Acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug reaction with eosinophilia and systemic symptoms are all severe hypersensitivity reactions to medications. While each of these reactions is a well-established entity with specific diagnostic criteria, clinicians see cases that fulfill criteria for more than one form, prompting discussion on the possibility of combined forms. Such overlapping clinical pictures meeting the criteria for 2 conditions have thus become a topic of debate in dermatology in recent years. We describe 2 patients with cutaneous drug reactions having the characteristics of both acute generalized exanthematous pustulosis and Stevens-Johnson syndrome -toxic epidermal necrolysis. We also review previously published cases and current thinking on such overlapping conditions.
La pustulosis exantemática generalizada aguda, el síndrome de Stevens-Johnson, la necrólisis epidérmica tóxica y el síndrome de hipersensibilidad a fármacos con síntomas sistémicos y eosinofilia son reacciones de hipersensibilidad grave a fármacos. Aunque cada una de ellas se describe como una entidad bien constituida con criterios diagnósticos propios, en la práctica clínica se encuentran algunos casos que manifiestan características de 2 de estas entidades, abriendo el diálogo ante la posible existencia de formas combinadas. La existencia de estas formas solapadas entre 2 toxicodermias graves es motivo de controversia en los últimos años en diferentes foros de dermatología.
En el artículo se aportan 2 nuevos casos de formas solapadas entre pustulosis exantemática generalizada aguda y síndrome de Stevens-Johnson/necrólisis epidérmica tóxica, se revisan los casos previos publicados y el estado actual de esta controversia en la literatura.
Adverse drug reactions refer to any unwanted clinical manifestation after administration of a drug or chemical substance with prophylactic, diagnostic, or therapeutic ends. Such reactions can be classed as expected or unexpected (Table 1).
Types of Adverse Drug Reactions.
| Expected reactions: these can be observed in any patients after sufficient exposure. These are the most frequent | |
| Overdose | Toxicity due to an excessive dose, impaired excretion, or both (for example, metabolic acidosis due to acetyl salicylic acid) |
| Adverse drug effect | Different action to the primary effect of the drug derived from the primary pharmacological effect of a drug (for example, somnolence with antihistamine agents) |
| Side effect | Undesirable pharmacological effect occurring due to the drug's action at recommended doses (for example, candidiasis associated with inhaled corticosteroids) |
| Drug-drug interactions | Action of a drug on the efficacy or toxicity of another (for example, theophylline and macrolides) |
| Unexpected reactions: occur unexpectedly in a subgroup of patients, regardless of dose | |
| Drug intolerance | Effect due to low tolerance to the pharmacological action of a drug. Similar to an adverse effect at subtherapeutic doses (example: tinnitus after a single dose of acetyl salicylic acid) |
| Idiosyncrasy | Qualitatively abnormal reaction to a drug related to a metabolic or enzyme deficiency (for example, hemolytic anemia associated with primaquine due to G6PDH deficit) |
| Allergy | Immune-mediated reaction characterized by its specificity, transferability by antibodies or lymphocytes, and recurrences after re-exposure (for example, Stevens-Johnson syndrome due to sulfamethoxazole/trimethoprim) |
The term cutaneous drug reaction is used for dermatoses with involvement of the skin, mucosas, and/or skin appendages, caused by the effect of a substance, usually a drug, which comes into contact with the organism by a range of pathways. Serious cutaneous drug reactions are immune-mediated events that are classed as unexpected adverse drug reactions.
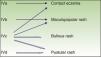
Immune-mediated hypersensitivity reactions vary according to the type of cutaneous drug reaction (Table 2).2,3 Thus, in urticarial drug reactions, type i IgE-mediated reactions are activated (Fig. 1) with a rapid clinical onset. In maculopapular exanthema, which is the most frequent type of cutaneous drug reaction, cell-mediated immune reactions are activated (Gell and Coombs type iv hypersensitivity) (Fig. 2).
Underlying Hypersensitivity Mechanism for Different Forms of Cutaneous Drug Reactions According to Gell and Coombs Classification.
| Lymphocytes Implicated | Type (Gell and Coombs) | Immune Mediator | Pathogenesis | Clinical Characteristics |
|---|---|---|---|---|
| B lymphocyte | I | IgE | Mastocyte and basophil degranulation | Urticaria, angioedema, bronchospasm, anaphylaxis |
| II | IgG or IgM | Cell lysis | Cytopenias | |
| III | IgG or IgM or complement | Immunocomplex deposit | Vasculitis, serum disease, arthritis | |
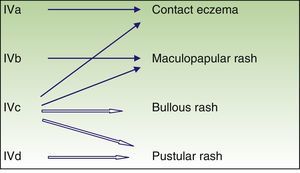
| T lymphocyte | IVa | IFN-gamma (Th1) | Monocyte and macrophage activation | Eczema |
| IVb | IL 4, IL 5 (Th2) | Eosinophil activation | Maculopapular or bullous exanthema | |
| IVc | Perforin, granzyme (cytotoxic T lymphocytes) | Cytotoxicity | Maculopapular or bullous or pustular exanthema | |
| IVd | IL 8 | Chemiotaxis and neutrophil activation | Pustular rash |
Pichler et al.3 reviewed the immune mechanisms underlying the different types of cutaneous drug reaction.
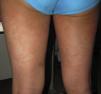
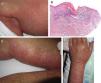
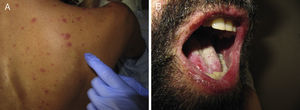
Acute Generalized Exanthematous PustulosisAcute generalized exanthematous pustulosis (AGEP) is a fast-onset cutaneous skin reaction, usually occurring within 3 to 5 days of starting administration of the drug responsible. The lesions present as exanthema mainly in flexures and are composed of sterile, nonfollicular pustules no larger than a couple of millimeters, with coalescence on strongly erythematous plaques (Fig. 3A and Fig. 3B). Mucosal involvement is uncommon and, if present, usually only affects the oral mucosa.4
The process is often accompanied by fever and neutrophilia in peripheral blood. Resolution is also rapid after suspension of the drug, and prognosis is usually good.5 AGEP occurs more frequently in women and antibiotics are among the drugs most frequently responsible. Pathogenesis involves activation of T lymphocytes, which in turn activate polymorphonuclear cells (Gell and Coombs type IVd reaction) mediated by interleukin 8 (via CXCL-8 and GM-CSF).3 Internal organs are rarely involved with AGEP. Differential diagnosis should include pustular psoriasis and other subcorneal pustular dermatoses (Table 3).
Main Differential Diagnoses for Acute Generalized Exanthematous Pustulosis.
| Generalized pustular psoriasis | Subcorneal pustulosis (Sneddon-Wilkinson) |
| IgA pemphigus | Candidal intertrigo |
| Infectious folliculitis | Viral vesiculopustular exanthema |
| Sweet syndrome | Bullous impetigo |
| Dermatophyte skin infections | Pustular contact eczema |
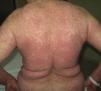
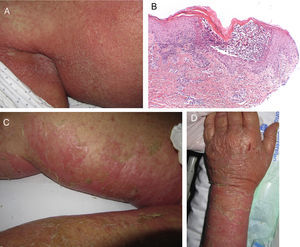
Stevens-Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN), although initially described separately, are currently considered as lying at 2 ends of the same spectrum.6 The incidence of these conditions is higher in certain groups (HIV-positive patients and those with certain HLA types).7,8 A prodromic phase with fever and poor general health usually precedes skin lesions, which present as touch-sensitive erythematous macules on the trunk and limbs. These progress rapidly to bullous or erosive lesions (Fig. 4A and Fig. 4B). The extent of epidermal detachment (erosions, blisters, or areas with positive Nikolsky sign) is used to classify patients into the following 3 groups (Table 4).9
Classification of the Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis Spectrum.
| EMM | SJS | SJS/TEN Overlap | TEN | |
|---|---|---|---|---|
| Type of Lesion | ||||
| Typical bullseye | Yes | |||
| Atypical bullseye | ||||
| Raised | Yes | |||
| Flat | Yes | Yes | Yes | |
| Macules | ||||
| Erythematous | Yes | |||
| Purpuric | Yes | Yes | Yes | |
| Lesion Distribution | ||||
| Localized | Yes | |||
| Generalized | Yes | Yes | Yes | |
| Mucosal Involvement | Yes | Yes | Yes | Yes |
| Systemic Symptoms | Often | Always | Always | |
| Extent of Detachment | <10% | <10% | 10-30% | >30% |
Abbreviations: EMM, erythema multiforme major; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrosis.
Mucosal erosions may leave substantial sequelae after recovery from the acute phase. Differential diagnosis is very broad and should include all bullous dermatoses. Histopathology shows necrosis of keratinocytes. Cytotoxic molecules such as perforin and granzyme and the FAS/FASL pathway (Gell and Coombs type IVc hypersensitivity) have been implicated in this process thanks to molecular studies. Among the most frequently implicated drugs are antibiotics, anticonvulsants, and allopurinol. Mortality in SJS is estimated to be between 1% and 5% while that of TEN is between 25% and 35%.10 The SCORTEN scale is the most widely used for stratifying risk in these patients.11
Management in intensive care units and rapid withdrawal of the responsible drug are crucial for a good outcome (Table 5).12 The utility of the other therapeutic measures is still under discussion in the literature.
Drugs With Greater Risk of Inducing Cutaneous Drug Reactions in the Spectrum of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis.
| Sulfamethoxazole/Trimethoprim | Cephalosporins | Phenytoin | Phenobarbital | NSAID/COXIB |
|---|---|---|---|---|
| Aminopenicillins | Quinolones | Carbamazepine | Nevirapine | Allopurinol |
Although these cutaneous drug reactions are different in terms of pathogenesis, presentation, and prognosis, in clinical practice we find overlapping forms and these are a diagnostic challenge. We now present 2 cases with overlapping clinical characteristics.
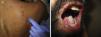
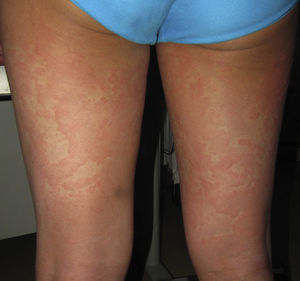
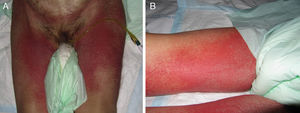
Case 1An 87-year-old woman with hypersensitivity to Anisakis was admitted to hospital with a urinary tract infection and recurrence of renal failure. Of relevance in her personal history were chronic renal disease, atrial flutter, dilated cardiomyopathy, and stroke with recovery without sequelae 7 years earlier. During admission, she received furosemide, ceftriaxone, amlodipine, and enoxaparin, among other drugs. Approximately 1 week after starting treatment with these drugs, erythematous plaques began to appear in the flexures. Oral fluconazole was added to her treatment. After 24hours, plaques continued to present on the trunk and limbs (Fig. 5A), some with a violaceous center. Within hours, these progressed to detachment (Fig 5B and C), with positive Nikolsky sign and appearance of bullous lesions on the lower limbs. Perilabial erosions were also present. With TEN-type serious cutaneous drug reaction suspected, a biopsy was taken. Amlodipine, fluconazole, and furosemide were discontinued and treatment initiated with gammaglobulin IV agents. On the fifth day, in view of the slow recovery, systemic corticosteroids were added. The lesions resolved slowly over the course of 2 weeks.
Histological study of 2 incipient plaques with bullseye morphology (Fig. 5D) showed subcorneal plaques with a mild perivascular dermal infiltrate, scant eosinophils and neutrophils, and negative immunofluorescence. These histological findings are characteristic of AGEP-type cutaneous skin reactions. The laboratory analysis showed leukocytosis with neutrophilia, mild transaminase elevation, and ANA positivity at 1/320 dilution with a homogeneous pattern. After discharge, the patient was lost to follow-up.
Case 2A 75-year-old woman, with known history of allergy to diclofenac, was admitted to hospital after infection of a surgical wound in the left hip resulting from acetabular replacement 15 days earlier. Her personal history included arterial hypertension, biliary lithiasis, and breast cancer in complete remission.
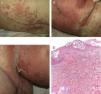
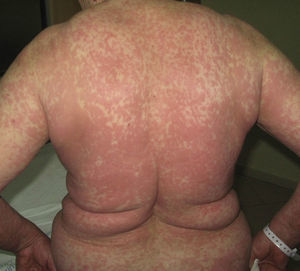
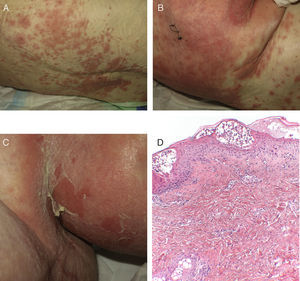
On admission, she had fever and local signs of infection with purulent exudate from which methicillin-resistant Staphylococcus aureus was isolated. She had leukocytosis with neutrophilia and elevated levels of other acute phase reactants. Treatment was started with meropenem, omeprazole, paracetamol, Nolotil, tramadol, bemiparin sodium, losartan, and amlodipine. After 48hours, meropenem was suspended and she started with teicoplanin. The following day, erythematous lesions appeared on the submammary fold and began to spread. Three days later, a papulopustular exanthema presented on the trunk and limbs, with confluence in the axillary and inguinal folds, while the mucosas were respected (Fig. 6). Skin biopsy was taken given the suspicion of AGEP. Treatment with teicoplanin and metamizole was suspended and linezolid and systemic corticosteroid therapy initiated.
The histological study (Fig. 6D) showed the presence of a pustule composed of neutrophils and neutrophil fragments in the subcorneal layer, with spongiosis in the peripheral part of the lesion and a dense superficial perivascular inflammatory infiltrate associated with neutrophils at points on the dermis. All these findings were consistent with AGEP.
The cutaneous lesions progressively merged and the patient once again had fever, which remitted with antibiotic treatment. The laboratory analysis showed neutrophilia, very mild eosinophilia, cholestatic enzyme elevation, and acute phase reactants. In the next 48hours, epidermal detachment occurred on 30% of the body surface area (Fig. 6B and C), with positive Nikolsky sign and erosions of the labial mucosa. The clinical manifestations resembled a TEN process. On the third day, her condition stabilized and her clinical symptoms and laboratory values improved until complete resolution.
In the allergy study, a lymphocyte transformation test was positive for teicoplanin7,18; skin patch test was positive for amlodipine and negative for teicoplanin, metamizole, and omeprazole; skin prick tests were negative for meropenem, PPL, and MDM; and tests of controlled exposure were negative for meropenem, omeprazole, and tramadol. Thus, her process was attributed to a cutaneous drug reaction to teicoplanin and/or amlodipine, while allergy to meropenem, omeprazole, and tramadol was ruled out.
DiscussionIt is important to differentiate between AGEP and SJS/TEN given their different prognosis and management. SJS/TEN should preferably be managed in burns or intensive care units. Cases of overlap between AGEP and SJS/TEN have been published before, but they have been shrouded in some debate.
We describe 2 patients with manifestations suggestive of serious cutaneous drug reaction with characteristics overlapping between AGEP and TEN. The manifestations of the first case were those of SJS/TEN, while the histology, although the sample was not from a pustular lesion, was that of AGEP. In the second case, the manifestations and initial histology pointed to AGEP, but the full clinical picture, given the morphology of the lesions, mucosal involvement, substantial skin detachment, and Nikolsky sign, resembled a superimposed TEN, although this interpretation is open to debate. Improvement occurred more quickly than in classic TEN.
We have reviewed the cases of overlap between AGEP and TEN reported in the literature (Table 6). Most cases were associated with antibiotics. We found 11 articles describing 15 cases.14–24 In 6 of the articles, the cases are described as AGEP with TEN characteristics,14–19 whereas in other 5 the authors suggest a true overlap between AGEP and TEN was present.20–24
Cases Published With Overlapping Acute Generalized Exanthematous Pustulosis and Toxic Epidermal Necrolysis Characteristics.
| Source | Suspected Drug | Clinical and Laboratory Findings | Histology and Biopsy Site |
|---|---|---|---|
| Cohen et al.,16 2001 | Cefuroxime, paracetamol | No fever. Leukocytosis. Generalized rash with nonfollicular pustules. No bullseye lesions. Blisters and positive Nikolsky sign | Pustules: spongiform pustules, edema in the papillary dermis, perivascular mononuclear infiltrate, leukocytoclastic vasculitis. No necrotic keratinocytes |
| Scheinfeld et al.,17 2003 | Famotidine | Leukocytosis and neutrophilia. Diffuse erythema, erosions, and pustules. Positive Nikolsky sign | Not specified: subcorneal blisters, without necrotic keratinocytes, spongiosis, or acantholysis |
| Byerly et al.,15 2005 | Valdecoxib | Fever. Hypotension. Leukocytosis. Generalized rash with nonfollicular pustules and plaques. Negative Nikolsky sign | Not specified: spongiform pustules. Perivascular neutrophilic and eosinophilic infiltrate |
| Meiss et al.,21 2007 | Ampicillin+sulbactam Clindamycin. Amoxicillin (3 cases) | Rash with edema and multiple pustules with positive Nikolsky sign. Progression of pustular rash with poor general state of health, blister formation, and subsequent generalized peeling. | Pustule (only in one of the available cases): subcorneal pustule, neutrophilic infiltrate, and necrosis of some basal keratinocytes |
| Goh et al.,20 2008 | Carbamazepine | Fever, elevated liver enzymes. Maculopapular exanthema that progressed to nonfollicular pustules and blisters. Positive Nikolsky sign. Multiple mucosal involvement | Pustule: subcorneal pustule, epidermal necrosis, mild spongiosis, perivascular lymphocytic infiltrate. Blister: epidermal necrosis and subepidermal blister |
| Lateef et al.,22 2009 | Hydroxychloroquine | Fever, leukocytosis with neutrophilia. Itchy papulopustular exanthema that progressed to bullseye lesions and multiple mucosal involvement | Pustule: epidermal spongiosis, intraepidermal neutrophils, lymphocyte infiltrate, and perivascular neutrophils |
| Peermohamed et al.,18 2011 | Piperacillin-tazobactam | Rash composed of nonfollicular pustules, vesicles, and blisters. Positive Nikolsky sign. No mucosal involvement | Pustule: intraepidermal pustules with neutrophils. Intraepidermal blisters with neutrophils, edema in papillary dermis, no epidermal necrosis |
| Kardaun et al.,14 2011 | Morphine | Fever. Leukocytosis with neutrophilia. Rash predominantly affecting flexures and face, with multiple pustules and surface erosions at confluence of pustules (pseudo-Nikolsky positive sign) | Pustule: spongiform subcorneal pustules, neutrophilic spongiosis, limited necrotic keratinocytes, dermal edema, and neutrophil-rich perivascular and interstitial infiltrate |
| Lee et al.,23 2010 | Ceftriaxone. Cloxacillin. Ceftriaxone (3 cases). | Fever. Leukocytosis with neutrophilia. Onset with erythematous confluent rash with nonfollicular pustules and subsequent development of erosions and blisters. Mucositis also present in 2 cases | Not specified, case 1: intraepidermal neutrophilic blisters with spongiosis and vacuolar degeneration of the basal layer, lymphocyte and eosinophil infiltrate. Not specified, case 2: subcorneal pustules with acanthosis and spongiosis. Superficial perivascular infiltrate with lymphocytes, neutrophils, and eosinophils. Not specified, case 3: subcorneal pustule with acanthosis and perivascular infiltrate of neutrophils and lymphocytes |
| van Hattem et al.,19 2013 | Flucloxacillin | Fever. Leukocytosis with neutrophilia. Hemodynamic instability. Erythematous rash with pustules, large blisters, and surface erosions at the confluence of pustules (pseudo-Nikolsky sign positive) | Pustules and erythema: spongiform subcorneal pustules with neutrophils, spongiosis, edema in the papillary dermis and mixed perivascular and interstitial infiltrate with eosinophils |
| Moling et al.,24 2014 | Amoxicillin-clavulanic acid or cefixime or ibuprofen or ketoprofen or paracetamol or metamizole | Leukocytosis. Pustular exanthema with subsequent development of erosions, blisters, and mucositis. Pseudo-positive Nikolsky sign | Not available |
| Case 1 | Ceftriaxone or enoxaparin or amlodipine or furosemide | Fever. Leukocytosis with neutrophilia. Transaminase elevation. Erythematous plaques on the trunk and limbs, some with violaceous center, with detachment, blisters, and positive Nikolsky sign | Bullseye-like plaque: subcorneal pustules, mild perivascular infiltrate with occasional eosinophils and neutrophils |
| Case 2 | Teicoplanin and/or amlodipine | Fever, leukocytosis with neutrophilia and eosinophilia, cholestasis. Papulopustular exanthema with onset in skin folds. Progression to epidermal detachment with positive Nikolsky sign and erosions in labial mucosa | Pustule: subcorneal spongiotic pustule, dense inflammatory infiltrate superficial perivascular with neutrophils |
In most cases, the clinical picture is initially one with AGEP characteristics, and the fact that the manifestations do not remit after discontinuation of the suspected drug, but rather progresses to TEN-like forms, leads to classification of the process as an overlapping one. Some authors prefer to think of the process as AGEP that progresses to TEN rather than an overlap. The histopathological study was performed with the initial lesions in most cases, and shows subcorneal or intraepidermal pustules consistent with diagnosis of AGEP.
In a retrospective study of 216 serious cutaneous drug reactions, overlap with SJS/TEN was initially suspected in 9 of 64 cases of AGEP.25 Overlap of AGEP with DRESS was suspected in a further 19 cases, while in 25 of 97 cases of SJS/TEN, an overlap with DRESS was suspected. However, in an a posteriori study, applying criteria of the RegiSCAR group, the possibility of overlap between SJS/TEN and DRESS only remained for 3 cases, while there no cases of overlap between PEGA and TEN.
In all cases, the authors who point to the independence of these entities justify the existence of systemic symptoms and the severity of the skin manifestations of clinically overlapping forms with the presence of elevated peripheral neutrophil counts,26 and associate the erosions that occur with coalescence of pustules (pseudo-Nikolsky sign).
Some authors suggest that histological study can guide classification of AGEP or TEN in those cases with overlap of clinical manifestations.18,19 The presence of subcorneal pustules and dermal edema would be seen in cases of AGEP and in these cases, the epidermal necrosis characteristic of cases of TEN/SJS would not be observed. Confluence of pustules in AGEP can give rise to areas of skin detachment, but the histology is different to lesions with detachment due to epidermal necrosis. However, given that the pathological findings depend on the timing and site of the biopsy, both initial and progressed lesions should be studied to assess the cases suggestive of overlap. In some published cases, biopsies do present characteristics of both AGEP and TEN entities.20
In the first of our cases, the biopsy sample was taken from an erythematous bullseye-like plaque, with no clinical pustules. Even so, the biopsy showed histology typical of AGEP. We were therefore dealing with an overlapping case in which the typical clinical manifestations of the spectrum of SJS/TEN cutaneous drug reactions manifest histologically as AGEP. In the second case, however, the biopsy sample was taken from a fold with incipient pustules and so it was noteworthy that we did not encounter once again an AGEP-type histology. The subsequent clinical evolution was to an SJS/TEN form, with mucosal erosions, detachment, and positive Nikolsky sign. However, as we did not have a biopsy sample from the progressed lesions, it can be debated whether there was true overlap.
Another criterion for differentiation proposed by some authors19 is the a posteriori positivity of the patch test, as it has been reported that a positive result is much more common in cases of AGEP than in SJS/TEN.27,28 However, given that we are dealing with a question of frequencies, and we are considering not just the type of reaction but also the implicated drug,28 patch testing has not been validated to differentiate between one hypersensitivity reaction and another.20,21,23
In AGEP-type cutaneous skin reactions, patch tests have an intermediate sensitivity, which increases with an additional reading at 96 and 120h, in addition to the usual reading at 48 and 72h. At least a month should have passed before placing the patches, and when reading these, the formation of pustules can be observed. The risk of triggering generalized pustulosis with the skin patches is thought to be low. When the result of the patch testing is negative or unclear, the sensitivity of the tests can be increased by performing an ex vivo test such as the lymphocyte release test (LRT). These tests should be conducted in laboratories with experience given the difficulty in running and interpreting these tests.13
In cases of SJS-TEN, skin prick tests or rechallenge tests are contraindicated given the risk of inducing a new serious reaction. The role of patch tests in these cases is debatable, although they are routinely performed. The most widely used tests in these cases are the ex vivo tests such as LRT, even though these have a low sensitivity in such cutaneous drug reactions. The sensitivity can be increased by performing the test within a week of the serious reaction or subsequently adding a cytotoxic test.13
The phenotypic diversity of cutaneous drug reactions due to delayed hypersensitivity is explained by the recruitment of different drug-specific T lymphocytes and a different cytokine expression pattern (Fig. 7). Biopsies of the early stages of serious cutaneous drug reactions in AGEP have shown an initial blistering mediated by keratin cytolytic cytokines (including granzyme and perforin) produced by drug-specific cytotoxic T lymphocytes, with subsequent expression of IL8, leading to the formation of pustules and accumulation of neutrophils. Initial cytolysis due to granzymes and perforins produced by specific T lymphocytes also occurs in SJS/TEN. The process continues with massive apoptosis of keratinocytes due to activation of FAS/FASL and tumor necrosis factor (TNF). The mechanisms that guide the initial keratinocytolysis to one path or the other are not known.20
In AGEP, in addition to increased IL-8, variable expression of TNF has been demonstrated. In TEN, increased expression of TNF has been associated with apoptosis of the keratinocytes. It is possible that increased levels of TNF in a subgroup of patients with AGEP facilitate skin detachment and blister formation. Support for this affirmation comes from Meiss et al.23 who showed increased levels of TNF in patients with overlapping clinical manifestations of AGEP/TEN. These patients show good response to anti-TNF treatments in cases of AGEP/TEN overlap and in TEN.
In addition to the cases reported of overlap of AGEP and SJS/TEN, other cases of suspected overlap of serious cutaneous drug reactions, such as between AGEP and DRESS, have been reported.29 In these cases, both eosinophilia and neutrophilia are present in peripheral blood.13 This is a further cause for debate, as pustules can be observed as a skin manifestation in up to 20% of cases with DRESS and there may be cases that meet the diagnostic criteria for both entities.25
ConclusionWe present 2 new cases of serious cutaneous drug reaction with overlapping clinical manifestations between AGEP and TEN and we review the existing literature. It is hard to determine whether this is a separate overlapping entity or 2 consecutive skin reactions. Differentiation is important given the different approach to management and the different prognosis. If the combined forms are thought to be present, they should be treated as SJS/TEN, given the more serious nature of this condition. Further studies would be needed to complete the interpretation of these combined presentations.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank Dr. Juncal Ruiz Rivero, Dr. Virna Rodríguez Soria, and Dr. Verónica Parra Blanco for their assistence with the figures.
Please cite this article as: Horcajada-Reales C, Pulido-Pérez A, Suárez-Fernández R. Toxicodermias graves: ¿existen las formas combinadas?. Actas Dermosifiliogr. 2016;107:23–33.