Antimalarial drugs have been in common use in dermatology since the 1950s. Their mechanism of action is complex, and it is now known that they act through various pathways. We review the indications for antimalarials in dermatology, their adverse effects, and some less well-known effects, such as their antithrombotic and hypolipidemic action. The most recent recommendations concerning ophthalmological screening in patients on antimalarials are also reviewed.
Los antipalúdicos (AP) son fármacos de uso habitual en dermatología desde la década de los 50. Su mecanismo de acción es complejo, y actualmente se sabe que actúan por diversas vías. En este artículo se revisan las indicaciones de los antimaláricos en dermatología, sus efectos secundarios y algunos efectos menos conocidos, como el antitrombótico o el hipolipidemiante. Se recogen también las recomendaciones más recientes acerca del seguimiento oftalmológico de estos pacientes.
Antimalarial drugs have been known for more than 300 years. The antipyretic properties of bark from cinchona, a tree native to South America, were already known in the 17th century. The first natural antimalarial agent, quinine, was obtained from this tree and was used by European colonists in tropical countries as protection against malaria. The beneficial effects of quinine in patients with lupus erythematosus (LE) were published in 1894 by Payne, who reported the successful treatment of discoid LE with this drug.1 The first synthetic antimalarial, quinacrine (QC), was made in 1930. Chloroquine (CQ) and hydroxychloroquine (HCQ) followed (CQ was first synthesized in 1934 and HCQ in 1955).2 The use of antimalarials as a treatment for LE became widespread in 1951, with the publication of an article by Page,3 which described good response to QC treatment in 18 patients with LE.
The antimalarials used in dermatology are CQ, HCQ, and QC. The latter of these is not commercially available in Spain and can only be used by making an application for a foreign medicine.
PharmacokineticsCQ and HCQ are 4-aminoquinoleins which differ from each other in that HCQ has a hydroxylated side chain. CQ is formulated as a diphosphate for oral administration. Between 90% and 100% is absorbed in the gastrointestinal tract. Stable plasma concentrations are reached after 4 to 6 weeks, and so most cases require drug administration for at least this duration to obtain therapeutic response. CQ binds strongly to plasma proteins which are deposited in tissues such as the liver, spleen, kidneys, and lungs, and in blood cells.4,5 Avidity is particularly high for skin and melanin-containing retinal cells, where concentrations are between 100 and 200 times the plasma concentration. Between 45% and 50% of these plasma proteins are eliminated in urine. CQ has a long half-life, which can vary between 74 hours and 50 days depending on the cumulative dose. The drug can remain in the skin for 6 to 7 months after discontinuation of therapy.6 Both drugs cross the placenta and are excreted in small amounts in breast milk.7
QC is a 9-aminocridin and its pharmacokinetics are similar to those of the 4-aminoquinoleins. It is also quickly absorbed after oral administration and steady-state concentrations are reached after 4 weeks.
Mechanism of ActionThe mechanism of action has not been fully elucidated, but they are known to act on a range of pathways and have immunomodulatory, anti-inflammatory, antiproliferative, and photoprotective effects. Antimalarials are weak bases that can readily cross cell membranes and accumulate in acidic cytoplasmic vesicles (lysosomes or endosomes), where they remain trapped in a protonated state.8 This mechanism of action places antimalarials in the therapeutic group known as lysosomotropic drugs.9 The pH in lysosomes increases as antimalarials accumulate there, subsequently interfering in binding of antigenic peptides with class II molecules of the major histocompatibility complex. Presentation to CD4+ T lymphocytes is thus avoided, leading to inhibition of the production of cytokines that participate in the generation of inflammatory response.10,11 A mechanism has been proposed whereby antimalarials can act as immunomodulators without causing immunosuppression. According to this proposed mechanism, inhibition of binding with the major histocompatibility complex only occurs for autoantigens and not for exogenous peptides. Autoantigens are low affinity peptides. Thus, they do not bind to the alpha and beta chains of the major histocompatibility complex molecules when the pH of the vesicle increases. Exogenous peptides, in contrast, have a high affinity, and so binding does occur and they are presented to the T lymphocytes.12,13
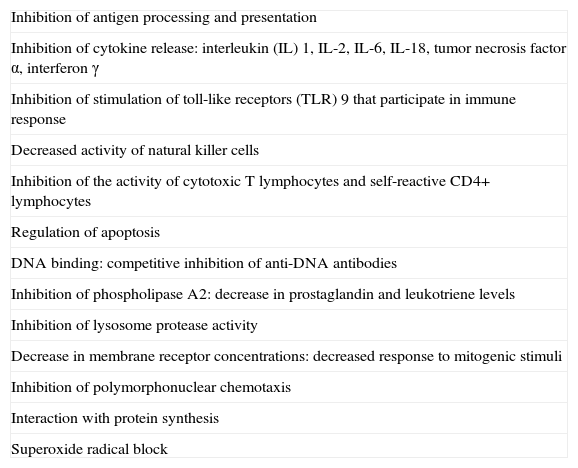
In addition, antimalarials act through other immunomodulatory, anti-inflammatory, and antiproliferative mechanisms (Table 1).10,14–18
Mechanisms of Action of Antimalarial Agents.
| Inhibition of antigen processing and presentation |
| Inhibition of cytokine release: interleukin (IL) 1, IL-2, IL-6, IL-18, tumor necrosis factor α, interferon γ |
| Inhibition of stimulation of toll-like receptors (TLR) 9 that participate in immune response |
| Decreased activity of natural killer cells |
| Inhibition of the activity of cytotoxic T lymphocytes and self-reactive CD4+ lymphocytes |
| Regulation of apoptosis |
| DNA binding: competitive inhibition of anti-DNA antibodies |
| Inhibition of phospholipase A2: decrease in prostaglandin and leukotriene levels |
| Inhibition of lysosome protease activity |
| Decrease in membrane receptor concentrations: decreased response to mitogenic stimuli |
| Inhibition of polymorphonuclear chemotaxis |
| Interaction with protein synthesis |
| Superoxide radical block |
Finally, a photoprotective effect has been attributed to these drugs, although the mechanism is not yet understood. One possibility is that these drugs may have a certain screen effect, absorbing certain wavelengths of sunlight.17 Another is that antimalarials inhibit the inflammatory response of keratinocytes which is triggered by exposure to sunlight through induction of apoptosis and subsequent exposure to keratinocyte antigens.11 Moreover, the drug may enhance natural photoprotection of the epidermis through induction of c-jun transcription.19
Other Antimalarial ActionsOther actions have also been attributed to antimalarials. These include antithrombotic, lipid-lowering, and glucose-lowering effects and effects on bone metabolism. The studies cited below have been conducted mainly in patients with systemic lupus erythematosus (SLE), and in most cases the agent used was HCQ.
Antithrombotic ActionThe antithrombotic effect of antimalarials has been attributed to a range of mechanisms. First, a reduction in red blood cell aggregation has been observed.20 Second, antimalarials inhibit platelet aggregation,21,22 and might also reduce blood viscosity.23 Antimalarials also hinder platelet aggregation induced by antiphospholipid antibody,24 and inhibit the production of thromboxane A2 through deactivation of phospholipase A2 and prostaglandins in platelet membranes.25 In addition, some authors have suggested that these drugs have a synergistic antiatherosclerotic effect which prevents thrombus formation, although the strength of scientific evidence in this case is limited.26
The antithrombotic action of antimalarials has been known since the 1970s, when some antimalarials were investigated for the prevention of thrombosis after surgery.27–29 In these studies, however, the doses of HCQ used ranged from 600 to 1000mg, which is considerably higher than those usually used in patients with LE.
Since the end of the 1980s, numerous studies have been performed to investigate the possible antithrombotic effect of antimalarials in patients with SLE both with and without associated antiphospholipid syndrome.30–38 In general, these studies indicate that antimalarials have an effect on the prevention of thrombotic phenomena in patients with SLE. There are insufficient data to draw conclusions about whether the antithrombotic effect is greater for arterial or venous events.26
Lipid-Lowering EffectSeveral studies have been performed to assess the effect of antimalarials on the lipid profile.39–47 In all except 2,43,47 there were significant differences between patients who were taking antimalarials and those who were not. Effects on levels of total cholesterol, low-density lipoprotein cholesterol, very-low-density lipoprotein cholesterol, and triglycerides have been observed.41,42
Glucose-Lowering EffectPatients with SLE treated with antimalarials have shown a trend towards better control of glucose levels, decreased insulin resistance, and lower levels of hemoglobin A1c.48–50
Effects on Bone MetabolismTwo studies with small cohorts of patients with SLE have investigated the effect of HCQ on bone mineral density.51,52 In both studies, the patients treated with HCQ had a greater bone mineral density measured in the spine51 and in the hips.52
DosingFor most indications, including LE, the normal maintenance doses of CQ and HCQ are 250mg/d and 200mg/d, respectively. These doses are usually doubled in the first 15 to 30 days of drug administration and in the event of lesion flare-ups. QC, which is not usually used in Spain, is administered at a dose of 100mg/d.
Formulas are available to adjust the daily dose that can be administered to reduce the risk of retinopathy. These doses are calculated according to the ideal weight of the patient. Examples of these formulas for calculating this ideal weight are as follows: for women (height in cm - 100) - 15% and for men (height in cm - 100) - 10%. If the patient weighs less than the ideal weight, the doses are adjusted according to the real weight. The dose to be administered is calculated as follows: 3.5-4mg × ideal body weight in kg for CQ and 6-6.5mg × ideal body weight in kg for HCQ. With these formulas, the HCQ dose will easily surpass 200mg/d; in this case, 400mg/d can be administered on the number of days of the week necessary. For example, for an ideal weight of 40-43kg, 400mg/d is administered twice a week and 200mg/d for the remaining days.53 In the case of CQ, in patients with a low ideal body weight, the daily dose will be below 250mg/d. Given that tablets with a dose less than 250mg are not available in Spain, one possibility would be not to take the drug one or several days of the week.
Dose calculation using these formulas is particularly important for long-term treatment and in small patients, in whom there is a risk of overdose with the standard antimalarial doses. These doses should be reduced and individualized in patients with impaired liver or renal function.53
Indications in DermatologyFor practical purposes, we can divide the indications of antimalarials into indications as first-line treatment, indications as second- or third-line treatments, and miscellaneous (use in isolated clinical cases). The indications of antimalarials in rheumatologic diseases such as rheumatoid arthritis or Sjörgren syndrome will not be discussed here.
Indications As First-Line DrugsLupus ErythematosusLE is the only dermatologic indication approved by the US Food and Drug Administration.
Antimalarials are effective for the treatment of the specific skin lesions of cutaneous LE (CLE), whether acute, subacute, or chronic.54-56 However, in the case of chronic CLE, a worse response has been observed in the hypertrophic and verrucous forms, disseminated lesions, and lesions associated with SLE.10,56 Lesions do respond well in cases of tumid LE, even with the chronic forms.57 In patients with SLE, antimalarials also improve certain systemic manifestations, such as arthralgia, myalgia, and serositis.58 They also have an effect on nonspecific lesions of the disease, such as oral ulcers, photosensitivity, and calcinosis cutis.59 In patients with CLE, it is normal clinical practice for patients to follow intermittent treatments, with antimalarials administered during the months of highest exposure to sunlight, when flare-ups are most frequent, and suspended during fall and winter. The drug is therefore suspended in those periods of inactivity of the cutaneous disease.
In cases of LE in which there is no response to the first-line antimalarial treatment, a switch to another antimalarial can be tried (from HCQ to CQ or vice versa). In patients with refractory cutaneous disease, a combination of antimalarials may be appropriate, combining QC with any of the other 2 antimalarials.60 The action of QC is not associated with a greater risk of retinal toxicity.
Smoking has been shown to be associated with decreased efficacy of antimalarials in several studies,61–63 although in a recent retrospective study the authors found no relationship between smoking and HCQ response.56
Porphyria Cutanea TardaPhlebotomy is the mainstay treatment for porphyria cutanea tarda. Nevertheless, in certain cases, such as in patients who do not response or in whom this procedure is contraindicated, antimalarials can be administered.64–66 Antimalarials enhance hepatic depletion and urinary excretion of porphyrins while also inhibiting their synthesis.18 The doses used should be low to avoid massive porphyrin depletion and liver damage. Therefore, a dose of 2mg/kg twice a week is recommended in the case of CQ and of 3.5mg/kg twice a week in the case of HCQ.10,17,18 This dose is usually slowly increased according to response. Some authors recommend administering a test dose of 125-250mg of CQ and performing an analysis of liver enzymes before continuing with treatment.67
According to some studies, 1 to 4 phlebotomies, performed prior to administration of the antimalarials, may enhance drug response and reduce the risk of liver damage.68,69
Chronic Ulcerative StomatitisChronic ulcerative stomatitis (CUS) was first described as an entity in 1990.70,71 The disease occurs mainly in elderly women and follows a course of painful ulceration in the oral mucosa. The clinical and pathologic features are similar to erosive lichen planus. The disease has been shown to be caused by antinuclear antibodies reactive exclusively to the squamous epithelium and specifically to the delta Np64 alpha protein, expressed in epithelial basal cells.72–75
A characteristic feature of CUS is that it is refractory to topical and systemic corticosteroids. In contrast, CUS responds well to antimalarials, and so these are the first-line treatment.18
Indications as Second- and Third-line TreatmentsDermatomyositisAntimalarials have been shown to be effective in the treatment of dermatomyositis with substantial skin involvement. They are administered either as monotherapy in cases of dermatomyositis without muscle weakening or as a coadjuvant treatment in those with associated muscle disease.76–80 Although antimalarials mainly improve the skin manifestations, they have been shown to also improve muscle strength in combination with oral corticosteroids.81 In patients who do not respond to monotherapy with antimalarials, combined therapy with QC can be administered.79 It should be pointed out that patients with dermatomyositis have a higher risk of cutaneous side effects from taking antimalarials.82
SarcoidosisSystemic or topical corticosteroids are the first-line treatment for sarcoidosis. However, antimalarials are effective in the treatment not only of skin manifestations of this disease83–86 but also of pulmonary manifestations87–89 and certain systemic complications.90–94
Polymorphous Light EruptionAlthough antimalarials are not the first-line treatment for polymorphous light eruption (PLE), several studies have reported good therapeutic outcomes with these drugs.95–97 Two controlled studies have found an increased tolerance to sunlight exposure and a decrease in rash; itching, however, was alleviated to a lesser extent.98,99
Antimalarials are indicated in patients with PLE who do not respond to usual therapy, such as photoprotection, hardening associated with phototherapy, and topical corticosteroids.10,18 Some authors suggest starting HCQ at a dose of 400mg/d from a few days prior to exposure to sunlight and reducing the dose to 200mg/d once clinical stability is attained.18
Disseminated Granuloma AnnularePublications on the use of antimalarials in disseminated granuloma annulare are limited to a few case series.100–102 However, use of antimalarials is recommended in patients with disseminated granuloma annulare that does not respond to topical corticosteroids or when corticosteroids cannot be used due to the extension of the lesions.10,18
Miscellaneous ConditionsTable 2 summarizes some conditions in which antimalarials have been used, although the scientific evidence is scant and limited to the publication of isolated case reports.103–119
Indications for Antimalarial Agents: Miscellaneous Conditions.
| Erosive lichen planus of oral mucosa |
| Actinic lichen planus |
| Lichen planopilaris |
| Lichen sclerosus et atrophicus |
| Frontal fibrosing alopecia |
| Necrobiosis lipoidica |
| Annular elastolytic giant cell granuloma |
| Chronic actinic dermatitis |
| Actinic reticuloid |
| Actinic prurigo |
| Solar urticaria |
| Eosinophilic fasciitis |
| Epidermolysis bullosa |
| Graft-versus-host disease |
| Chronic erythema nodosum |
| Weber-Christian panniculitis |
Antimalarials can cause several reversible ocular side effects. Retinopathy is the most feared side effect of these agents and can, at times, lead to an irreversible vision loss.
Reversible ocular effects include corneal deposits, which can be asymptomatic or cause blurred vision and halos of colors around lights. These deposits are observed in up to 90% of patients who take CQ and 5% of those who take HCQ.120 There is no direct relationship with retinopathy, but the presence of such deposits can be a warning for the need for closer follow-up. Accomodation disorders and diplopia are other reversible ocular side effects of these agents. Diplopia occurs more frequently with CQ and usually resolves in time or with a decrease in dose.
Retinopathy is the most serious ocular side effect and, if detected, treatment should be suspended. Antimalarials are known to have avidity for melanin in the pigmentary epithelium of the retina; however, the mechanism by which retinopathy occurs is not well known.121 In the early stages, functional loss in the paracentral region of the retina may occur. The screening methods used should be able to detect patients in this stage, at which vision loss does not progress if the drug is withdrawn. If exposure to the drug continues, the subsequent retinopathy gives rise to an image with a bull's eye appearance, which consists of macular paleness surrounded by several rings. In this case, the damage is irreversible. Depigmentation often occurs along with progressive functional loss up to 1 year after suspending treatment. Advanced cases show diffuse retinal atrophy associated with loss of visual acuity, peripheral vision, and night vision.121
The prevalence of retinopathy caused by antimalarials is low, particularly in the first 5 years of treatment. However, with longer treatment durations, it can rise to 1%.122 The risk of ocular toxicity thus increases in patients with cumulative doses greater than 1000 g of HCQ and 460 g of CQ. This risk is also greater in patients who exceed the dose of 3.5-4mg/kg of ideal body weight for CQ and 6-6.5mg/kg of × ideal body weight for HCQ.121 As mentioned above, these daily doses are not exceeded in most patients who take 400mg/d of HCQ or 250mg/d of CQ, but small patients may exceed this threshold. However, we should bear in mind that cases of retinal toxicity have been described despite taking doses below these limits and for a short period of time.123 In general, it is accepted that the risk of developing retinopathy is greater in patients treated with CQ than in those treated with HCQ, while the risk in those receiving QC is almost nonexistent.26,124 However, the recommendations for screening of the American Academy of Ophthalmology (AAO) are the same for CQ and HQC. The most recent update of these recommendations, published in 2011, recommend baseline ocular control using biomicroscopy, automated perimetry, fundus examination and at least one of the following tests: spectral domain optical coherence tomography, fundus autofluorescence imaging, and multifocal electroretinogram. Annual screening should start after 5 years of treatment with antimalarials in patients without any risk factors, and from the start of treatment in those patients with the risk factors shown in Table 3. According to the AAO, annual screening should include automated perimetry and, if possible, at least 1 of the 3 aforementioned tests: spectral domain optical coherence tomography, fundus autofluorescence imaging, and multifocal electroretinogram.121 The authors of this review insist, however, that these are the minimum number of recommended controls and the treating physician is free to increase the frequency of these visits or the number of tests ordered.
Risk Factors for Retinopathy with Antimalarial Therapy.
| Daily dose |
| ○ HCQ:>400mg/d (>6.5mg/kg of ideal body weight in small individuals) |
| ○ CQ:>250mg/d (>3mg/kg of ideal body weight in small individuals) |
| Cumulative dose |
| ○ HCQ:>1000 g |
| ○ CQ:>460 g |
| Age>60 years |
| History of retinopathy or maculopathy |
| Renal or hepatic dysfunction |
Gastrointestinal effects are the most common reactions to antimalarials, although they are usually mild in severity and can be managed by dose reduction. They occur most frequently in patients treated with QC (30%), followed by those who take CQ (20%) and HCQ (10%).2 The most common gastrointestinal events are nausea, vomiting, and diarrhea. Less frequent gastrointestinal events include anorexia, abdominal distension, and transaminitis.10
Cutaneous EffectsSome patients who take antimalarials in the long term develop a grey-blue skin pigmentation, which is more evident on the face, palate, forearms, and legs. QC causes darker pigmentation than CQ and HCQ. In addition, the hair roots may become whitened and transversal bands may appear in the nails.125 These disorders resolve a few months after suspending treatment.
Other cutaneous side effects are pruritus, rash, erythroderma, exfoliative dermatitis, urticaria, eczema, alopecia, photosensitivity, and erythema annulare centrifugum.10
Neuromuscular EffectsAntimalarials have been reported to cause some psychiatric side effects, such as psychosis, irritability, depression, insomnia, and nightmares. These events, however, usually occur in patients treated at higher doses than those used in dermatology.126,127 Antimalarials can also induce seizure in predisposed individuals,128 and cause myotoxicity due to the accumulation of the drug in the vacuoles of the myofibrils of the striated muscle. This myopathy, which affects the proximal muscles and may be accompanied by peripheral neuropathy, usually resolves with discontinuation of the antimalarial.129
Cardiac EffectsOccasionally, antimalarials can cause conduction disorders and hypertrophic cardiomyopathy.10,130 However, 2 studies that specifically assessed cardiotoxicity in patients treated with antimalarials did not find any significant cardiac side effects.131,132
Hematologic EffectsHematologic side effects are uncommon. Hemolysis in patients with glucose-6-phosphate dehydrogenase deficiency, aplastic anemia, and leukopenia has been reported.10 These disorders occur more frequently in patients treated with QC, especially when used at higher doses.133 The need for blood tests during treatment is subject to debate, although testing is generally not considered necessary unless the patient has an underlying hematologic disease.
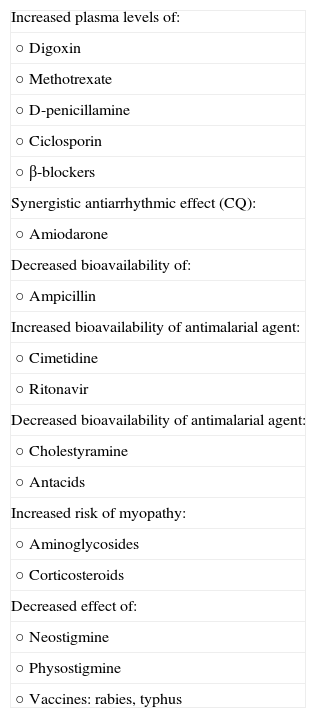
Drug-Drug InteractionsAntimalarials can interact with some drugs. These interactions are listed in Table 4.
Drug-Drug Interactions Involving Antimalarial Agents.
| Increased plasma levels of: |
| ○ Digoxin |
| ○ Methotrexate |
| ○ D-penicillamine |
| ○ Ciclosporin |
| ○ β-blockers |
| Synergistic antiarrhythmic effect (CQ): |
| ○ Amiodarone |
| Decreased bioavailability of: |
| ○ Ampicillin |
| Increased bioavailability of antimalarial agent: |
| ○ Cimetidine |
| ○ Ritonavir |
| Decreased bioavailability of antimalarial agent: |
| ○ Cholestyramine |
| ○ Antacids |
| Increased risk of myopathy: |
| ○ Aminoglycosides |
| ○ Corticosteroids |
| Decreased effect of: |
| ○ Neostigmine |
| ○ Physostigmine |
| ○ Vaccines: rabies, typhus |
The absolute and relative contraindications of antimalarials are summarized in Table 5.
Contraindications of Antimalarial Agents.
| Absolute Contraindications |
| History of retinopathy |
| Known hypersensitivity |
| Concomitant bone marrow suppressive therapy |
| Relative Contraindications |
| Renal failure |
| Hepatic disease |
| Hematologic disease |
| Glucose 6-phosphato-dehydrogenase deficiency |
| Neuromuscular disease |
| Psychiatric disease |
| Psoriasis? |
Antimalarials can cross the placenta and reach the fetus.134 However, several studies have assessed the safety of these drugs during pregnancy and none have detected a greater risk of congenital malformations or ocular, neurologic, or auditory toxicity.135–141 The level of evidence for this absence of effect is greater for HCQ than for CQ, as HCQ was the drug administered to most of the pregnant women studied.26 In studies conducted in patients with SLE, the benefit of continuing treatment with antimalarials during pregnancy is generally acceptable because the risk of disease complications during pregnancy is greater than the risk the drug poses to the fetus.10
Ethical ResponsibilitiesProtection of human and animal subjects.The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of data.The authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consent.The authors declare that patient data do not appear in this article.
Conflicts of InterestDr. Clara Rodriguez-Caruncho has received research grants from Laboratorios Rubió. I Bilsa Marsol declares that she has no conflicts of interest.
Please cite this article as: Rodriguez-Caruncho C, Bielsa Marsol I. Antipalúdicos en dermatología: mecanismo de acción, indicaciones y efectos secundarios. Actas Dermosifiliogr. 2014;105:243–252.