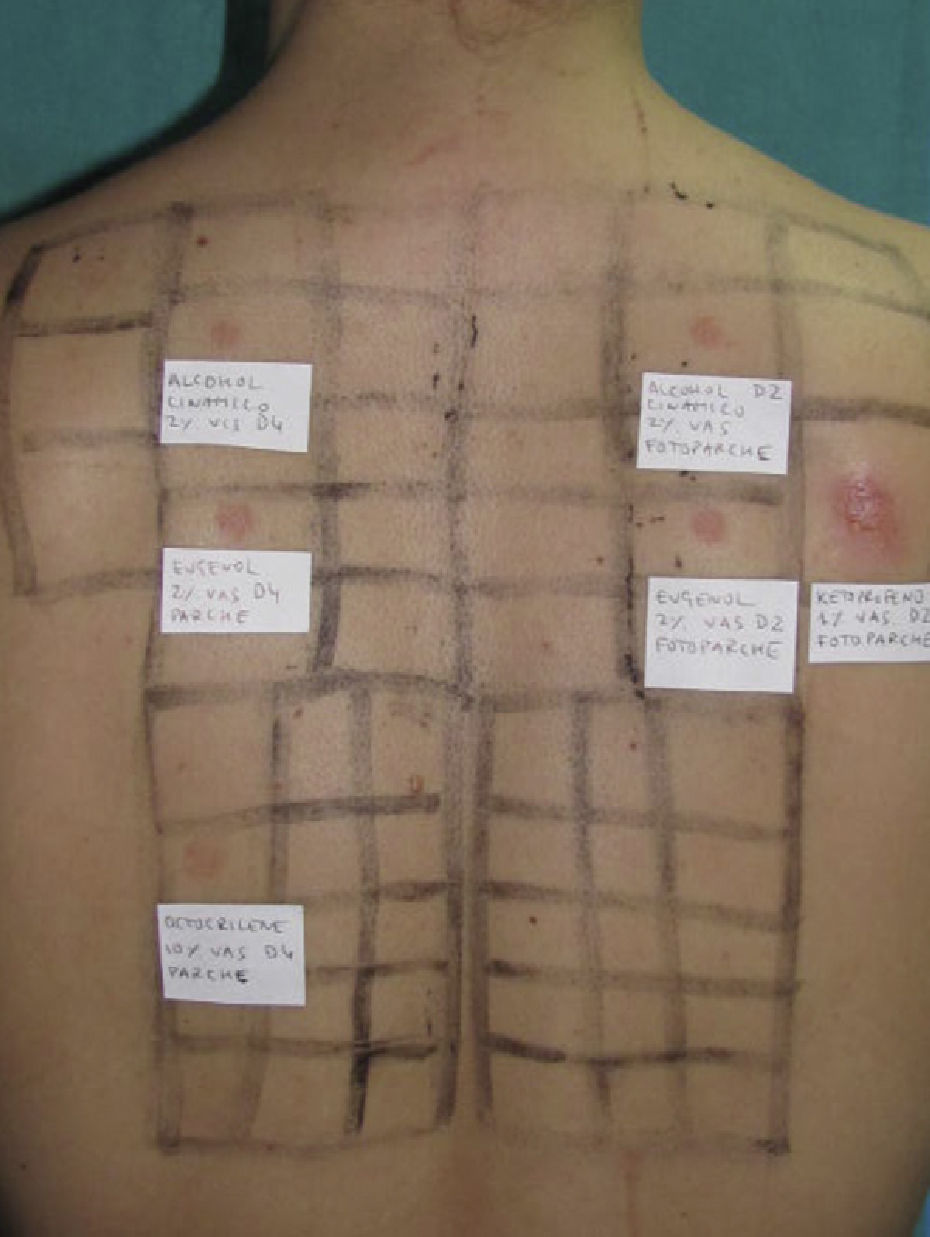
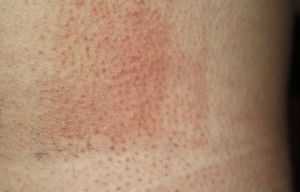
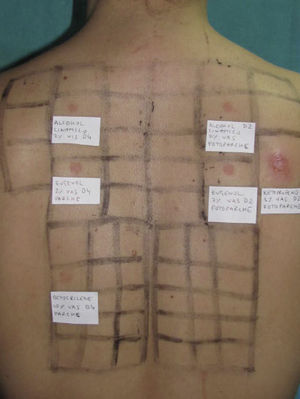
Photopatch tests should be performed in patients with suspected phototoxic and photoallergic reactions (Fig. 4). The results should be interpreted as follows8,10:
- 1.
Positive patch test on irradiated side and no reaction on nonirradiated side: contact photoallergy
- 2.
Positive patch test on irradiated and nonirradiated sides: contact allergy
- 3.
No reaction on either side: negative result
- 4.
Stronger reaction on irradiated side than on nonirradiated side: photoaggravated contact allergy
- 5.
Weaker reaction on nonirradiated side than on irradiated side: photoinhibited contact allergy
- 6.
Reaction with a morphologic pattern and time course typically seen in skin irritation: irritant reaction
Photopatch test.
Panel of UV filters and fragrance mix I components. Allergic contact photodermatitis due to nonsteroidal anti-inflammatory drug: positive photopatch test to ketoprofen. The reaction to octocrylene (UV filter series) in the nonirradiated patch set is probably an irritant reaction and the negative result in the irradiated patch set might be due to an inhibitory UV-A effect.
One group of authors described 4 morphologic phototest patterns to aid in the distinction between photoallergic and phototoxic reactions. The patterns were a classic photoallergic crescendo pattern, an immediate reaction with a decrescendo pattern (phototoxicity), a plateau pattern, and a combined pattern with an immediate and delayed reaction of unknown origin.53
Finally, it is essential to determine the relevance of all positive photopatch test results within the context of the patient's history, the presence of the allergen, and the existence of a temporal relationship between UV exposure and onset of photosensitivity. The most common photoallergens identified in the 1960s and 1970s were halogenated salicylanilides and chlorinated phenols (fenticlor), used as antibacterial agents in soaps. Musk ambrette was withdrawn from the market several years ago, but this fragrance is still an ingredient in products imported from Asia54 and therefore might be relevant in cases of contact photoallergy.
The prevalence of positive photopatch test results is currently low.5,13,53 UV filters8 and NSAIDs9 are the most common allergens involved in photoallergic contact dermatitis, followed by fragrances,10 promethazine,11 and certain plants.48,55 Ketoprofen tops the list of photoallergens in Spain, followed at quite a distance by benzydamine and etofenamate.13 Fragrance mixes tend to cause contact rather than photoallergic reactions. Photoallergy to ketoprofen is also common in other countries, such as Belgium56 and Sweden57; the prevalence of this photoallergen has been attributed, at least in part, to prescribing habits and the drug's photosensitizing capacity.58 Ketoprofen cross-reacts with benzophenone-3, cinnamic aldehyde (fragrance mix component), and phenofibrate.56,57 According to a European multicenter study published in 2012, ketoprofen, etofenamate, octocrylene, and butyl methoxydibenzoylmethane were the most common chemical substances involved in contact photoallergy.14
Light Tolerance or UV HardeningExposure to small doses of UV radiation induces a certain degree of natural sun protection. Photoadaptation, or UV hardening, is also used as prophylactic treatment in photodermatoses and photosensitivity. Its use in PLE and solar urticaria is well established.
The mechanism underlying UV hardening is unknown, but it is thought to possibly involve increased melanin synthesis, a thickening of the stratum corneum, a decrease in the hypothetical antigen, or a combination of these factors. However, immunologic mechanisms certainly also have a role. UV radiation has been shown to induce changes in Langerhans cells and other inflammatory skin cells such as lymphocytes, macrophages, and neutrophils.
In patients about to start UV hardening therapy, photobiologic studies are necessary in order to establish the starting dose and subsequent increments (by determining degree of photosensitivity).
Polymorphic Light EruptionSkin alterations in patients with PLE can be resolved by repeated exposure to UV-B radiation, which enhances UV-induced cell migratory responses, particularly in patients with a positive photoprovocation test.60
The higher component of UV-B and UV-A radiation during the summer months could explain why PLE lesions reduce in number in late summer (inhibitory effect of UV-B-induced immunosuppression on the skin).61 NB–UV-B and psoralen-UV-A (PUVA) therapy in the spring can induce light tolerance and reduce the number and intensity of flares in certain photodermatoses; the 2 types of treatment have shown similar effectiveness.61,62 NB–UV-B can even increase the UV-A threshold in patients with a low threshold. In other words, in addition to preventing PLE lesions, NB–UV-B improves sensitivity to UV-A light. The therapy generally consists of short periods of daily exposure to natural sunlight or artificial light during the summer. The success of this desensitization process is independent of a history of tolerance or intolerance to natural sunlight.62
Solar UrticariaThe induction of light tolerance in patients with solar urticaria could be related to the inhibition of responses observed before or after irradiation with longer wavelengths than those of the action spectrum. These wavelengths might inactivate the endogenous allergen or stabilize the membrane of mast cells, which are the main effector cells. However, the fact that the inhibitory effect occurs after preirradiation with light from the action spectrum suggests that the mechanism behind tolerance induction somehow involves blockage of the photoallergen's ability to bind to IgE and mast cells, or a reduction of specific IgE production.63
Tolerance has been induced in patients with solar urticaria with UV-A phototherapy,64 multiple incremental doses of UV-A radiation for 2 to 3 days,65 a combination of PUVA and UV-A therapy (used as induction and maintenance treatments, respectively),66 and NB–UV-B.67 The regimen consisted of between 8 and 15 sessions and in all cases the patients were advised to continue with short periods of daily sun exposure during the summer months. Induction of tolerance does not seem to depend on the action spectrum, but UV-A and UV-B MUDs must be determined so that the starting and subsequent doses can be established. Tolerance, however, tends to last for just a few days so treatment should continue with regular exposure to sunlight or light from phototherapy booths.
Ethical DisclosuresProtection of humans and animalsThe authors declare that the procedures followed complied with the ethical standards of the corresponding human experimentation committee and the World Medical Association and with the principles of the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors obtained informed consent from the patients and/or subjects referred to in this article. This document is held by the corresponding author.
FundingNo funding was received for this study.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: De Argila D, Aguilera J, Sánchez J, García-Díez A. Estudio de las fotodermatosis idiopáticas y exógenas. Parte II: el estudio fotobiológico. Actas Dermosifiliogr. 2014;105:233–242.