Most economic evaluations in the literature on the subject of biologic therapy for the treatment of psoriasis do not reflect normal clinical practice or consider the cost of patient management.
ObjectiveThe objective of the present study is to establish a model for assessing the efficiency of biologic therapies in the treatment of psoriasis taking into account the cost of managing treatment which, in routine clinical practice, depends on patient response.
MethodsWe developed a model based on a decision tree that incorporates the probability of treatment response or failure with adalimumab, etanercept, infliximab, and ustekinumab after 24 weeks of therapy (end of the induction phase). The probability in each case was calculated using data from a meta-analysis. The following direct health costs were taken into account: the cost of drugs and their administration in euro (2015). Our analysis was based on the cost of 12 months of treatment administered in a hospital setting.
ResultsAccording to the proposed model, the mean cost per year by initial treatment strategy was lowest for patients who started treatment with ustekinumab, although the percentage cost difference between ustekinumab and infliximab or adalimumab was less than 3%. With a fixed budget of €1000000, the initial treatment option that would achieve success in the largest number of patients for one year would, according to this model, be ustekinumab (66 patients), followed by infliximab (n=62), adalimumab (n=59), and etanercept (n=50). Sensitivity analysis confirmed the reliability of these results. However, considering the confidence intervals of the incremental efficacy observed in the meta-analysis, the differences found are probably not significant in all the possible binary comparisons. Likewise, possible differences in actual price structures, populations, and the strategies and therapeutic objectives of each hospital could all give rise to considerable variations in real life.
ConclusionsThe cost of managing treatment in patients who fail to achieve an acceptable response during the induction phase should also be considered since such costs are a determining factor in any assessment of treatment efficiency. To achieve the optimum allocation of resources and to treat more patients efficiently, the information provided by this analysis should be cross-checked with real data taken from actual clinical practice in Spain collected in each geographical region and hospital.
La mayoría de las evaluaciones económicas publicadas sobre terapias biológicas en el tratamiento de la psoriasis no reflejan la práctica clínica habitual ni incorporan el coste asociado al manejo de los pacientes.
ObjetivoEl objetivo del presente estudio es establecer un modelo de la eficiencia de los fármacos biológicos en el tratamiento de la psoriasis, incorporando el coste asociado al manejo de los pacientes en función de la respuesta que se hace en la práctica clínica habitual.
MétodosSe ha desarrollado un modelo de árbol de decisión que incorpora la probabilidad de respuesta y fracaso de adalimumab, etanercept, infliximab y ustekinumab tras 24 semanas de tratamiento (final de la fase de inducción) obtenida a partir de un metaanálisis. Se han considerado costes sanitarios directos: farmacológicos y de administración (euros, 2015). Este análisis se ha llevado a cabo desde la perspectiva del hospital, considerando un horizonte temporal de un año.
ResultadosSegún el modelo propuesto, el coste medio anual por estrategia de tratamiento de inicio más bajo corresponde a aquellos pacientes que inician tratamiento con ustekinumab, aunque las diferencias porcentuales con infliximab y adalimumab no llegan al 3%. Considerando un presupuesto fijo de 1.000.000€, la estrategia de inicio que permite tratar con éxito a mayor número de pacientes durante un año según este modelo sería ustekinumab (66), seguido de infliximab (62), adalimumab (59) y etanercept (50). Los análisis de sensibilidad confirman la consistencia de estos resultados, aunque, teniendo en cuenta los intervalos de confianza de la eficacia incremental observada en el metaanálisis, las diferencias encontradas probablemente no sean significativas en todas las posibles comparaciones binarias, y las posibles modificaciones en la estructura real de precios, en las características de la población o en las estrategias y objetivos terapéuticos en cada centro pueden dar lugar a variaciones importantes en la vida real.
ConclusionesEl coste del manejo de los pacientes que no alcanzan una respuesta adecuada durante la fase de inducción no debe ser ignorado, puesto que es determinante a la hora de establecer la eficiencia del tratamiento. La información que proporciona este análisis debe contrastarse con los datos reales de la práctica clínica española en cada contexto geográfico y hospitalario para optimizar la asignación de recursos y tratar a un mayor número de pacientes de manera eficiente.
Biologic drugs represent a major advance in the treatment of moderate to severe psoriasis, making greater control of the disease possible. Today, there are 4 biologic drugs on the Spanish market indicated for the treatment of psoriasis: adalimumab, etanercept, infliximab and ustekinumab.1
The high cost of biologic therapies makes it particularly important to assess their efficiency2 (the relationship between the health outcomes achieved and the cost of treatment). The results of such studies can help us to optimize the use of these drugs in clinical practice, facilitating a more effective allocation of resources, which can maximize the benefit obtained by the patients. To do this we must combine data on the outcomes achieved in clinical practice with theoretical models based on assumptions derived from the available scientific evidence. Since indirect costs are difficult to assess in both clinical practice and economic models, analysis based on the acquisition cost of the drug (and the cost of administration, when applicable) represents a valid alternative. However, the authors of the economic studies in the literature have focused on the dosage regimen recommended in the Summary of Product Characteristics or have based their analyses on data from the records of hospital pharmacies. Consequently, their results do not reflect the realities of routine clinical practice because they fail to take into account the variations in cost associated with the management of different treatments depending on the patient's response.3–11
One peculiarity of the treatment of psoriasis with biologic drugs is that it involves an induction phase, at the end of which decisions on subsequent treatment are taken on a case by case basis depending on the patient's response to treatment. If the response is inadequate, treatment may be escalated (by increasing the dose or the frequency of administration) or the agent originally prescribed may be replaced by another biologic drug. Both of these options entail a significant increase in cost.9,10 Conversely, in patients with an excellent response, the regimen may be de-escalated (by reducing the dose or the frequency of administration),11 resulting in a reduction in the overall cost of therapy.
The objective of the present study was to evaluate a model for assessing the efficiency of the biologic therapies used in the treatment of psoriasis, taking into account the cost of managing treatment which, in routine clinical practice, depends on patient response.
MethodsWe developed a model that considered the cost associated with pharmacological treatment for 1 year from the start of treatment taking into account the implications of the comparative efficacy of the biological drugs after the first 24 weeks of treatment (the point at which, typically, a decision is taken on whether to maintain, reduce, or escalate the regimen, or switch the patient to another drug11).
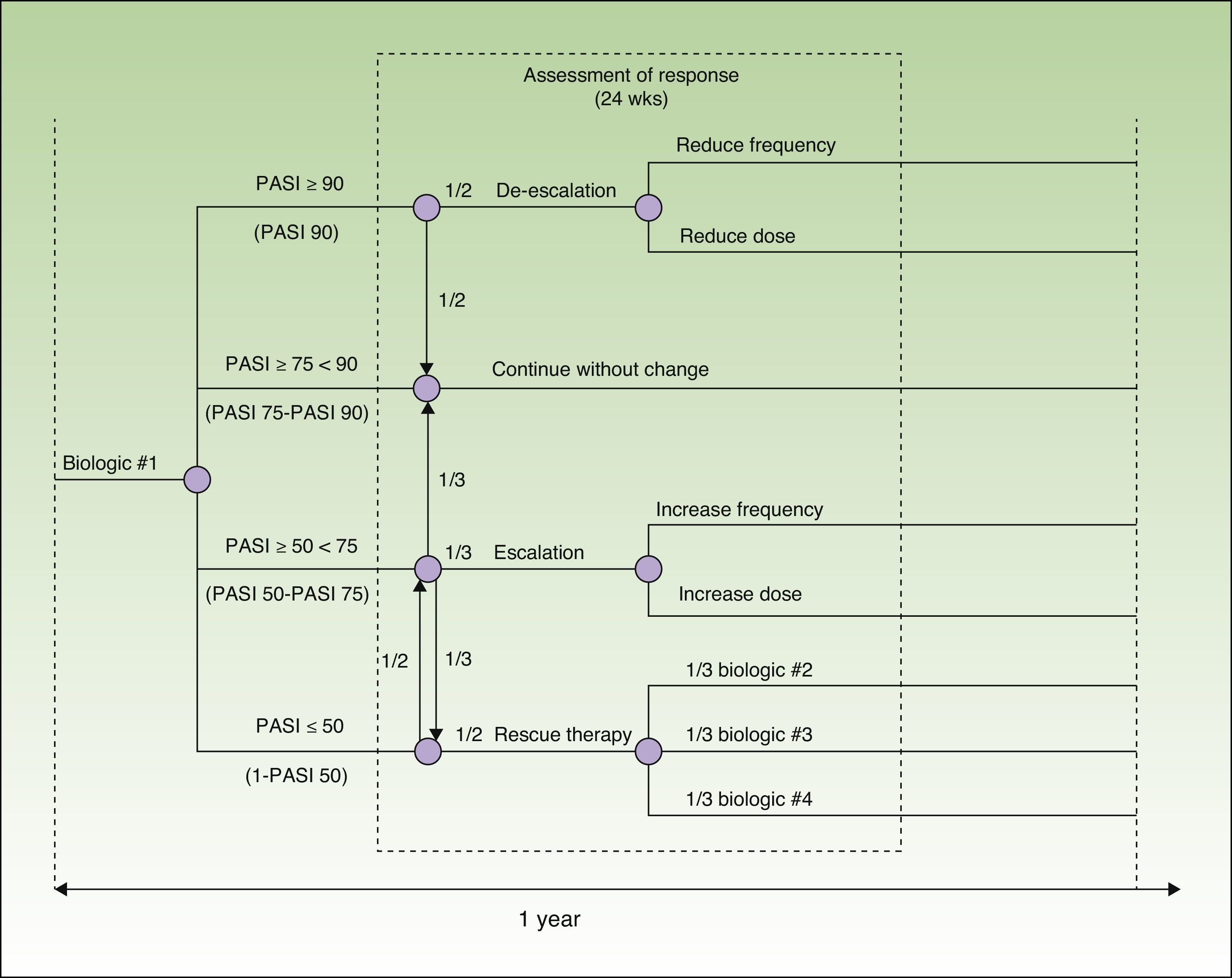
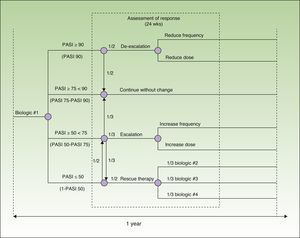
Model DesignThe model was designed to assess 52 weeks of treatment in a hospital setting (hospital pharmacy). It is based on a decision tree that incorporates each one of the drugs evaluated (adalimumab, etanercept, infliximab, and ustekinumab) and takes into account the possible escalation or de-escalation of the regimen and the need to switch drugs estimated on the basis of the probability of different particular responses at week 24. The probability in each case was calculated using data from a recently published meta-analysis12 (Fig. 1).
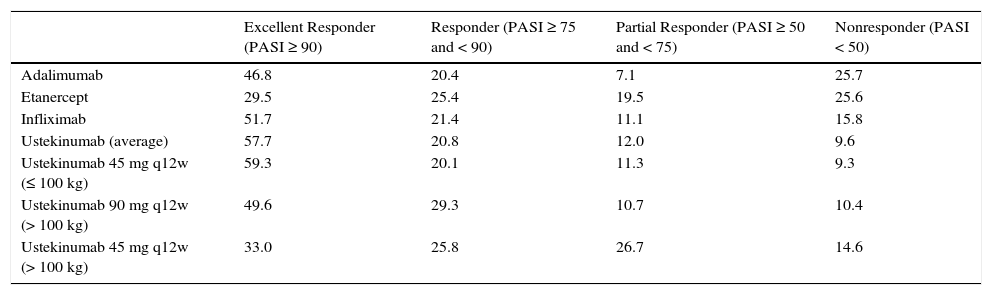
The reference document used to create the model was the European consensus document on treatment goals,13 which we adapted to the clinical practice of our country. In this model, patients are classified into 4 groups according to their response to treatment, which is assessed in terms of an improvement in the Psoriasis Area and Severity Index [PASI] of at least 50%, 75% or 90% (PASI 50, PASI 75, and PASI 90, respectively). In patients who achieve a PASI 90 response (excellent responders), the options are to continue the initial regimen or to reduce the dose or frequency of administration of the current biologic agent. Patients with a PASI 75 response who do not achieve a PASI 90 response (responders) continue on the initial regimen. In patients whose response is between PASI 50 and PASI 75 (partial responders), the choices are to continue on the initial regimen, to escalate the regimen (by increasing the dose or frequency of administration), or to switch to a different biologic agent. In patients who do not achieve a PASI 50 response, the options are to escalate the dosage regimen or to switch the patient to another biologic agent.
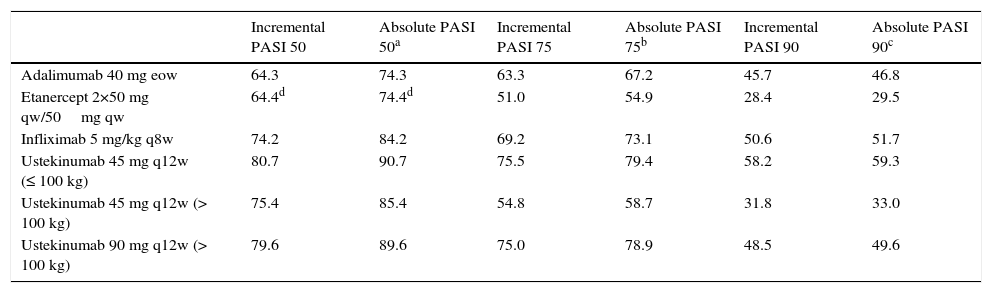
Efficacy DataThe distribution of patients according to response (Table 1) was determined on the basis of the absolute rates of efficacy for each biologic drug obtained from the rates of incremental efficacy over placebo published in the meta-analysis of reference12 (Table 2). Absolute efficacy rates were estimated on the basis of an average rate of response to placebo of 10.0%, 3.9%, and 1.1% for a PASI 50, PASI 75, and PASI 90 response, respectively (obtained by imputing the last observation before week 24 of all the patients treated with placebo in the meta-analysis12).
Distribution of Patients by Response to Treatment.
| Excellent Responder (PASI ≥ 90) | Responder (PASI ≥ 75 and < 90) | Partial Responder (PASI ≥ 50 and < 75) | Nonresponder (PASI < 50) | |
|---|---|---|---|---|
| Adalimumab | 46.8 | 20.4 | 7.1 | 25.7 |
| Etanercept | 29.5 | 25.4 | 19.5 | 25.6 |
| Infliximab | 51.7 | 21.4 | 11.1 | 15.8 |
| Ustekinumab (average) | 57.7 | 20.8 | 12.0 | 9.6 |
| Ustekinumab 45 mg q12w (≤ 100 kg) | 59.3 | 20.1 | 11.3 | 9.3 |
| Ustekinumab 90 mg q12w (> 100 kg) | 49.6 | 29.3 | 10.7 | 10.4 |
| Ustekinumab 45 mg q12w (> 100 kg) | 33.0 | 25.8 | 26.7 | 14.6 |
Abbreviation: q12w, every 12 weeks.
Percentage Response Rates at Week 24.
| Incremental PASI 50 | Absolute PASI 50a | Incremental PASI 75 | Absolute PASI 75b | Incremental PASI 90 | Absolute PASI 90c | |
|---|---|---|---|---|---|---|
| Adalimumab 40 mg eow | 64.3 | 74.3 | 63.3 | 67.2 | 45.7 | 46.8 |
| Etanercept 2×50 mg qw/50mg qw | 64.4d | 74.4d | 51.0 | 54.9 | 28.4 | 29.5 |
| Infliximab 5 mg/kg q8w | 74.2 | 84.2 | 69.2 | 73.1 | 50.6 | 51.7 |
| Ustekinumab 45 mg q12w (≤ 100 kg) | 80.7 | 90.7 | 75.5 | 79.4 | 58.2 | 59.3 |
| Ustekinumab 45 mg q12w (> 100 kg) | 75.4 | 85.4 | 54.8 | 58.7 | 31.8 | 33.0 |
| Ustekinumab 90 mg q12w (> 100 kg) | 79.6 | 89.6 | 75.0 | 78.9 | 48.5 | 49.6 |
Abbreviations: eow, every other week; PASI, Psoriasis Area and Severity Index; qw, weekly; q8w, every 8 weeks; q12w, every 12 weeks.
In this study, we analyzed the efficiency of treatment in a hospital setting on the basis of the direct cost (acquisition cost plus the cost of administration, if applicable) of each one of the biologic drugs at the manufacturer's price, as funded by the national health system as of April 2015,14 less the corresponding obligatory deduction15 plus 4% VAT. In calculating the cost of infliximab, we took into account the optimization of vial consumption (using the leftover portion of a vial in the treatment of the following patient) and the cost associated with intravenous infusion (€259.21).16 Annual costs were calculated by prorating the cost of the last dose received to the number of weeks remaining up to and including week 52.
The costs of treatment were calculated on the basis of the dosing regimens recommended in the Summary of Product Characteristics17–20: adalimumab, 80mg induction dose followed by 40 mg every other week starting 1 week after the initial dose; etanercept, 50 mg twice weekly for 12 weeks followed by 50 mg weekly; infliximab, 5 mg/kg administered at weeks 0, 2, and 6, followed by 5 mg/kg every 8 weeks; and ustekinumab, 45 mg or 90 mg at weeks 0 and 4, followed by a dose every 12 weeks. From week 24 onwards, we calculated the costs of treatment according to the dosing regimen that would be used depending on patient response to treatment (Fig. 1).
In the case of the agents that use dosing regimens adjusted to the patient's body weight (ustekinumab and infliximab), we calculated an average cost using the distribution of body weight in the Spanish population with psoriasis treated with biologics. This information was taken from the Spanish Registry of Systemic Therapy in Psoriasis (BIOBADADERM)21: < 60kg (13.2%); 61-80 kg (41.6%); 81-100kg (36.0%); 101-120kg (6.8%); and > 120kg (2.5%).
Assessment of EfficiencyOn the basis of the mean annual cost of treatment and the proportion of patients who continue on the initial biologic agent prescribed, we calculated the cost per successfully treated patient after one year for each of the 4 initial treatment strategies.
The mean annual cost per initial treatment strategy reflects the cost associated with the decision to start treatment with a particular drug and includes the cost of treatment with the biologic drug administered initially plus the cost of subsequent treatment based on the response observed at week 24.
Efficiency can also be evaluated by determining which treatment option offers the best health outcomes (the maximum number of patients treated successfully and the minimum number of patients treated unsuccessfully) for a specific budget. Using that approach, we determined how many patients would continue on the initial drug (successful treatment) and how many would be switched to a different biologic agent (unsuccessful treatment) at 1 year based on a fixed annual budget of €1000000 and using the data on mean annual cost taking into consideration the response at week 24.
Base Case ModelPatients who are considered to be excellent responders (those who have a PASI 90 response, that is, an improvement of ≥ 90% over the baseline PASI score), either continue with the initial dosing regimen or change to a reduced regimen at a ratio of 1:1. Responders (those with a PASI 75-90 response, that is, a ≥ 75% but < 90% improvement over baseline PASI), continue on the initial dosing regimen. Partial responders (PASI 50-75 response, that is an improvement of ≥ 50% but < 75% over baseline PASI), continue on the initial treatment regimen, change to an escalated regimen, or switch to another biologic agent at a ratio of 1:1:1. In the case of nonresponders (those who do not achieve a PASI 50 response), the initial dosing regimen is escalated or the patient is switched to another biologic agent at a ratio of 1:1. The biologic agent selected for rescue therapy is assumed to be any of the other 3 available treatments at a ratio of 1:1:1. In the absence of real world data, these ratios are assumed to be representative of the average across different clinical practice settings.
The model assumes that patients on ustekinumab whose body weight is greater than 100kg (9.3%) are treated with 90 mg or 45mg in equal proportions (1:1) because both of these doses are effective for such patients and recommended in the Summary of Product Characteristics.20
De-escalation of treatment in excellent responders involves a reduction in either the dose or the frequency of administration. In this respect, the model is based on the assumptions enumerated below. In the case of adalimumab, frequency of administration is reduced to every 3 (33% reduction in the cost of acquisition) or every 4 weeks (50% reduction) at a ratio of 3:1. In the case of etanercept, the dose is reduced to 25 mg weekly or the frequency to 50 mg every other week (50% reduction in the acquisition cost) at a ratio of 3:1. In the case of etanercept, for which the Summary of Product Characteristics includes the option of intermittent therapy, responders (PASI 75-90 response) and excellent responders (at least a PASI 90 response) in whom therapy is not de-escalated by a reduction in dose or dosing frequency, receive continuous or intermittent treatment at a ratio of 3:1. Because infliximab is potentially immunogenic, many clinicians prefer not to reduce the frequency or dose of this drug. This preference is reflected in the model by the following assumptions: in at least 50% of optimum responders (at least PASI 90), neither the dose nor the interval between doses is modified; in 25%, the interval between doses is changed to 10 weeks (the equivalent of reducing the dose to 4 mg/kg/d if the dosing frequency were maintained); and in the remaining 25%, the interval is changed to 12 weeks (a 33% reduction). In the case of ustekinumab 45 mg, the dosing frequency is reduced to every 18 or every 24 weeks at a ratio of 3:1. With ustekinumab 90 mg, the dose is reduced to 45 mg or the dosing frequency is reduced at a ratio of 1:1; the patients in whom the frequency is reduced are changed to a regimen of every 18 weeks or every 24 weeks at a ratio of 3:1.
Escalation of the treatment regimen implies an increase in dose or frequency of administration. In the case of adalimumab, the model assumes that the prescribing physician either increases the dosing frequency to once a week or changes the dosage to 80 mg weekly at a ratio of 3:1. With etanercept, the frequency is increased to 50 mg twice weekly. With infliximab, the frequency is increased to every 6 or every 4 weeks at a ratio of 3:1. With ustekinumab 45 mg, the dose is increased to 90 mg or the frequency is increased at a ratio of 1:3; and the increase in frequency is achieved by administration every 10 weeks or every 8 weeks at a ratio of 3:1. With ustekinumab 90 mg, the frequency is increased to every 10 weeks or every 8 weeks at a ratio of 3:1.
The base case model assumes that there is no washout period when a patient is switched to a different biologic drug (when the initial treatment is ineffective), and that the first dose of the new drug is administered on the day the next dose of the initial treatment would have been administered.
Sensitivity AnalysisTo determine the consistency of the model, a number of deterministic sensitivity analyses were carried out on the most important parameters.
The first of these analyses presupposed a washout period of 4 half-lives when rescue therapy with any one of the other 3 biologic agents is implemented.
In the second sensitivity analysis, treatments with a dosing frequency in multiples of 4 weeks were changed to multiples of months9,22,23 (infliximab every 2 months and ustekinumab every 3 months, instead of every 8 and every 12 weeks, respectively).
In the third sensitivity analysis, we varied the proportion of patients with a body weight greater than 100 kg treated with ustekinumab 45 mg and 90 mg, applying 2 limit scenarios: all patients treated with 90 mg or all patients treated with 45 mg.
A fourth sensitivity analysis assumed the use of an induction dose (50 mg twice weekly for 12 weeks) in only 50% or in 0% of the patients treated with etanercept.
Since the base values used in the model for the percentages of escalation and de-escalation were arbitrary, several sensitivity analyses were conducted to explore the impact of variations in how the dosing regimen is modified according to initial patient response. In the case of excellent responders, 2 limit scenarios were analyzed: reduction in all cases and maintenance of the initial dosing regimen in all cases. The alternative scenario in partial responders was escalation of the dosing regimen in all cases, and in non-responders it was rescue therapy with another biologic agent in all cases.
To add more information and to prevent the model from becoming obsolete in the event of pricing changes, we incorporated two final scenarios, which contemplate a 5% and a 10% reduction in the prices of adalimumab and etanercept. Since the prices of both infliximab and ustekinumab have recently changed, no price variation was considered for those 2 treatments.
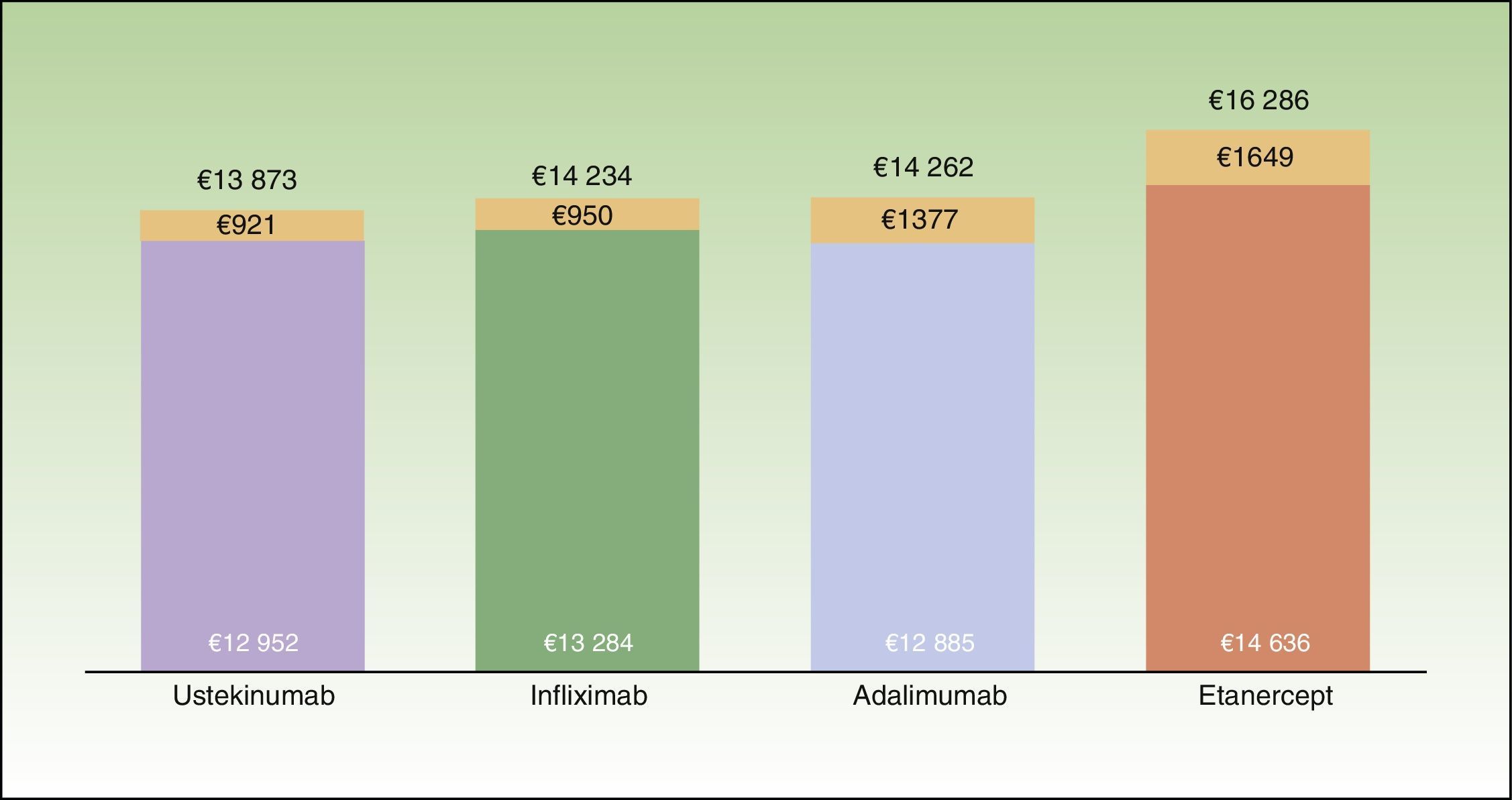
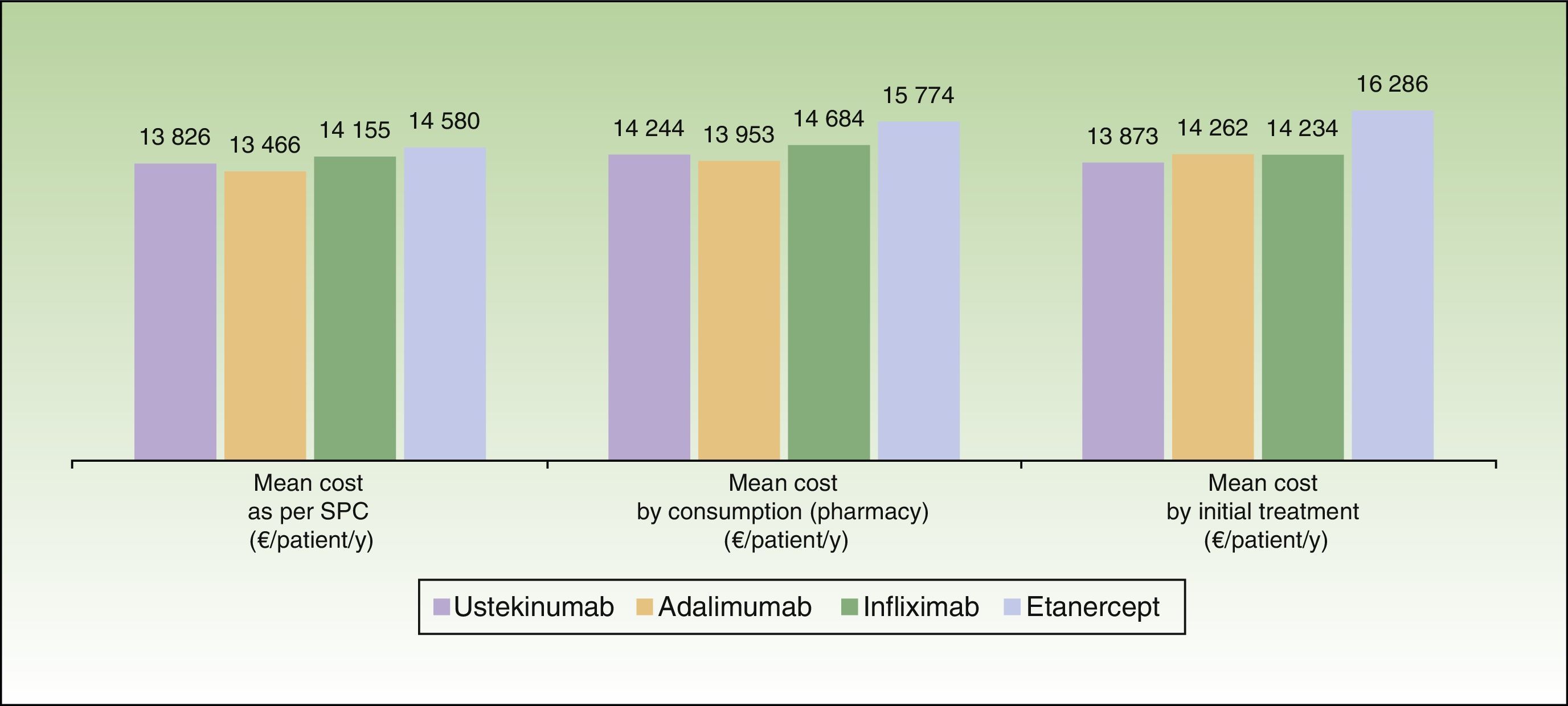
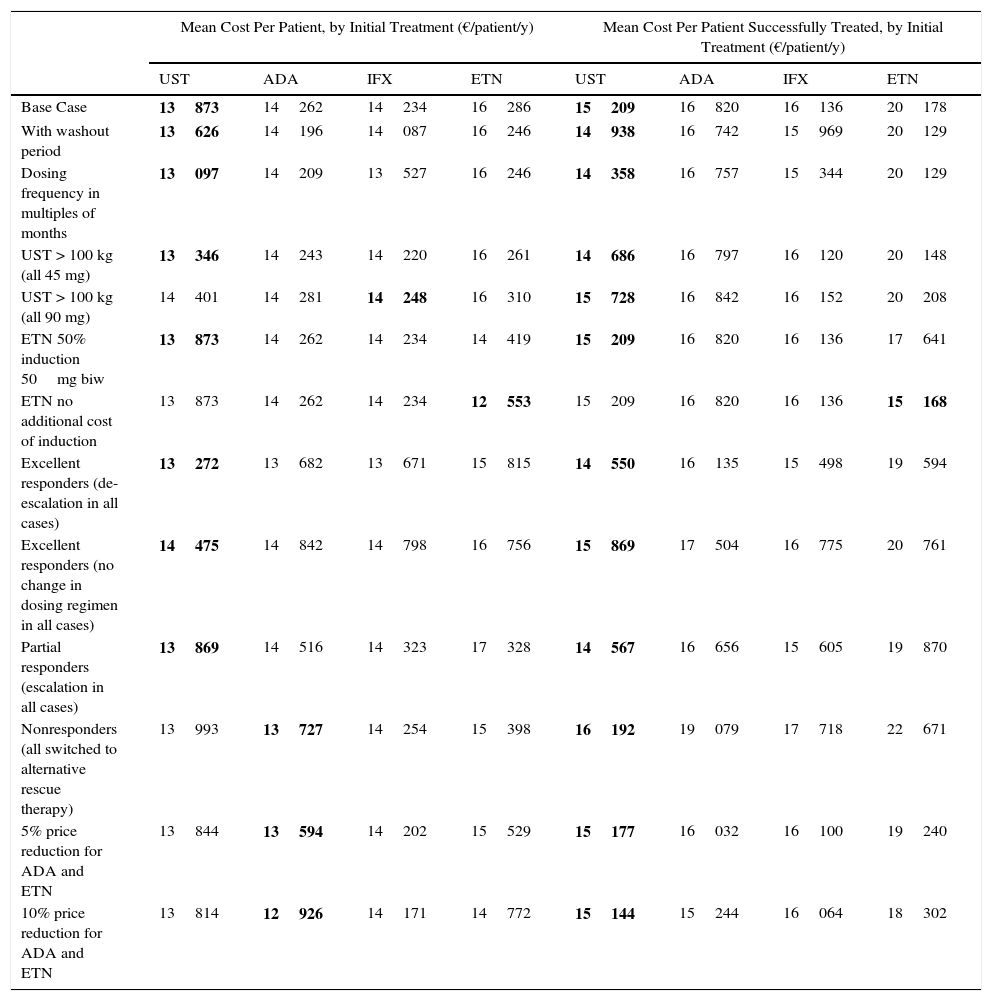
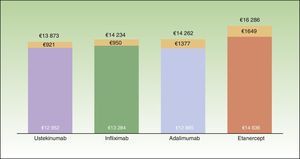
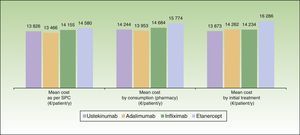
ResultsBase CaseThe mean cost for 1 year of treatment according to the model, was lowest for patients who started treatment with ustekinumab (€13873), followed by infliximab (€14234), adalimumab (€14262), and etanercept (€16286) (Fig. 2). However, the percentage differences with respect to the most cost-effective treatment were minimal in the case of infliximab (2.6%) and adalimumab (2.8%). The costs per initial treatment strategy in the proposed model differ from the mean theoretical cost calculated solely on the basis of the dosing regimen recommended in the Summary of Product Characteristics (which does not consider possible dose reductions in optimal responders or the need for escalated treatment or rescue therapy) and from per patient consumption derived from pharmacy records (which does not take into account that part of the consumption of each drug corresponds to rescue therapy for patients in whom treatment with another biological agent has failed) (Fig. 3).
Average cost per patient at the end of the first year of treatment according to the biologic therapy initially prescribed. The colored portion of the bar represents the cost for patients who complete the year on the same therapy; the orange portion represents the cost of rescue therapy with a different biologic agent.
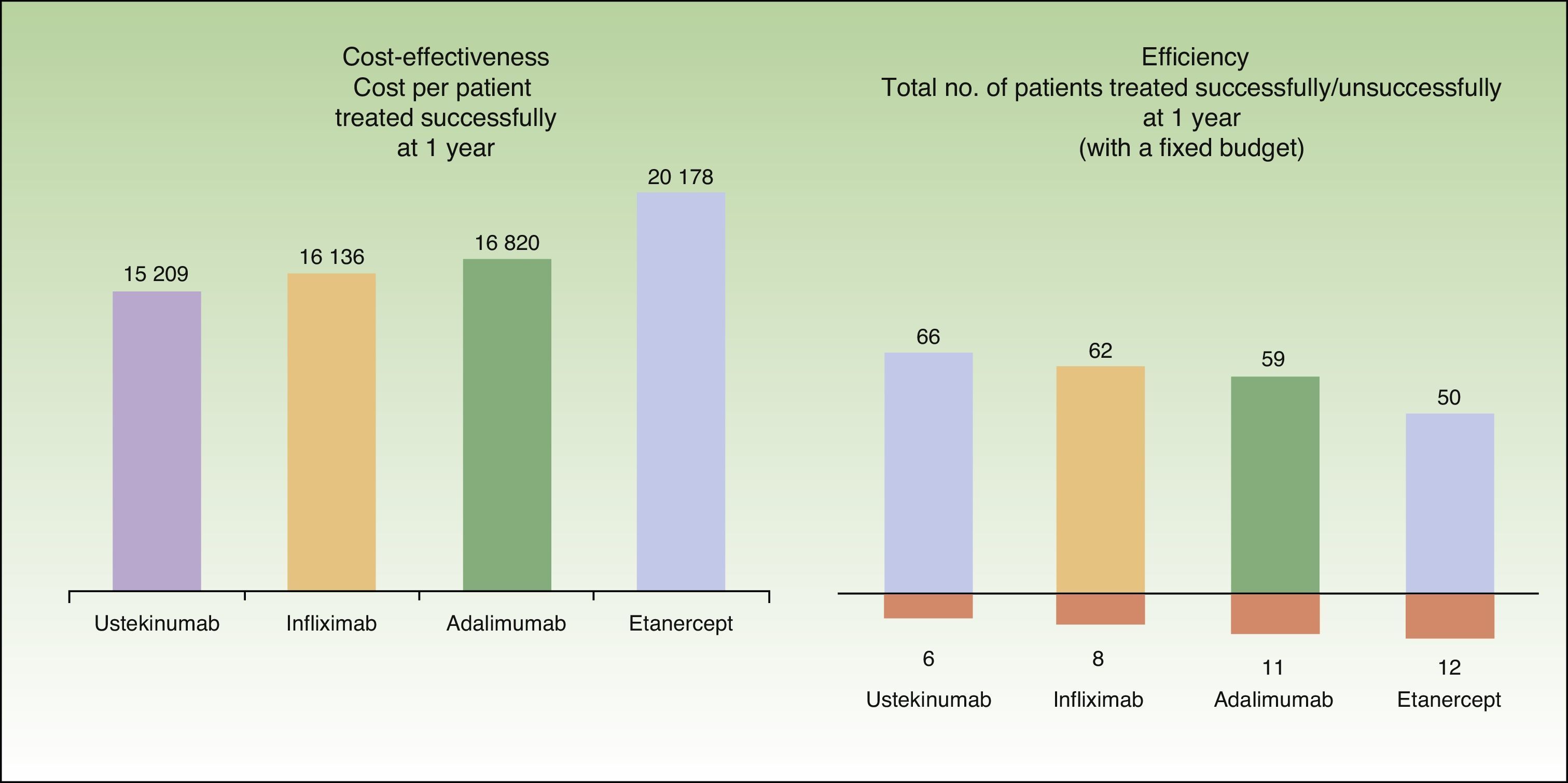
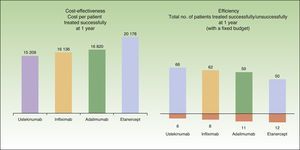
In the present model, the mean cost per patient treated successfully at the end of 1 year is lowest in patients who start treatment with ustekinumab (€15209), followed by infliximab (€16136), adalimumab (€16820), and etanercept (€20178) (Fig. 4.)
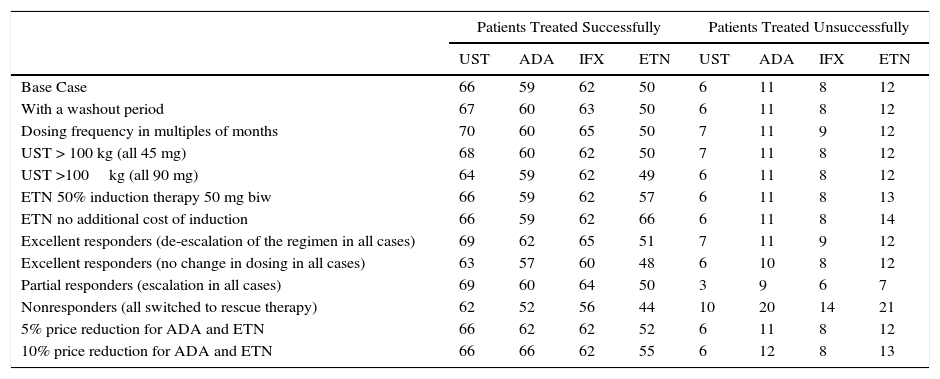
According to the present model, for a fixed budget of €1000000, the number of patients treated successfully (those who continue with the initial biologic agent) at the end of 1 year is greatest for the treatment regimen starting with ustekinumab (66), followed by infliximab (62), adalimumab (59), and etanercept (50). For the same budget, the number of patients treated without success (those who must be switched to rescue therapy with another biologic agent) after 1 year is lowest for the treatment regimen starting with ustekinumab (6), followed by infliximab (8), adalimumab (11), and etanercept (12) (Fig. 4).
Sensitivity AnalysisThe results of the sensitivity analyses (Table 3) show that ustekinumab presents the lowest mean annualized cost per initial treatment in all but the following cases: a 90 mg dose for all patients weighing over 100 kg (in that scenario, infliximab is the lowest cost option); no additional cost for induction therapy with etanercept (in which case etanercept would be the lowest cost option); rescue therapy with another biologic agent for all nonresponders (no escalation of the initial regimen); and a 5% or a 10% reduction in the price of adalimumab and etanercept. In the last 3 cases, adalimumab would become the dominant alternative, with the lowest annual cost of treatment.
Sensitivity Analysis: Mean Annualized Cost Per Patient and Per Patient Treated Successfully by Initial Treatment.a
| Mean Cost Per Patient, by Initial Treatment (€/patient/y) | Mean Cost Per Patient Successfully Treated, by Initial Treatment (€/patient/y) | |||||||
|---|---|---|---|---|---|---|---|---|
| UST | ADA | IFX | ETN | UST | ADA | IFX | ETN | |
| Base Case | 13873 | 14262 | 14234 | 16286 | 15209 | 16820 | 16136 | 20178 |
| With washout period | 13626 | 14196 | 14087 | 16246 | 14938 | 16742 | 15969 | 20129 |
| Dosing frequency in multiples of months | 13097 | 14209 | 13527 | 16246 | 14358 | 16757 | 15344 | 20129 |
| UST > 100 kg (all 45 mg) | 13346 | 14243 | 14220 | 16261 | 14686 | 16797 | 16120 | 20148 |
| UST > 100 kg (all 90 mg) | 14401 | 14281 | 14248 | 16310 | 15728 | 16842 | 16152 | 20208 |
| ETN 50% induction 50mg biw | 13873 | 14262 | 14234 | 14419 | 15209 | 16820 | 16136 | 17641 |
| ETN no additional cost of induction | 13873 | 14262 | 14234 | 12553 | 15209 | 16820 | 16136 | 15168 |
| Excellent responders (de-escalation in all cases) | 13272 | 13682 | 13671 | 15815 | 14550 | 16135 | 15498 | 19594 |
| Excellent responders (no change in dosing regimen in all cases) | 14475 | 14842 | 14798 | 16756 | 15869 | 17504 | 16775 | 20761 |
| Partial responders (escalation in all cases) | 13869 | 14516 | 14323 | 17328 | 14567 | 16656 | 15605 | 19870 |
| Nonresponders (all switched to alternative rescue therapy) | 13993 | 13727 | 14254 | 15398 | 16192 | 19079 | 17718 | 22671 |
| 5% price reduction for ADA and ETN | 13844 | 13594 | 14202 | 15529 | 15177 | 16032 | 16100 | 19240 |
| 10% price reduction for ADA and ETN | 13814 | 12926 | 14171 | 14772 | 15144 | 15244 | 16064 | 18302 |
Abbreviations: ADA: adalimumab; biw: twice weekly; ETN: etanercept; IFX: infliximab; UST: ustekinumab.
Ustekinumab would also have the lowest mean annualized cost per patient treated successfully in all the sensitivity analysis scenarios except when induction with etanercept does not involve any additional cost, in which case etanercept would be the lowest cost option (Table 3). On the basis of a fixed budget, ustekinumab (followed by infliximab, adalimumab, and etanercept) would achieve successful treatment in the largest number of patients and would be associated with the lowest number of patients experiencing primary treatment failure in most of the scenarios evaluated (Table 4).
Sensitivity Analysis: Number of Patients Treated Successfully and Unsuccessfully on an Annual Budget of €1000000, by Initial Treatment.
| Patients Treated Successfully | Patients Treated Unsuccessfully | |||||||
|---|---|---|---|---|---|---|---|---|
| UST | ADA | IFX | ETN | UST | ADA | IFX | ETN | |
| Base Case | 66 | 59 | 62 | 50 | 6 | 11 | 8 | 12 |
| With a washout period | 67 | 60 | 63 | 50 | 6 | 11 | 8 | 12 |
| Dosing frequency in multiples of months | 70 | 60 | 65 | 50 | 7 | 11 | 9 | 12 |
| UST > 100 kg (all 45 mg) | 68 | 60 | 62 | 50 | 7 | 11 | 8 | 12 |
| UST >100kg (all 90 mg) | 64 | 59 | 62 | 49 | 6 | 11 | 8 | 12 |
| ETN 50% induction therapy 50 mg biw | 66 | 59 | 62 | 57 | 6 | 11 | 8 | 13 |
| ETN no additional cost of induction | 66 | 59 | 62 | 66 | 6 | 11 | 8 | 14 |
| Excellent responders (de-escalation of the regimen in all cases) | 69 | 62 | 65 | 51 | 7 | 11 | 9 | 12 |
| Excellent responders (no change in dosing in all cases) | 63 | 57 | 60 | 48 | 6 | 10 | 8 | 12 |
| Partial responders (escalation in all cases) | 69 | 60 | 64 | 50 | 3 | 9 | 6 | 7 |
| Nonresponders (all switched to rescue therapy) | 62 | 52 | 56 | 44 | 10 | 20 | 14 | 21 |
| 5% price reduction for ADA and ETN | 66 | 62 | 62 | 52 | 6 | 11 | 8 | 12 |
| 10% price reduction for ADA and ETN | 66 | 66 | 62 | 55 | 6 | 12 | 8 | 13 |
Abbreviations: ADA: adalimumab; biw, twice weekly; ETN, etanercept; IFX, infliximab; UST, ustekinumab.
While the literature includes various studies that analyze the cost-effectiveness of the biologic drugs used to treat psoriasis,3,4,7,8,24–28 none of those authors have assessed the situation at 24 weeks, the point at which it is recommended that efficacy be assessed in clinical practice, nor have they taken into account the costs associated with the management of treatment in patients in routine clinical practice. Furthermore, there are very few studies of the costs of such treatment in Spain.
The proposed model offers a novel method for assessing the comparative efficiency of biologic therapies in psoriasis because it incorporates the cost associated with the management of treatment in clinical practice. In this case, for a similar cost of treatment and estimated on the basis of the Summary of Product Characteristics (between €13466 and €14580), efficiency is determined primarily by the rates of response and the possible ways each biologic regimen may be modified during the course of treatment. The consistency of our results is confirmed by the sensitivity analyses, which reflect diverse real alternative scenarios in the management of treatment, thus providing data of more practical use than other studies published to date.
According to our model, the choice of ustekinumab as an initial treatment is the scenario that would entail the lowest cost per successfully treated patient, would maintain the largest number of patients in treatment at the end of 1 year, and would minimize the number of patients needing rescue therapy with a different biologic agent. The relative efficacy of ustekinumab, together with other factors, such as its scant immunogenicity and 3-month dosing interval, help to explain the results observed in several registries and patient cohorts in routine clinical practice, which indicate that ustekinumab is the biologic agent with the highest rate of retention at 1 year.29–32
The present study shows how differences in efficacy between biologic agents12,31–35 determine the efficiency of each treatment and have important implications for budget optimization, such that therapeutic equivalence should not, in general, be assumed.35 The analysis also shows that neither the theoretical cost based on the Summary of Product Characteristics nor consumption observed in hospital pharmacies are appropriate methods for assessing the efficiency of biologic agents or can serve as a basis for decisions because both methods fail to take into consideration the cost associated in routine clinical practice with the evolution of treatment in patients who present either an excellent or an unsatisfactory response.
One of the principal limitations of this analysis is that the model does not contemplate possible variations in the efficacy of the treatment between weeks 24 and 52, a shortcoming that is prejudicial to the biologic agents with the highest rate of retention at 1 year. Likewise, since the literature offers insufficient data and the efficacy of rescue treatment was assessed at the end of the first year, it has been assumed that all patients respond adequately to the modification of the regimen or to an alternative biologic treatment prescribed as a second-line therapy. The addition of classic systemic drugs to biologic therapy has not been incorporated into this model because of the lack of relevant data and the minimal economic impact of such combinations. It would be useful to incorporate data on effectiveness taken from national or institutional registries, which would facilitate an analysis that would even more accurately reflect routine clinical practice.
On the basis of the official state funded prices currently applicable in Spain and comparative response rates derived from the meta-analysis used as a reference in this model, ustekinumab is the most cost-effective biologic therapy in patients with moderate to severe psoriasis in a 1-year timeframe However, considering the confidence intervals of the incremental efficacy of the different drugs in the meta-analysis,12 we cannot affirm that the differences observed would be significant in all the possible binary comparisons. Likewise, changes in the current price structure or variations in the characteristics of the population or in the therapeutic strategies and objectives of individual hospitals could give rise to considerable variations.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals during the course of this study.
Data confidentialityThe authors declare that no private patient data are disclosed in this article.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Funding SourcesJanssen-Cilag, SA financed the independent analysis of the data by Pértica, Spain.
Conflicts of InterestLluís Puig has received consulting fees and has participated in clinical trials sponsored by Abbvie, Pfizer, Janssen, and Merck. Anna López-Ferrer has received speaker fees from Janssen. Eva Vilarrasa has participated in clinical trials sponsored by Janssen. Ignacio García and Raquel Fernández del Olmo are employees of Janssen.
Please cite this article as: Puig L, López-Ferrer A, Vilarrasa E, García I, Fernández-del-Olmo R. Modelo de eficiencia de los fármacos biológicos en el tratamiento de la psoriasis moderada-grave durante un año en las condiciones de uso en España. Actas Dermosifiliogr. 2016;107:34–43.