Psoriatic patients who are being treated with biologic drugs and have a history of hepatitis B virus (HBV) infection are at risk of viral reactivation.1
To evaluate the safety of secukinumab treatment in psoriasis patients with a history of previous or chronic HBV infection, we retrospectively collected from 3 national centers clinical and laboratory data for psoriasis patients that met the following criteria: serological markers compatible with previous or chronic HBV infection; psoriasis treated with secukinumab for at least 3 months; and periodic follow-up with serology and viral load monitoring. The outcomes measured were viral reactivation and the development of hepatitis during secukinumab treatment.
HBV reactivation was defined as an increase in serum viral DNA levels of at least 1 log10 copies/mL or a change from undetectable to detectable. Hepatitis was defined as an increase in transaminase levels of at least 5 times the upper limit. HBV infection was considered chronic in patients who were positive for the surface antigen of HBV (HBsAg or Australian antigen) for at least 6 months. Previous HBV infection was defined by positivity for anti-core antibodies (HBcAb) and antibodies against surface antigen (HBsAb), and negativity for HBsAg. Isolated HBcAb was defined as positive HBcAb with negative HBsAb and HBsAg.
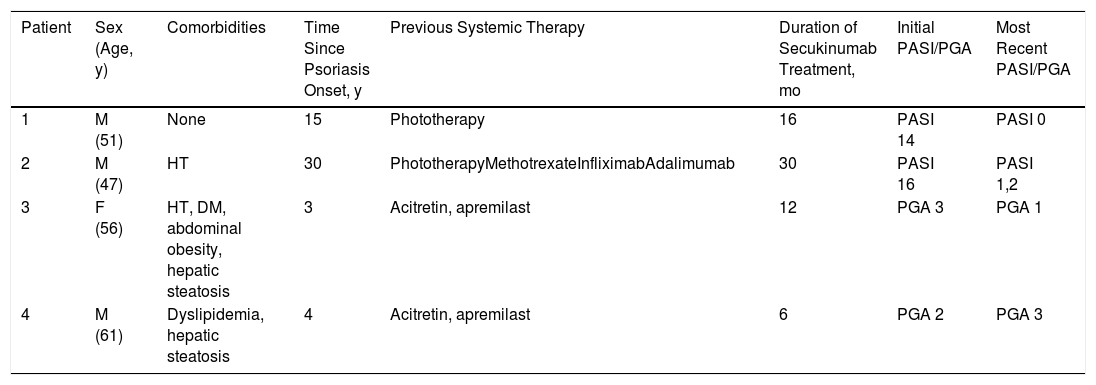
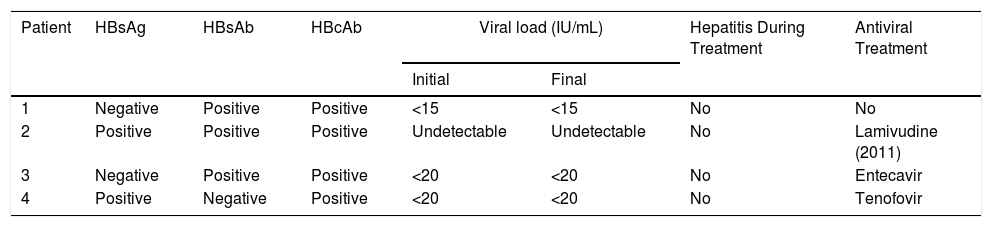
Four patients fulfilled the inclusion criteria: 3 women and 1 man (mean age, 53 y). Tables 1 and 2 summarize the clinical and serological characteristics of the patients.
General Characteristics of the Patients
| Patient | Sex (Age, y) | Comorbidities | Time Since Psoriasis Onset, y | Previous Systemic Therapy | Duration of Secukinumab Treatment, mo | Initial PASI/PGA | Most Recent PASI/PGA |
|---|---|---|---|---|---|---|---|
| 1 | M (51) | None | 15 | Phototherapy | 16 | PASI 14 | PASI 0 |
| 2 | M (47) | HT | 30 | PhototherapyMethotrexateInfliximabAdalimumab | 30 | PASI 16 | PASI 1,2 |
| 3 | F (56) | HT, DM, abdominal obesity, hepatic steatosis | 3 | Acitretin, apremilast | 12 | PGA 3 | PGA 1 |
| 4 | M (61) | Dyslipidemia, hepatic steatosis | 4 | Acitretin, apremilast | 6 | PGA 2 | PGA 3 |
Abbreviations: DM, diabetes mellitus; F, female; HT, hypertension; M, male; PASI, psoriasis area severity index; PGA, physician global assessment.
Patients With HBV Infection: Laboratory Findings
| Patient | HBsAg | HBsAb | HBcAb | Viral load (IU/mL) | Hepatitis During Treatment | Antiviral Treatment | |
|---|---|---|---|---|---|---|---|
| Initial | Final | ||||||
| 1 | Negative | Positive | Positive | <15 | <15 | No | No |
| 2 | Positive | Positive | Positive | Undetectable | Undetectable | No | Lamivudine (2011) |
| 3 | Negative | Positive | Positive | <20 | <20 | No | Entecavir |
| 4 | Positive | Negative | Positive | <20 | <20 | No | Tenofovir |
Abbreviations: HBsAb, antibody against hepatitis B surface antigen; HBsAg, hepatitis B surface antigen; HBcAb, hepatitis B anti-core antibody.
Patients 1 and 2 were diagnosed with plaque psoriasis and patients 3 and 4 with palmoplantar psoriasis. All had undergone at least 1 systemic treatment prior to beginning secukinumab therapy (300mg/mo).
Patients 1 and 3 had previous HBV infections and patients 2 and 4 had chronic HBV infections. Patients 3 and 4 also had hepatic steatosis. Viral load was undetectable in all patients at the start of treatment. Patient 1 had received lamivudine treatment for a HBV infection in 2011. Patients 3 and 4 began antiviral and secukinumab concomitantly to avoid possible HBV reactivation. None of the patients experienced viral reactivation or hepatitis during follow-up (mean, 20 mo; range, 6–30 mo). Patient 4 discontinued treatment due to primary drug failure. The other 3 patients are still in treatment, to which their psoriasis has responded well.
Owing to their immunosuppressive effect, biologic drugs increase the risk of reactivation of HBV infection in psoriatic patients.1–5
Few studies have evaluated the safety of secukinumab for the treatment of psoriatic patients with HBV infection.1,6,7
The largest study to assess the effects of secukinumab treatment in psoriasis patients with concomitant HBV (n=49)6 found that of 22 patients who had chronic infections and did not receive prophylaxis, 27% experienced viral reactivation. None of the 3 patients with chronic infection who received antiviral prophylaxis experienced HBV reactivation. Of the 7 patients that experienced viral reactivation, 3 were treated with antivirals (entecavir or telvibudine) and 4 underwent strict follow-up. In all cases viral load decreased or stabilized, without the development of hepatitis. The authors concluded that antiviral prophylactic treatment should be administered in patients with chronic infection (HBsAg-positive patients) and in those with isolated HBcAb associated with a detectable viral load.
In our study, only 1 of the 2 HBsAg-positive patients received antivirals during secukinumab treatment. Of the 2 patients with previous HBV infections, 1 received prophylactic antiviral treatment. This reflects disparities in patient management between different healthcare centers and underscores the need to unify criteria for the indication of antiviral prophylaxis in psoriatic patients undergoing treatment with new biologic therapies.
The limitations of our study are those inherent to retrospective studies, as well as the small sample size.
In conclusion, previous or chronic HBV infection does not contraindicate the use of secukinumab in patients with psoriasis. Adequate selection of treatment candidates is recommended, in addition to periodic monitoring with viral load, serological, and liver function tests. The frequency with which these tests should be performed is unclear. However, given that the reactivations described in the literature in psoriatic patients treated with secukinumab occur an average of 3 months after starting treatment, we propose that these tests be performed initially every 3 months, after which the frequency can be reduced where appropriate.6 In patients with inactive chronic infections, antiviral treatment independent of viral load is advisable given the increased risk of HBV reactivation.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Moneva-Leniz LM, Sahuquillo-Torralba A, Vila-Payeras A, Mateu-Puchades A. Riesgo de reactivación de infección por virus hepatitis B en pacientes con psoriasis en tratamiento con secukinumab: una serie de 4 casos. Actas Dermosifiliogr. 2020;111:613–614.