We report 3 cases of solar urticaria in which there was no response or limited response to first-line treatments with high-dose H1 antihistamines or phototherapy. The patients were then treated with omalizumab. Symptoms improved in 2 patients, whose tolerance to sunlight increased considerably; quality of life clearly improved for 1 of these patients. The third experienced no improvement and developed a mild local reaction to the injected medication. We conclude that omalizumab may offer a potentially safe, useful alternative for patients with solar urticaria who do not respond to conventional therapy.
Se presentan 3 pacientes con urticaria solar que o no habían respondido adecuadamente o presentaban limitaciones a los tratamientos de primera línea (antihistamínicos H1 a dosis altas o fototerapia), que fueron tratados con omalizumab. Dos de ellos mejoraron clínicamente con un aumento muy importante de la tolerancia a la luz, uno de ellos con clara mejoría de la calidad de vida. El otro paciente no mejoró y desarrolló una reacción local leve a la medicación inyectada. Omalizumab puede ser por tanto una alternativa terapéutica potencialmente útil y segura en urticarias solares graves no respondedoras al tratamiento convencional.
Solar urticaria (SU) is an uncommon chronic inducible urticaria characterized by the development of wheals after exposure to sun radiation, visible light, or UV radiation. Because the condition is rare, there are no epidemiological data on incidence or prevalence rates,1 although varying figures of between 2.3% and 17.8% have been reported within the group of photodermatoses.1 Lesions typically appear within minutes of exposure and the most common action spectra are visible light and UV-A. Action spectrum and minimal urticaria dose (MUD) are important for diagnosis, treatment, and prognosis, and can be used to rule out other photodermatoses.2
Second-generation H1 antihistamines are the first-line treatment for inducible chronic SU, just as they are for other forms of inducible chronic urticaria. However, most patients require either high doses or combinations of different antihistamines.2 An alternative first-line approach is tolerance induction through phototherapy.2 The use of omalizumab has also been described in several case reports and small case series of patients in recent years, with varying results. The principle underlying this treatment is based on a hypothetic role for immunoglobulin (Ig) E in the pathogenesis of inducible chronic urticaria.3
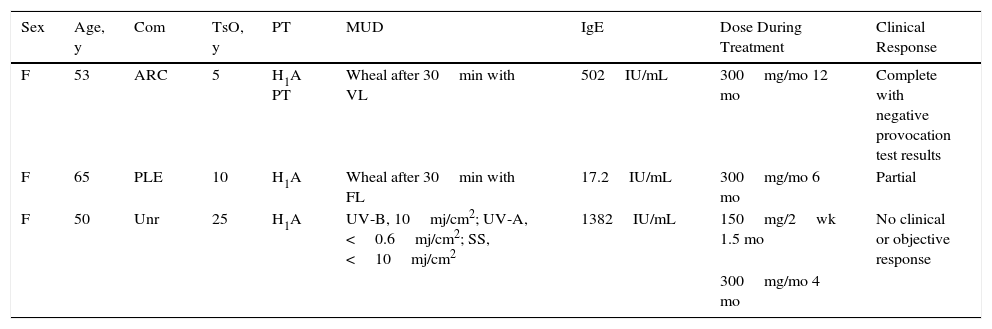
Case DescriptionsThe clinical data for 3 patients with severe SU refractory to H1 antihistamines treated in our department over a period of 5 years are summarized in Table 1.
Cases of Solar Urticaria Treated With Omalizumab.
| Sex | Age, y | Com | TsO, y | PT | MUD | IgE | Dose During Treatment | Clinical Response |
|---|---|---|---|---|---|---|---|---|
| F | 53 | ARC | 5 | H1A PT | Wheal after 30min with VL | 502IU/mL | 300mg/mo 12 mo | Complete with negative provocation test results |
| F | 65 | PLE | 10 | H1A | Wheal after 30min with FL | 17.2IU/mL | 300mg/mo 6 mo | Partial |
| F | 50 | Unr | 25 | H1A | UV-B, 10mj/cm2; UV-A, <0.6mj/cm2; SS, <10mj/cm2 | 1382IU/mL | 150mg/2wk 1.5 mo | No clinical or objective response |
| 300mg/mo 4 mo |
Abbreviations: ARC, allergic rhinoconjunctivitis; Com, comorbid conditions; FL, fluorescent light; H1A, H1 antihistamines; IgE, immunoglobulin E; MUD, minimal urticaria dose; PLE, polymorphic light eruption; PT, previous treatment; TsO, time since onset; Unr, unremarkable; VL, visible light.
Patient 1 had SU induced by visible light in which neither H1 antihistamines nor phototherapy proved effective. Following treatment with omalizumab, however, she exhibited clear signs of clinical improvement (she was able to tolerate sunlight for 10 times longer than before) and improved test results after photoprovocation testing. Patient 2 had SU to visible light triggered by indoor lights (fluorescents and LEDs) that prevented her from spending time in the sun. After treatment with omalizumab, she experienced an increase in tolerance of exposure to both indoor lights and sunlight. She is now able to remain in the sun for up to 5hours, something that she had not been able to do for 15 years. She also showed improved objective health-related quality of life scores on the Skindex-29 compared with baseline, with a 23% improvement noted for overall quality of life, and additional improvements in the Symptoms, Emotions, and Functioning domains. Patient 3, whose case has been previously published,4 had SU due to UV-B and UV-A and a very low MUD that limited the use of phototherapy. She had responded poorly to H1 antihistamines. She was treated twice with omalizumab but showed no response on either occasion. In addition, she developed a mild local reaction after the first injection in the form of pruritic wheals that resolved spontaneously.
DiscussionIn our review of the literature, we identified 16 patients with SU treated with omalizumab (8 case reports and 3 case series).3–14 Fourteen of the patients were adults and 2 were children.5,6 The treatment doses varied between 150mg/mo7 and 800mg/mo.8 Varying measures of clinical response were used, and not all authors reported on this aspect of treatment. Most authors used subjective criteria based on patient-reported manifestations or other health-related quality of life measures. Others used phototesting6–9 or the Urticaria Activity Score 7, which is a validated tool for evaluating chronic urticaria.10 Of the 16 patients, 12 (75%) responded either partially or completely to treatment and 5 of these (31.2%) additionally showed negative provocation results. Follow-up time varied from 1 month, in a patient who showed complete response after a single dose,7 to 1 year, in a patient who received 12 monthly doses.6
On analyzing the cases reviewed, we observed a certain tendency towards an association between high total baseline IgE levels, albeit variable, and greater response to treatment. Seven of the 8 patients in this subgroup responded to treatment, although it should be noted that some of the responders had normal IgE levels, while some of the nonresponders (like patient #3 in our series) had elevated levels.
No severe adverse effects were mentioned in the cases reviewed, and it is noteworthy that the treatment proved safe in the 2 pediatric cases described.5,6
Finally, Aubin et al.15 recently reported on results from a phase II clinical trial investigating the use of omalizumab (300mg/mo for 2 months) in 10 patients with SU studied by phototesting and photoprovocation (action spectra: UV-A, UV-B, and polychromatic solar spectrum). The primary endpoint was the proportion of patients who did not develop SU lesions after photoprovocation with a UV radiation dose 10 times higher than the baseline MUD after 12 weeks of treatment. Approximately 40% of the patients showed an initial clinical improvement, but the efficacy results based on the primary endpoint showed no significant differences.
In conclusion, despite the limited data available from case reports and small case series on the characteristics and results of SU treatment with omalizumab, the clinical response rate of 75% based on reports in the literature to date is promising. The 3 patients described in our series are a selection of patients with severe SU treated at our department and omalizumab proved effective in 2 of them. Response was slower than that typically described for spontaneous chronic urticaria, with improvement observed after 3 doses (patient #1) or 5 doses (patient #2). Both patients are satisfied with the results and wish to continue treatment. Omalizumab may therefore be a potentially safe and useful treatment for patients with severe SU that is refractory to conventional treatment.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestDr de Argila has worked as a clinical advisor for Novartis and also participated in clinical trials sponsored by this company. The other authors declare no conflicts of interest.
Please cite this article as: Rodríguez-Jiménez P, Chicharro P, Pérez-Plaza A, de Argila D. Respuesta a omalizumab en 3 casos de urticaria solar. Actas Dermosifiliogr. 2017;108:e53–e55.