Preservatives are added to cosmetic, household cleaning, and other industrial products to prevent the growth of microorganisms. Unfortunately, exposure to these substances can cause sensitization.
Material and methodsBetween January and June 2015, we analyzed the ingredients of 2300 products commercially available in Spain to identify the frequency of a wide variety of preservatives in different product categories. We analyzed 1093 skin care and cosmetic products sold exclusively in pharmacies (dermocosmetics), 458 household cleaning and personal hygiene and cosmetic products sold in supermarkets, 636 topical medications, and 113 cosmetic products sold in a herbal shop.
ResultsPhenoxyethanol, citric acid, sodium benzoate, and potassium sorbate were very common in all the cosmetic product categories. Parabens were present in 16.1% of dermocosmetic products, 14.45% of cosmetic products available in supermarkets, 0.88% of cosmetic products available in the herbal shop, 5.18% of topical medications, and in none of the cleaning products. Isothiazolinones were identified in 2.56% of dermocosmetic products, 18% of cosmetic products in supermarkets, 7.9% of cosmetic products in the herbal shop, 63.63% of household cleaners, and in none of the topical medications. Formaldehyde releasers were detected in 5.76% of dermocosmetic products, 6.42% of cosmetic products sold in supermarkets, 7.96% of cosmetic products sold in the herbal shop, 3.93% of topical medications, and 16.74% of household cleaners.
ConclusionsEvaluation of the presence of preservatives in everyday products allows us to indirectly estimate exposure levels to each one. Measures restricting the use of the most problematic preservatives need to be strengthened.
Los conservantes se agregan a cosméticos, limpiadores domésticos y otros productos industriales para impedir el crecimiento de microorganismos. Desafortunadamente, pueden sensibilizar a usuarios expuestos
Material y métodosEntre enero y junio de 2015 se analizaron las listas de los ingredientes de 2.300 productos de venta en España: 1.093 dermocosméticos, 458 productos de higiene, cosméticos y limpiadores domésticos de venta en supermercados, 636 medicamentos tópicos y 113 cosméticos de herbolario. Se evaluó la distribución de una amplia variedad de conservantes en las distintas categorías de productos.
ResultadosConservantes tales como el fenoxietanol, el ácido cítrico, el benzoato sódico y el sorbato potásico estuvieron ampliamente representados en todas las categorías de cosméticos. Se detectaron parabenos en el 16,1% de los dermocosméticos, el 14,45% de los cosméticos de supermercado, el 0,88% de los cosméticos de herbolario, el 5,18% de los medicamentos tópicos y ningún producto de limpieza. Se objetivaron isotiazolinonas en el 2,56% de los dermocosméticos, el 18% de los cosméticos de supermercado, el 7,9% de los cosméticos de herbolario, el 63,63% de los limpiadores domésticos y en ningún medicamento tópico. Contenían liberadores de formaldehído el 5,76% de los dermocosméticos, el 6,42% de los cosméticos de supermercado, el 7,96% de los cosméticos de herbolario, el 3,93% de los medicamentos tópicos y el 16,74% de los limpiadores.
ConclusionesLa evaluación de la frecuencia de los conservantes en los productos de nuestro entorno permite una estimación indirecta del grado de exposición a cada uno de ellos. Se precisa impulsar medidas que conduzcan a una restricción en el uso de los conservantes más problemáticos.
Preservatives are added to cosmetic, household cleaning, and other products to prevent the growth of microorganisms. Inadequate use of preservatives can lead the product to deteriorate. Excessive use of preservatives, on the other hand, could increase the risk of sensitization. Approximately 6% of the population is sensitized to the ingredients of cosmetics, especially preservatives and fragrances.1,2 When a risk of sensitization to a preservative is reported, expert committees (Scientific Committee on Consumer Products in Europe and Cosmetic Ingredient Review in North America) issue opinions that lead to changes in legislation to ensure that the maximum permitted concentrations are restricted or even banned.2 Consequently, the cosmetics industry encourages the use of alternative types of preservative (new substances or combinations). Occasionally, the most recently developed preservatives trigger new cases of sensitization, which are as serious as, or more serious than, those arising from the preservative they had replaced. Thus, over the years, there have been several “epidemics” of sensitization to the following preservatives: formaldehyde (1950-1960s), methylchloroisothiazolinone/methylisothiazolinone (MCI/MI) (1970-80s),3 and methyldibromo glutaronitrile (1990s).1 We are currently experiencing an epidemic of allergy to MI that began in 2005, when the substance was approved for use in cosmetics at a concentration of <100ppm in the belief that it was less sensitizing than MCI and despite the fact that the first cases of occupational dermatitis to MI had been reported by then.4,5 This belief was based on the publication of the results of an in vivo test that were later shown to be incorrect.6 The first cases of sensitization to MI in cosmetics began to be detected within only 5 years. The first report was by García-Gavín et al,7,8 who provided data from 6 patients with allergy to MI in moist toilet paper. Since then, rates of sensitization to MI have increased exponentially in Europe1,9 and the United States, where MI was crowned “Allergen of the Year” in 2013.10
Parabens, however, which are one of the oldest and still very widely used groups of biocides in cosmetics, are less sensitizing than most recently developed preservatives.1,2
We analyzed the distribution of the main preservatives in several categories of personal hygiene and cosmetic products, topical medications, and household cleaning products.
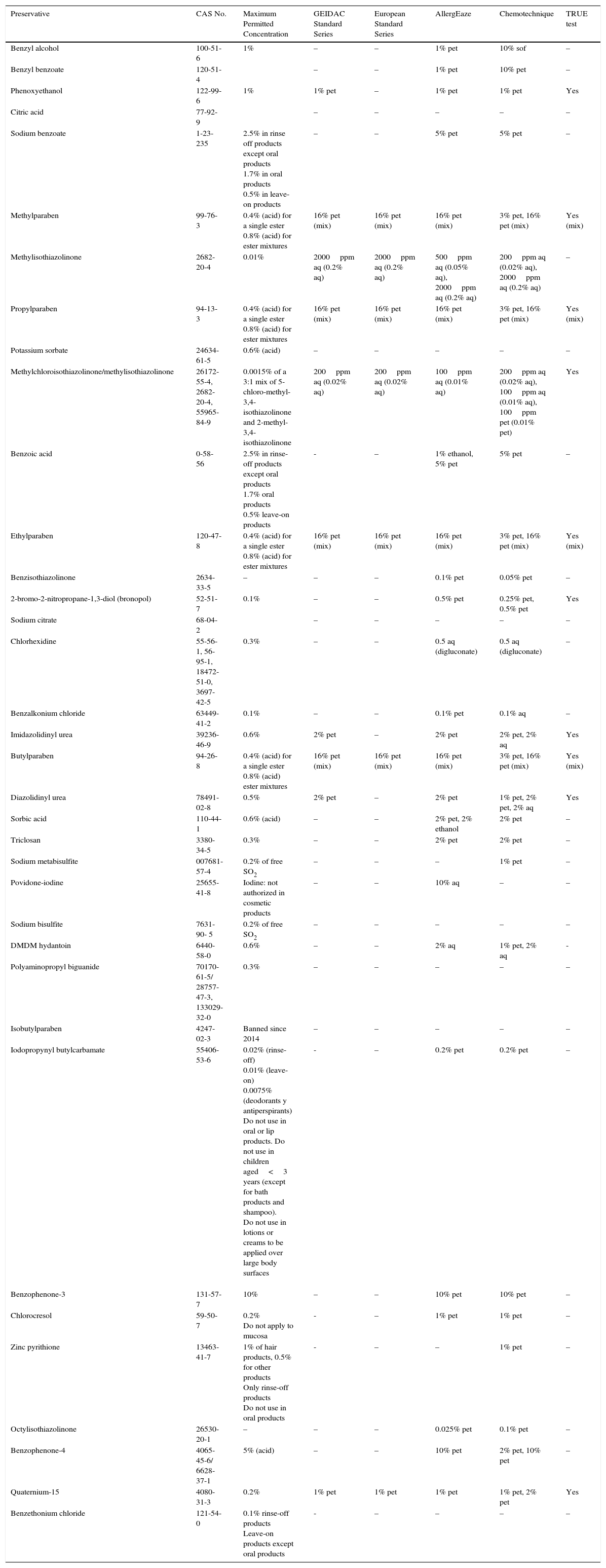
Primary ObjectiveTo evaluate the frequency of use of the main preservatives in each product category (Table 1).
Characteristics of the Preservatives Selected for Study.
| Preservative | CAS No. | Maximum Permitted Concentration | GEIDAC Standard Series | European Standard Series | AllergEaze | Chemotechnique | TRUE test |
|---|---|---|---|---|---|---|---|
| Benzyl alcohol | 100-51-6 | 1% | – | – | 1% pet | 10% sof | – |
| Benzyl benzoate | 120-51-4 | – | – | 1% pet | 10% pet | – | |
| Phenoxyethanol | 122-99-6 | 1% | 1% pet | – | 1% pet | 1% pet | Yes |
| Citric acid | 77-92-9 | – | – | – | – | – | |
| Sodium benzoate | 1-23-235 | 2.5% in rinse off products except oral products 1.7% in oral products 0.5% in leave-on products | – | – | 5% pet | 5% pet | – |
| Methylparaben | 99-76-3 | 0.4% (acid) for a single ester 0.8% (acid) for ester mixtures | 16% pet (mix) | 16% pet (mix) | 16% pet (mix) | 3% pet, 16% pet (mix) | Yes (mix) |
| Methylisothiazolinone | 2682-20-4 | 0.01% | 2000ppm aq (0.2% aq) | 2000ppm aq (0.2% aq) | 500ppm aq (0.05% aq), 2000ppm aq (0.2% aq) | 200ppm aq (0.02% aq), 2000ppm aq (0.2% aq) | – |
| Propylparaben | 94-13-3 | 0.4% (acid) for a single ester 0.8% (acid) for ester mixtures | 16% pet (mix) | 16% pet (mix) | 16% pet (mix) | 3% pet, 16% pet (mix) | Yes (mix) |
| Potassium sorbate | 24634-61-5 | 0.6% (acid) | – | – | – | – | – |
| Methylchloroisothiazolinone/methylisothiazolinone | 26172-55-4, 2682-20-4, 55965-84-9 | 0.0015% of a 3:1 mix of 5-chloro-methyl-3,4-isothiazolinone and 2-methyl-3,4-isothiazolinone | 200ppm aq (0.02% aq) | 200ppm aq (0.02% aq) | 100ppm aq (0.01% aq) | 200ppm aq (0.02% aq), 100ppm aq (0.01% aq), 100ppm pet (0.01% pet) | Yes |
| Benzoic acid | 0-58-56 | 2.5% in rinse-off products except oral products 1.7% oral products 0.5% leave-on products | - | – | 1% ethanol, 5% pet | 5% pet | – |
| Ethylparaben | 120-47-8 | 0.4% (acid) for a single ester 0.8% (acid) for ester mixtures | 16% pet (mix) | 16% pet (mix) | 16% pet (mix) | 3% pet, 16% pet (mix) | Yes (mix) |
| Benzisothiazolinone | 2634-33-5 | – | – | – | 0.1% pet | 0.05% pet | – |
| 2-bromo-2-nitropropane-1,3-diol (bronopol) | 52-51-7 | 0.1% | – | – | 0.5% pet | 0.25% pet, 0.5% pet | Yes |
| Sodium citrate | 68-04-2 | – | – | – | – | – | |
| Chlorhexidine | 55-56-1, 56-95-1, 18472-51-0, 3697-42-5 | 0.3% | – | – | 0.5 aq (digluconate) | 0.5 aq (digluconate) | – |
| Benzalkonium chloride | 63449-41-2 | 0.1% | – | – | 0.1% pet | 0.1% aq | – |
| Imidazolidinyl urea | 39236-46-9 | 0.6% | 2% pet | – | 2% pet | 2% pet, 2% aq | Yes |
| Butylparaben | 94-26-8 | 0.4% (acid) for a single ester 0.8% (acid) ester mixtures | 16% pet (mix) | 16% pet (mix) | 16% pet (mix) | 3% pet, 16% pet (mix) | Yes (mix) |
| Diazolidinyl urea | 78491-02-8 | 0.5% | 2% pet | – | 2% pet | 1% pet, 2% pet, 2% aq | Yes |
| Sorbic acid | 110-44-1 | 0.6% (acid) | – | – | 2% pet, 2% ethanol | 2% pet | – |
| Triclosan | 3380-34-5 | 0.3% | – | – | 2% pet | 2% pet | – |
| Sodium metabisulfite | 007681-57-4 | 0.2% of free SO2 | – | – | – | 1% pet | – |
| Povidone-iodine | 25655-41-8 | Iodine: not authorized in cosmetic products | – | – | 10% aq | – | – |
| Sodium bisulfite | 7631-90- 5 | 0.2% of free SO2 | – | – | – | – | – |
| DMDM hydantoin | 6440-58-0 | 0.6% | – | – | 2% aq | 1% pet, 2% aq | - |
| Polyaminopropyl biguanide | 70170-61-5/ 28757-47-3, 133029-32-0 | 0.3% | – | – | – | – | – |
| Isobutylparaben | 4247-02-3 | Banned since 2014 | – | – | – | – | – |
| Iodopropynyl butylcarbamate | 55406-53-6 | 0.02% (rinse-off) 0.01% (leave-on) 0.0075% (deodorants y antiperspirants) Do not use in oral or lip products. Do not use in children aged<3 years (except for bath products and shampoo). Do not use in lotions or creams to be applied over large body surfaces | - | – | 0.2% pet | 0.2% pet | – |
| Benzophenone-3 | 131-57-7 | 10% | – | – | 10% pet | 10% pet | – |
| Chlorocresol | 59-50-7 | 0.2% Do not apply to mucosa | - | – | 1% pet | 1% pet | – |
| Zinc pyrithione | 13463-41-7 | 1% of hair products, 0.5% for other products Only rinse-off products Do not use in oral products | - | – | – | 1% pet | – |
| Octylisothiazolinone | 26530-20-1 | – | – | – | 0.025% pet | 0.1% pet | – |
| Benzophenone-4 | 4065-45-6/ 6628-37-1 | 5% (acid) | – | – | 10% pet | 2% pet, 10% pet | – |
| Quaternium-15 | 4080-31-3 | 0.2% | 1% pet | 1% pet | 1% pet | 1% pet, 2% pet | Yes |
| Benzethonium chloride | 121-54-0 | 0.1% rinse-off products Leave-on products except oral products | - | – | – | – | – |
Abbreviations: CAS, Chemical Abstracts Service; GEIDAC, Grupo Español de Investigación en Dermatitis de Contacto y Alergia Cutánea (Spanish Contact Dermatitis Research Group); pet, petrolatum; sof, Softisan.
To compare the distribution of preservatives between the different product categories; to compare the percentage of leave-on products (ie, those that do not have to be rinsed off) for each preservative in the different product categories; to determine the percentage of dermocosmetic brands (ie, skin care and cosmetic brands sold exclusively in pharmacies) in which each preservative is used; to detect frequent combinations of preservatives; to detect sources of exposure to uncommon preservatives; to draw up lists of “prohibited” products in order to design avoidance protocols for allergic patients; and to review the literature and compare our results with those of other, similar studies.
Materials and MethodsBetween January and June 2015, we analyzed the ingredients of 2300 products belonging to various categories, as follows:
- 1
Dermocosmetic products.
- 2
Personal hygiene products and cosmetics sold in supermarkets.
- 3
Personal hygiene products and cosmetics sold in herbal shops.
- 4
Topical medications.
- 5
Household cleaning products.
We evaluated 1093 dermocosmetics sold in Spain by the following leading companies: Isdin (169); Avene (133); La Roche Posay (125); Eucerin (94); IFC (76); Cumlaude Dermopharm (71); Bioderma (69); Babé (62); Ducray (49); Uriage (49); Martiderm (41); Viñas (34); Roc (28); Bayer Hispania (20); Mustela (17); Vichy (15); Boderm Olyam Farma (11); Dermilid Farma (11); Bama Geve (7); Menarini (7); and Lutsine (5). The sources used were as follows:
- 1.
Vademecum de Dermocosmética, 2015 (Spanish handbook on dermocosmetics; 612 products, 12 brands).
- 2.
Materials published by pharmaceutical companies (287 products, 4 brands).
- 3.
Data that were not available online provided directly by the companies (187 products, 4 brands).
- 4.
Visit to a pharmacy to read product labels (5 products, 1 brand).
We evaluated personal hygiene and cosmetic products sold in supermarkets (249 products) and herbal shops (113), topical medications (636), and household cleaning products (209) by means of the following:
- 1.
Visits to 5 supermarkets in the province of Madrid, Spain (Alcampo, Aldi, Eroski, Lidl, and Mercadona) to read the labels of 249 cosmetic products and 209 cleaning products. We first selected all available products in a small supermarket of the Eroski chain in Madrid and recorded all the products of various categories belonging to the brands available in this specific center. We then selected products from other supermarkets (we included only house brand products for the sake of convenience).
- 2.
Visits to the web page of the Spanish Agency of Medicinal Products and Medical Devices, the Spanish handbook on medications, and online materials. Analysis of the summaries of product characteristics of 636 topical medications.
- 3.
Visit to a herbal shop in Guadalajara, Spain to read product labels (113 products). We recorded data for all the cosmetic products sold in the shop.
- 4.
Review of publications on the distribution of preservatives in cosmetic products, household cleaning products, and topical medications.
A priori, we selected the preservatives mentioned in the chapter on allergy to preservatives and vehicles in cosmetics and toiletries in Fisher's Contact Dermatitis.11 Data were collected using Microsoft Excel 2007. The statistical analysis was performed using Microsoft Excel 2007.
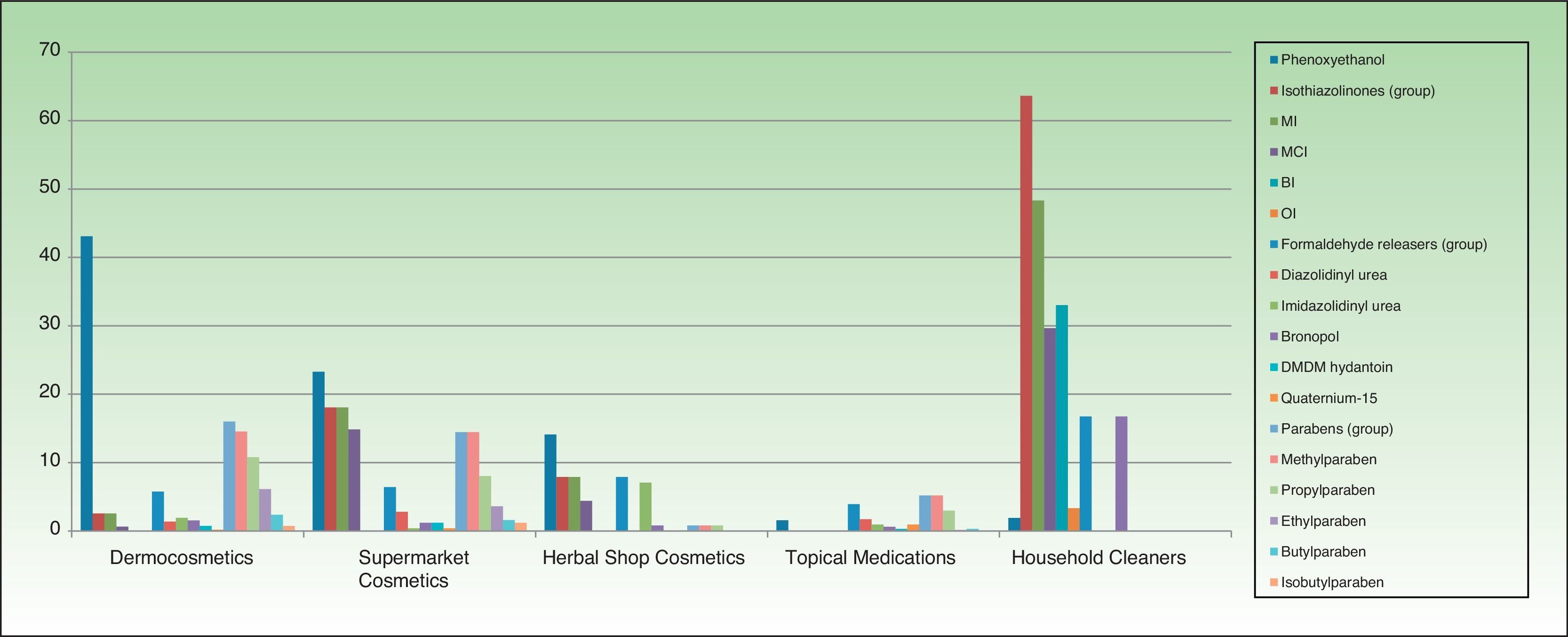
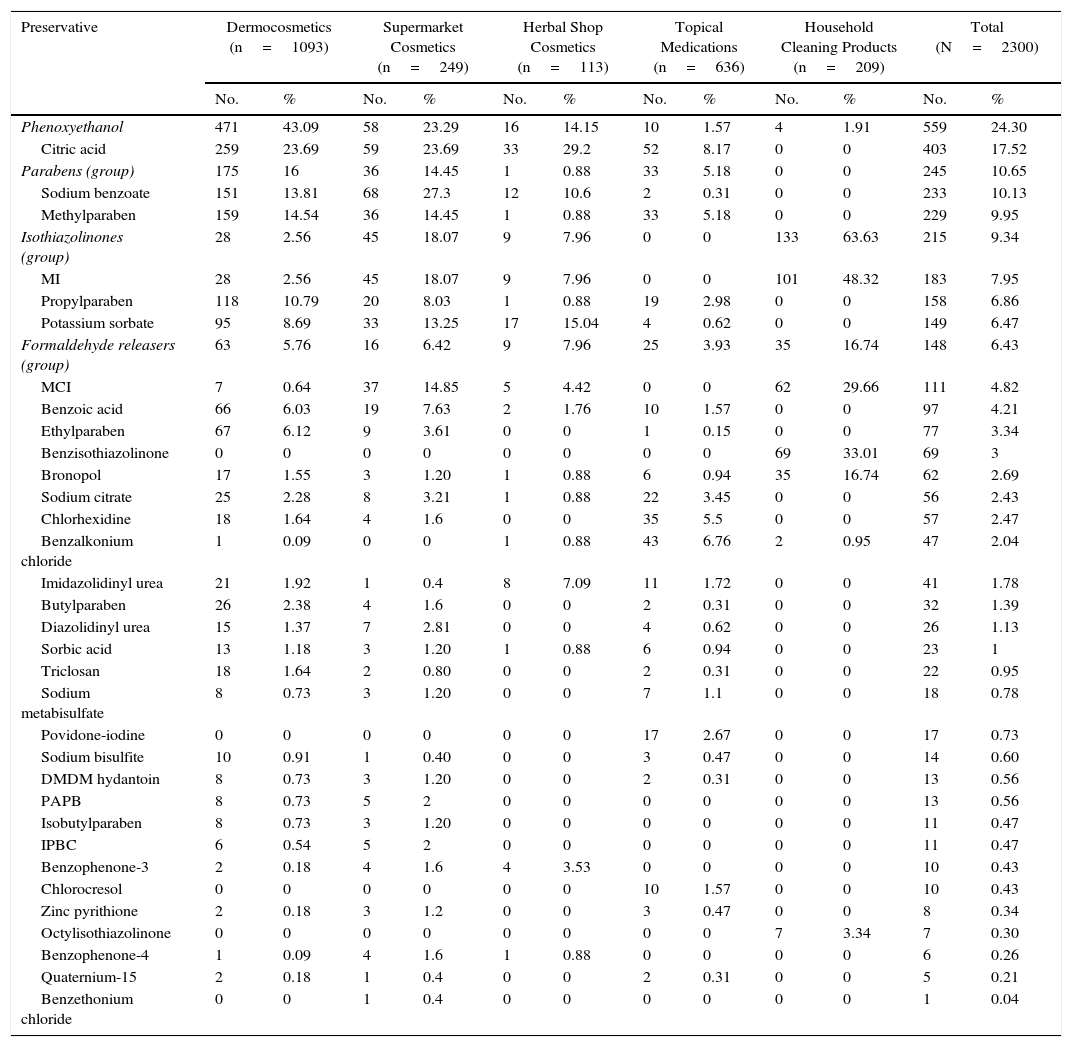
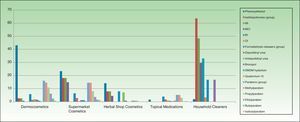
ResultsThe distribution of the preservatives in the different product categories (in both percentage and absolute terms) is shown in Table 2 and Figure 1. The order of frequency in which the different preservatives are used in each of the sectors analyzed is shown in Supplementary Material.
Distribution of Preservatives by Product Category.
| Preservative | Dermocosmetics (n=1093) | Supermarket Cosmetics (n=249) | Herbal Shop Cosmetics (n=113) | Topical Medications (n=636) | Household Cleaning Products (n=209) | Total (N=2300) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Phenoxyethanol | 471 | 43.09 | 58 | 23.29 | 16 | 14.15 | 10 | 1.57 | 4 | 1.91 | 559 | 24.30 |
| Citric acid | 259 | 23.69 | 59 | 23.69 | 33 | 29.2 | 52 | 8.17 | 0 | 0 | 403 | 17.52 |
| Parabens (group) | 175 | 16 | 36 | 14.45 | 1 | 0.88 | 33 | 5.18 | 0 | 0 | 245 | 10.65 |
| Sodium benzoate | 151 | 13.81 | 68 | 27.3 | 12 | 10.6 | 2 | 0.31 | 0 | 0 | 233 | 10.13 |
| Methylparaben | 159 | 14.54 | 36 | 14.45 | 1 | 0.88 | 33 | 5.18 | 0 | 0 | 229 | 9.95 |
| Isothiazolinones (group) | 28 | 2.56 | 45 | 18.07 | 9 | 7.96 | 0 | 0 | 133 | 63.63 | 215 | 9.34 |
| MI | 28 | 2.56 | 45 | 18.07 | 9 | 7.96 | 0 | 0 | 101 | 48.32 | 183 | 7.95 |
| Propylparaben | 118 | 10.79 | 20 | 8.03 | 1 | 0.88 | 19 | 2.98 | 0 | 0 | 158 | 6.86 |
| Potassium sorbate | 95 | 8.69 | 33 | 13.25 | 17 | 15.04 | 4 | 0.62 | 0 | 0 | 149 | 6.47 |
| Formaldehyde releasers (group) | 63 | 5.76 | 16 | 6.42 | 9 | 7.96 | 25 | 3.93 | 35 | 16.74 | 148 | 6.43 |
| MCI | 7 | 0.64 | 37 | 14.85 | 5 | 4.42 | 0 | 0 | 62 | 29.66 | 111 | 4.82 |
| Benzoic acid | 66 | 6.03 | 19 | 7.63 | 2 | 1.76 | 10 | 1.57 | 0 | 0 | 97 | 4.21 |
| Ethylparaben | 67 | 6.12 | 9 | 3.61 | 0 | 0 | 1 | 0.15 | 0 | 0 | 77 | 3.34 |
| Benzisothiazolinone | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 69 | 33.01 | 69 | 3 |
| Bronopol | 17 | 1.55 | 3 | 1.20 | 1 | 0.88 | 6 | 0.94 | 35 | 16.74 | 62 | 2.69 |
| Sodium citrate | 25 | 2.28 | 8 | 3.21 | 1 | 0.88 | 22 | 3.45 | 0 | 0 | 56 | 2.43 |
| Chlorhexidine | 18 | 1.64 | 4 | 1.6 | 0 | 0 | 35 | 5.5 | 0 | 0 | 57 | 2.47 |
| Benzalkonium chloride | 1 | 0.09 | 0 | 0 | 1 | 0.88 | 43 | 6.76 | 2 | 0.95 | 47 | 2.04 |
| Imidazolidinyl urea | 21 | 1.92 | 1 | 0.4 | 8 | 7.09 | 11 | 1.72 | 0 | 0 | 41 | 1.78 |
| Butylparaben | 26 | 2.38 | 4 | 1.6 | 0 | 0 | 2 | 0.31 | 0 | 0 | 32 | 1.39 |
| Diazolidinyl urea | 15 | 1.37 | 7 | 2.81 | 0 | 0 | 4 | 0.62 | 0 | 0 | 26 | 1.13 |
| Sorbic acid | 13 | 1.18 | 3 | 1.20 | 1 | 0.88 | 6 | 0.94 | 0 | 0 | 23 | 1 |
| Triclosan | 18 | 1.64 | 2 | 0.80 | 0 | 0 | 2 | 0.31 | 0 | 0 | 22 | 0.95 |
| Sodium metabisulfate | 8 | 0.73 | 3 | 1.20 | 0 | 0 | 7 | 1.1 | 0 | 0 | 18 | 0.78 |
| Povidone-iodine | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 2.67 | 0 | 0 | 17 | 0.73 |
| Sodium bisulfite | 10 | 0.91 | 1 | 0.40 | 0 | 0 | 3 | 0.47 | 0 | 0 | 14 | 0.60 |
| DMDM hydantoin | 8 | 0.73 | 3 | 1.20 | 0 | 0 | 2 | 0.31 | 0 | 0 | 13 | 0.56 |
| PAPB | 8 | 0.73 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0.56 |
| Isobutylparaben | 8 | 0.73 | 3 | 1.20 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0.47 |
| IPBC | 6 | 0.54 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0.47 |
| Benzophenone-3 | 2 | 0.18 | 4 | 1.6 | 4 | 3.53 | 0 | 0 | 0 | 0 | 10 | 0.43 |
| Chlorocresol | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 1.57 | 0 | 0 | 10 | 0.43 |
| Zinc pyrithione | 2 | 0.18 | 3 | 1.2 | 0 | 0 | 3 | 0.47 | 0 | 0 | 8 | 0.34 |
| Octylisothiazolinone | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 3.34 | 7 | 0.30 |
| Benzophenone-4 | 1 | 0.09 | 4 | 1.6 | 1 | 0.88 | 0 | 0 | 0 | 0 | 6 | 0.26 |
| Quaternium-15 | 2 | 0.18 | 1 | 0.4 | 0 | 0 | 2 | 0.31 | 0 | 0 | 5 | 0.21 |
| Benzethonium chloride | 0 | 0 | 1 | 0.4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.04 |
Abbreviations: bronopol, bromo nitropropane diol; DMDM, dimethyl dimethylol hydantoin; IPBC, iodopropynyl butylcarbamate; MI, methylisothiazolinone; MCI, methylchloroisothiazolinone; PAPB, polyaminopropyl biguanide.
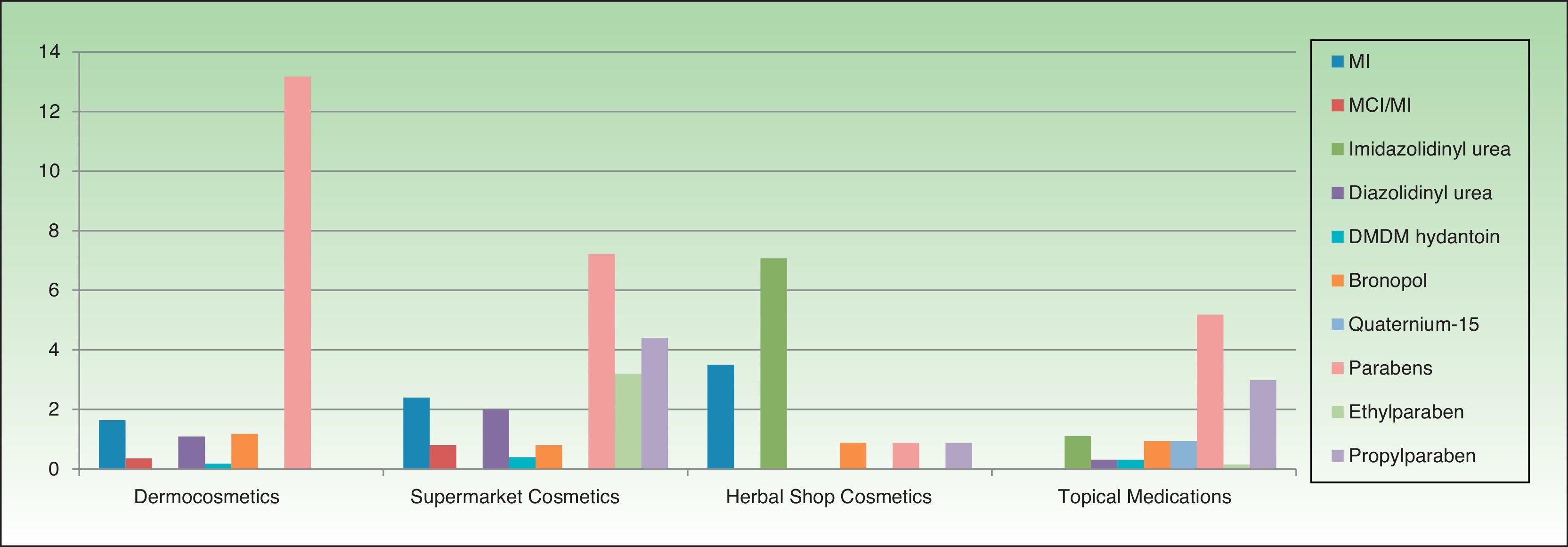
The most frequently used preservatives in dermocosmetics were phenoxyethanol (43.09%), citric acid (23.69%), methylparaben (14.54%), sodium benzoate (13.81%), and propylparaben (10.79%). Formaldehyde releasers were found in 6% of products, the most frequent being imidazolidinyl urea. MI and MCI were present, respectively, in 2.56% and 0.64%.
The most frequent preservatives in supermarket cosmetics were sodium benzoate (27.30%), citric acid (23.69%), phenoxyethanol (23.29%), MI (18.07%), MCI (14.85%), methylparaben (14.45%), potassium sorbate (13.25%), and benzyl alcohol (12.44%). More than 6% contained a formaldehyde releaser, the most frequent being diazolidinyl urea (2.81%) (Table 2).
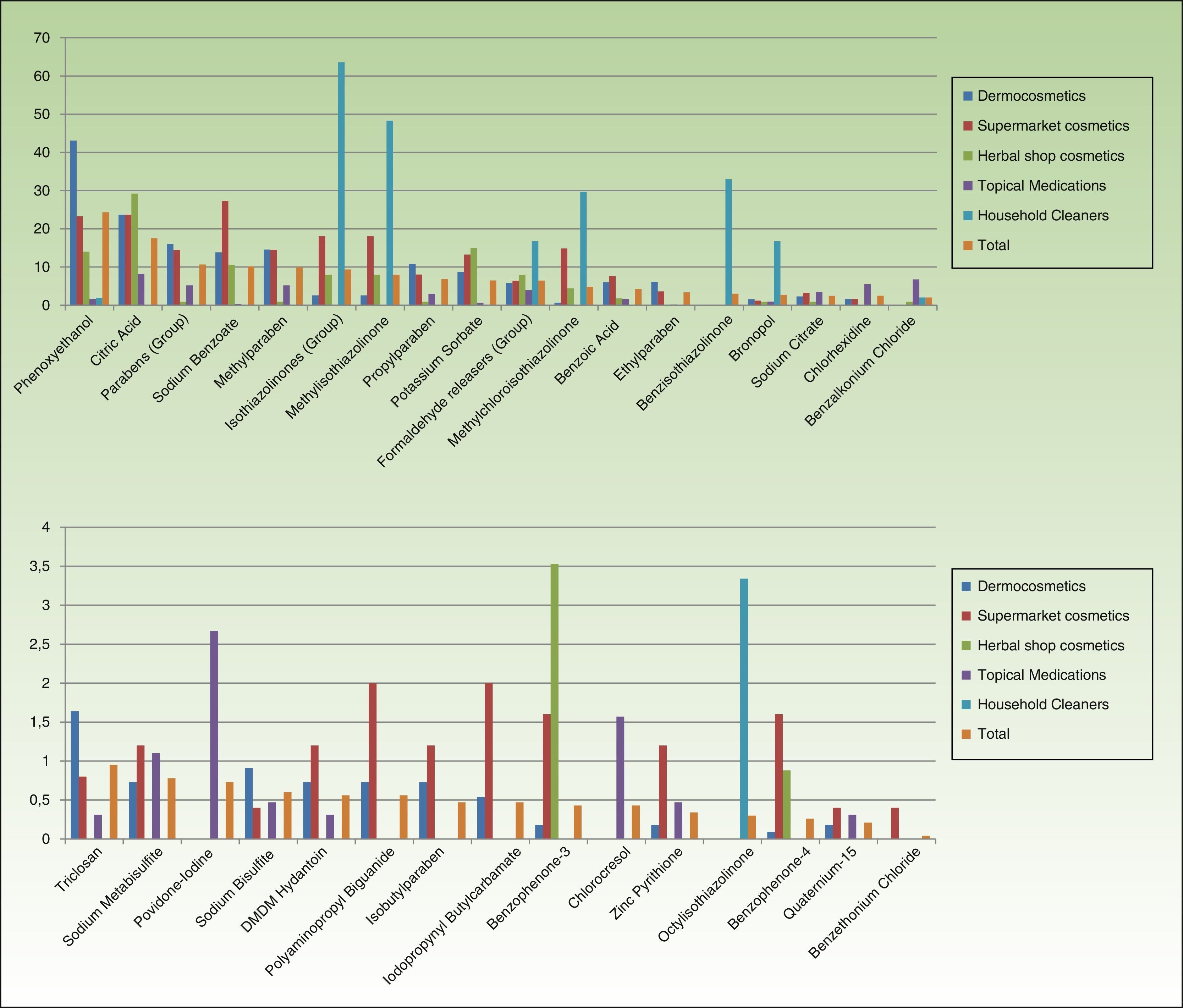
In cosmetics from herbal shops, the most frequent preservatives were citric acid (29.2%), potassium sorbate (15%), phenoxyethanol (14.1%), and sodium benzoate (10.6%). MI was detected in 8% and imidazolidinyl urea in 7% (these were all leave-on products of several brands, including an intimate lubricant). No product contained diazolidinyl urea, DMDM hydantoin, or quaternium-15. Parabens were only detected in 1 herbal product (0.8%), namely, a whitening facial moisturizer with methylparaben, propylparaben, and imidazolidinyl urea. Others such as MCI and benzophenones, which are unusual in dermocosmetics (<1%), and in particular benzophenones, which are uncommon in supermarket cosmetics (<2%), were detected in 4.4% of herbal products. Five leave-on products from 2 brands contained benzophenones: a hairspray containing benzophenone-4 and 4 creams containing benzophenone-3 (Table 2 and Fig. 2).
No medications contained isothiazolinones. However, parabens and formaldehyde releasers were detected in 5.18% and 3.93%, respectively; methylparaben, propylparaben, and imidazolidinyl urea in particular were detected in 5.18%, 2.98%, and 1.72% in this group. Some preservatives were almost absent from the other categories. Thus, 6.7% contained benzalkonium chloride (mainly ophthalmic medication and, to a lesser extent, oropharyngeal and otologic drugs). This preservative was detected in only 4 products from other sectors: a dermocosmetic cream for sensitive skin, an intimate hygiene foam from a herbal shop, and 2 cleaners. Chlorocresol, which was absent from other categories, was detected in 1.57% of medications, all of which were corticosteroids. Chlorhexidine and povidone-iodine were detected, respectively, in 5.5% and 2.67% of medications. Sodium metabisulfite was detected in 7 medications (1.1%), all of which were depigmenting agents, and in 3 hair dyes sold in supermarkets.
Almost two-thirds of household cleaning products contained isothiazolinones (133 products): MI in almost half and MCI in almost one-third. These products also contained other isothiazolinones, such as benzisothiazolinone (>30%), octylisothiazolinone (3.3%), and bronopol (16.74%) (Table 2 and Fig. 2).
Fabric conditioners accounted for 36.23% of household products containing benzisothiazolinone, and 5 of the 6 brands evaluated contained it. Octylisothiazolinone was detected in 7 household products (3.34%, 4 brands), of which 5 were for clothes care: 2 detergents and 2conditioners (of the same brand) and an odor remover (other brand). Octylisothiazolone was also found in a cleaner for induction cooktops and a bath cleaner (Fig. 1).
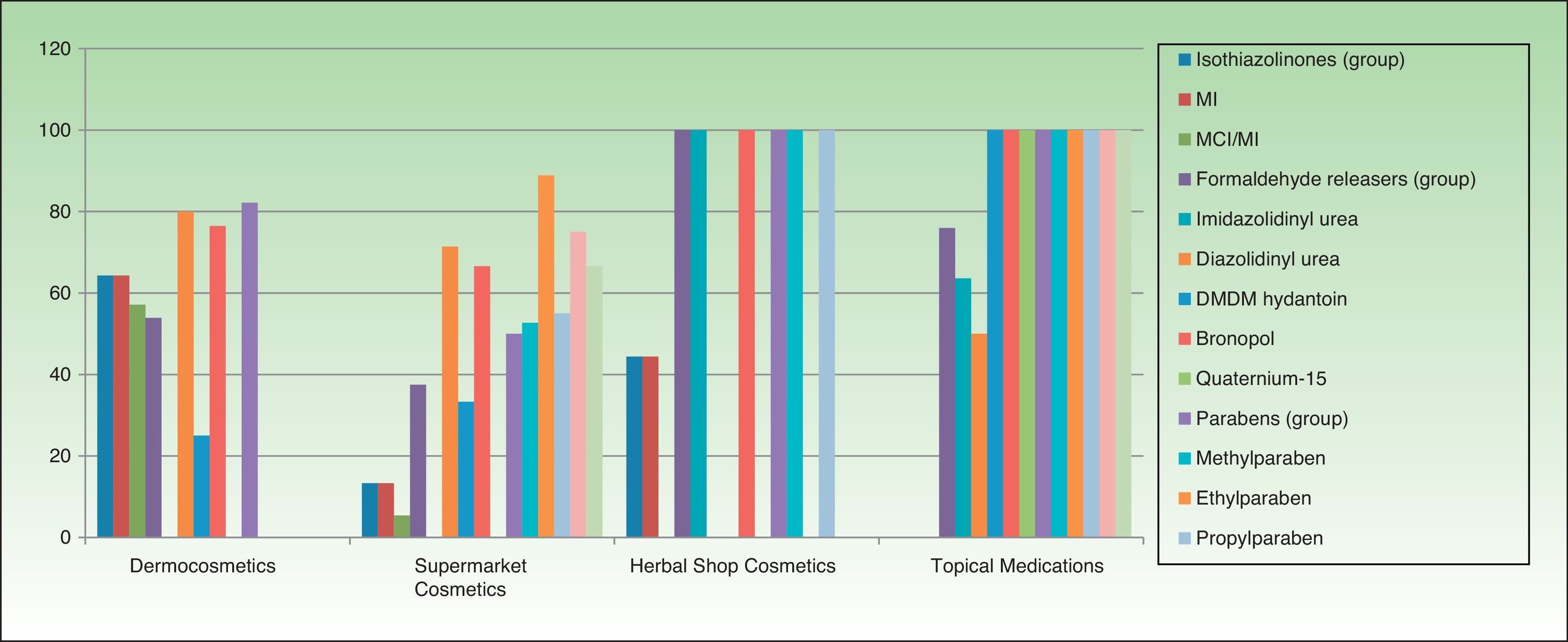
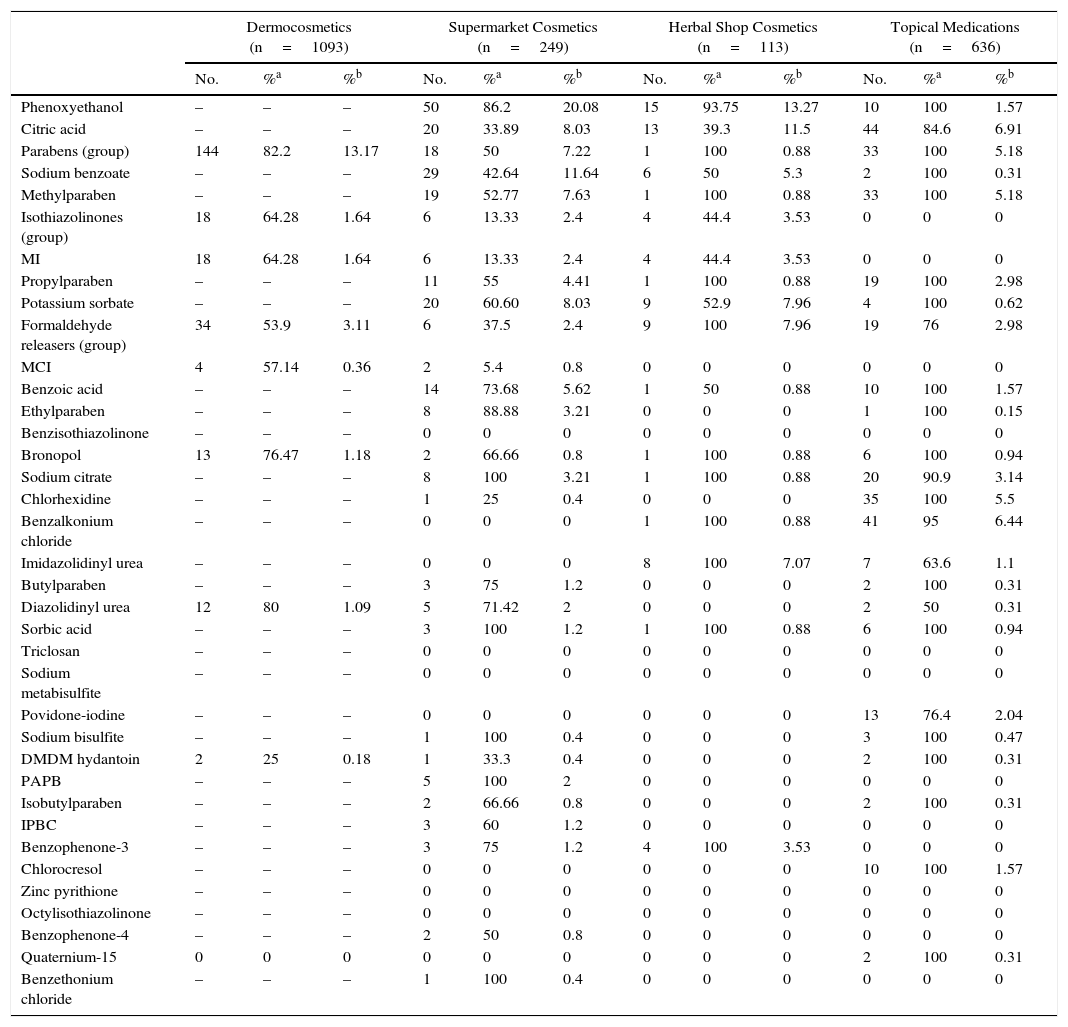
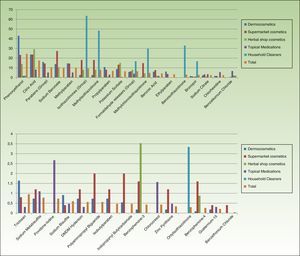
Table 3 and Figures 3 and 4 show the distribution of leave-on products (ie, those that do not have to be rinsed off) with each of the preservatives analyzed in each sector.
Percentage of Leave-on Products for Each Preservative by Product Category.
| Dermocosmetics (n=1093) | Supermarket Cosmetics (n=249) | Herbal Shop Cosmetics (n=113) | Topical Medications (n=636) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | %a | %b | No. | %a | %b | No. | %a | %b | No. | %a | %b | |
| Phenoxyethanol | – | – | – | 50 | 86.2 | 20.08 | 15 | 93.75 | 13.27 | 10 | 100 | 1.57 |
| Citric acid | – | – | – | 20 | 33.89 | 8.03 | 13 | 39.3 | 11.5 | 44 | 84.6 | 6.91 |
| Parabens (group) | 144 | 82.2 | 13.17 | 18 | 50 | 7.22 | 1 | 100 | 0.88 | 33 | 100 | 5.18 |
| Sodium benzoate | – | – | – | 29 | 42.64 | 11.64 | 6 | 50 | 5.3 | 2 | 100 | 0.31 |
| Methylparaben | – | – | – | 19 | 52.77 | 7.63 | 1 | 100 | 0.88 | 33 | 100 | 5.18 |
| Isothiazolinones (group) | 18 | 64.28 | 1.64 | 6 | 13.33 | 2.4 | 4 | 44.4 | 3.53 | 0 | 0 | 0 |
| MI | 18 | 64.28 | 1.64 | 6 | 13.33 | 2.4 | 4 | 44.4 | 3.53 | 0 | 0 | 0 |
| Propylparaben | – | – | – | 11 | 55 | 4.41 | 1 | 100 | 0.88 | 19 | 100 | 2.98 |
| Potassium sorbate | – | – | – | 20 | 60.60 | 8.03 | 9 | 52.9 | 7.96 | 4 | 100 | 0.62 |
| Formaldehyde releasers (group) | 34 | 53.9 | 3.11 | 6 | 37.5 | 2.4 | 9 | 100 | 7.96 | 19 | 76 | 2.98 |
| MCI | 4 | 57.14 | 0.36 | 2 | 5.4 | 0.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Benzoic acid | – | – | – | 14 | 73.68 | 5.62 | 1 | 50 | 0.88 | 10 | 100 | 1.57 |
| Ethylparaben | – | – | – | 8 | 88.88 | 3.21 | 0 | 0 | 0 | 1 | 100 | 0.15 |
| Benzisothiazolinone | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bronopol | 13 | 76.47 | 1.18 | 2 | 66.66 | 0.8 | 1 | 100 | 0.88 | 6 | 100 | 0.94 |
| Sodium citrate | – | – | – | 8 | 100 | 3.21 | 1 | 100 | 0.88 | 20 | 90.9 | 3.14 |
| Chlorhexidine | – | – | – | 1 | 25 | 0.4 | 0 | 0 | 0 | 35 | 100 | 5.5 |
| Benzalkonium chloride | – | – | – | 0 | 0 | 0 | 1 | 100 | 0.88 | 41 | 95 | 6.44 |
| Imidazolidinyl urea | – | – | – | 0 | 0 | 0 | 8 | 100 | 7.07 | 7 | 63.6 | 1.1 |
| Butylparaben | – | – | – | 3 | 75 | 1.2 | 0 | 0 | 0 | 2 | 100 | 0.31 |
| Diazolidinyl urea | 12 | 80 | 1.09 | 5 | 71.42 | 2 | 0 | 0 | 0 | 2 | 50 | 0.31 |
| Sorbic acid | – | – | – | 3 | 100 | 1.2 | 1 | 100 | 0.88 | 6 | 100 | 0.94 |
| Triclosan | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sodium metabisulfite | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Povidone-iodine | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 76.4 | 2.04 |
| Sodium bisulfite | – | – | – | 1 | 100 | 0.4 | 0 | 0 | 0 | 3 | 100 | 0.47 |
| DMDM hydantoin | 2 | 25 | 0.18 | 1 | 33.3 | 0.4 | 0 | 0 | 0 | 2 | 100 | 0.31 |
| PAPB | – | – | – | 5 | 100 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Isobutylparaben | – | – | – | 2 | 66.66 | 0.8 | 0 | 0 | 0 | 2 | 100 | 0.31 |
| IPBC | – | – | – | 3 | 60 | 1.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Benzophenone-3 | – | – | – | 3 | 75 | 1.2 | 4 | 100 | 3.53 | 0 | 0 | 0 |
| Chlorocresol | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 100 | 1.57 |
| Zinc pyrithione | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Octylisothiazolinone | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Benzophenone-4 | – | – | – | 2 | 50 | 0.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Quaternium-15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 100 | 0.31 |
| Benzethonium chloride | – | – | – | 1 | 100 | 0.4 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: bronopol, 2-bromo-2-nitro-1,3-propanediol; DMDM, dimethyldimethylol hydantoin; IPBC, iodopropynyl butylcarbamate; MI, methylisothiazolinone; MCI, methylchloroisothiazolinone; PAPB, polyaminopropyl biguanide.
Percentage of leave-on products containing each preservative by topical product category.
The percentages refer to the proportion of leave-on products containing a specific preservative with respect to the total number of products in this sector (no. of leave-on products with a preservative X/total no. of products with a preservative X [expressed as a percentage]). DMDM indicates dimethyl dimethylol; MCI, methylchloroisothiazolinone; MI, methylisothiazolinone.
Percentage of leave-on products containing each type of preservative with respect to the total number of topical products in each category.
The percentages refer to the proportion of leave-on products containing a specific preservative with respect to the total number of products in this sector (no. of leave-on products with a preservative X/total no. of products with preservative X [expressed as a percentage]). DMDM indicates dimethyl dimethylol; MCI, methylchloroisothiazolinone; MI, methylisothiazolinone.
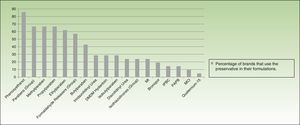
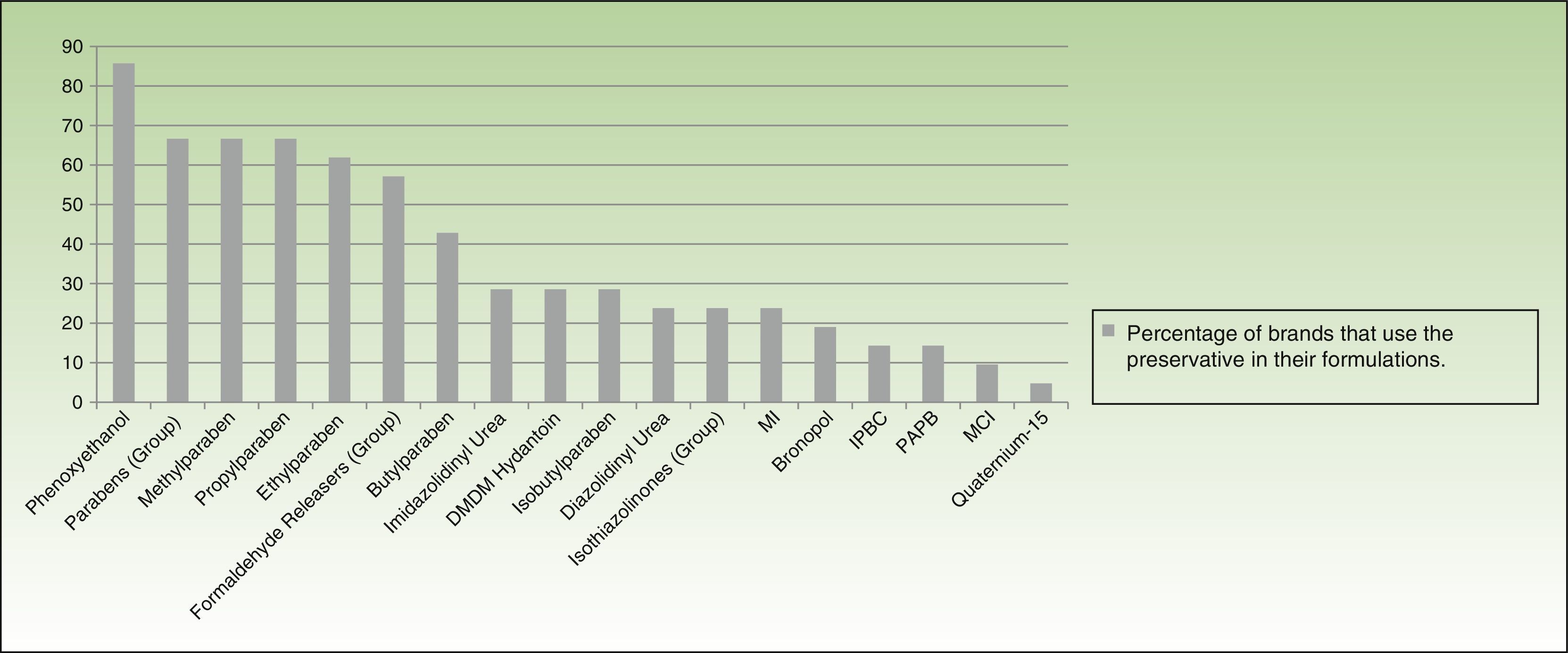
More than half of the companies producing dermocosmetics use phenoxyethanol (85.7% of brands), parabens (66.6%), or a formaldehyde releaser (57%) in their formulations. MI is used by 23.8% and MCI by 9.52%. When examined individually, formaldehyde releasers are used by less than one-third of brands, with imidazolidinyl urea and DMDM hydantoin being the most common (almost one-third) and bronopol and quaternium-15 the least common (19.04% and 4.76%, respectively) (Fig. 5).
Combinations of parabens were common in dermocosmetics (see Supplementary Material).
Phenoxyethanol was more commonly detected in all cosmetic sectors than the other main preservatives. Among dermocosmetics products, phenoxyethanol was found with 1 or more parabens in 27.81%, with MI in 3.6%, and with bronopol in 1.47% of products. Phenoxyethanol was very rarely found with other preservatives.
Combinations of the main preservatives were unusual, with the exception of the combination of phenoxyethanol+paraben(s) (10% of dermocosmetics and almost 3% of cosmetics sold in supermarkets) and bronopol+isothiazolinones (>10% of cleaning products). No combinations of formaldehyde releasers were observed, with the exception of a vaginal lubricant containing imidazolidinyl urea and bronopol sold in herbal shops.
As for combinations of isothiazolinones in cleaning products, the most frequent were MI+MCI (20%) and MI+benzisothiazolinone (>15%). No combinations of octylisothiazolinones with MI or benzisothiazolinone were detected. Similarly, no products contained all 4 isothiazolinones simultaneously.
We detected sources of exposure to less common preservatives, such as polyaminopropyl biguanide and iodopropynyl butylcarbamate.
Polyaminopropyl biguanide was detected in 8 products (4 dermocosmetics [2 brands], an eye make-up remover, 2 micellar solutions, and a moisturizer) and in 4 supermarket wet wipes (half of which were for children).
Iodopropynyl butylcarbamate was detected in 6 dermocosmetics (3 brands): 2 moisturizers, 1 deodorant, and 3 shampoos. It was also found in 4 supermarket cosmetic products: 2 emollients, a diaper cream, and baby soap. Neither of the 2 preservatives was found in herbal products, medications, or cleaning products.
Quaternium-15 was only detected in 5 products: 2 dermocosmetic shampoos (1 brand), 1 supermarket infant bath gel, and 2 medications (wart patches, 1 brand). Both medications were suspended on July 2, 2015, after our data were collected.
DiscussionVarious published studies from other countries have evaluated the market distribution of preservatives by analyzing ingredients12–18 (see Supplementary Material).
The major contribution of our study is its sample size (which is only exceeded by those analyzed in studies based on official registries as their data source), the variability of the categories (other studies only evaluate cosmetic and/or cleaning products), and the wide variety of preservatives analyzed. We did not find previous publications on the distribution of preservatives in topical medications.
The disparate results from these studies could reflect regional differences or changes in formulations over time. However, given the heterogeneity of the methodologies used, we believe that the results cannot be compared directly. For example, it is noteworthy that the distribution of parabens and formaldehyde releasers in these series is broader in some cases than that observed in our study. However, in articles evaluating household products, the presence of isothiazolinones was lower than that observed in our study.
Nevertheless, the analyses of ingredients have 2 disadvantages: (1) the lack of information on concentrations, which is the main weakness of our study; and (2) the fact that information on the availability of marketed products cannot be extrapolated directly to exposure, since some products and brands are more frequently bought by consumers than others.
Therefore, other types of analysis (chemicals and sales) should be performed to confirm the trends highlighted by the evaluations of the lists of ingredients in available products.
Knowing the market presence of preservatives makes it possible to indirectly estimate their sensitizing capacity by comparing it with the prevalence of sensitization. Thus, for example, sodium benzoate, citric acid, potassium sorbate, phenoxyethanol, and parabens would have a lower sensitizing capacity, given the low rate of sensitization to these allergens despite their widespread use.
The dermocosmetic industry has made an effort to reduce the use of problematic preservatives, such as isothiazolinones, in a small number of brands. However, no equivalent reduction has been observed in the use of formaldehyde releasers, for which percentages are similar to those recorded in other sectors.
Although the percentage of isothiazolinone-containing cosmetics sold in supermarkets is high, most of the products are rinse-off (ie, must be rinsed off the skin). These products remain on the skin for short periods and are removed immediately after application. They are usually water-based and have lower skin penetrability. Therefore, these products do not usually lead to sensitization, although they are capable of triggering contact dermatitis in previously sensitized individuals, especially after repeated application on previously damaged skin. Leave-on products (ie, those that do not have to be rinsed from the skin) carry a greater risk of sensitization and of triggering eczematous reactions in allergic individuals, since they are generally oily products (creams, lotions) with higher skin penetrability that remain in contact with the skin for longer periods.
Surprisingly, although fewer dermocosmetics and herbal products contain isothiazolinones, the percentage of leave on products containing this preservative is high. Given that these products are aimed at specific clients with sensitive or diseased skin, and, given their greater risk of primary sensitization after application, especially on damaged skin, it seems reasonable to restrict their use in these sectors. Many allergic patients do not realize that “pharmacy products,” “products for atopic patients,” “natural products,” and “paraben-free products” can aggravate their problem. In addition, since they make no connection between the product and reactions to it, continued application only serves to perpetuate their lesions.
It is worth noting the presence of benzisothiazolinone and octylisothiazolinone in household products, many of which are used for clothes care; therefore, although there are no precedents, cases of nonoccupational sensitization to these preservatives could arise in relation to this source.
In all categories, the percentage of products with releasers is lower than that of products with isothiazolinones. The reasons offered for excluding quaternium-15 from the European standard series include the scarce market presence of products containing this preservative and the low rates of sensitization. However, given that some cosmetic products—albeit very few—still contain quaternium-15, it does seem reasonable to maintain it, at least in our setting.19
It is interesting to note that cosmetic products from herbal shops are those that best reflect the unjustified paraben phobia that has taken hold in society. In order to respond to the demand for paraben-free products, parabens have been replaced by more problematic products, such as isothiazolinones, which are more frequently used in cosmetic products from herbal shops (8%) than in dermocosmetics (2.56%).
Of particular interest is the presence of isobutylparaben (banned from use in cosmetics since 2014) in 11 cosmetic products (0.47%) (8 dermocosmetic products and 3 supermarket cosmetics).20 Given that data were collected during the first months of 2015, this finding could reflect how despite being banned, a specific substance occasionally remains on the market for some time.
Sources of exposure to unusual but emerging preservatives such as polyaminopropylbiguanide and iodopropynyl butylcarbamate are reported.
It is also important to consider topical medications as sources of exposure to preservatives, especially parabens, formaldehyde releasers (especially imidazolidinyl urea), and phenoxyethanol, as well as other preservatives that are less common in or absent from other settings, such as benzalkonium chloride, chlorocresol, and sulfites.
Lastly, we believe that recording the ingredients of cosmetic products makes it possible to draw up a list of products that sensitized persons should be advised against. Almost half of all patients find it difficult to read the product label because of the small print and the complex chemical names.21 These lists could prove useful for helping patients to avoid problematic products and thus lead to an improvement in their condition (Table 4).
However, the lists are subject to 2 disadvantages: they are short-term in nature (continuous changes in the formulation mean that they must be constantly updated), and priority must be given to providing patients with appropriate training in how to avoid the preservatives.
In conclusion, we believe that until the necessary changes to legislation are made, both doctors and patients should be proactive in the identification and avoidance of potential sensitizing agents.
The evaluation of the presence of the main preservatives in products marketed in our setting provides us with an indirect estimation of the degree of exposure of the population to each of them and, in particular, to those that carry a greater health risk.
Studies such as the present one can serve as a guideline for expert committees and governmental bodies when taking the decision to limit the most problematic and widespread preservatives in our setting.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purposes of this study.
Confidentiality of dataThe authors declare that they have followed their institutional protocols on publication of patient data.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
I am grateful to the following people: María Teresa Losa for opening her herbal shop to me; Luis Conde, Mariela Gatica, Ana Giménez-Arnau, and Juan Francisco Silvestre for their encouragement; Lucía Martín Moreno for her defense of common sense and integrity in the exercise of our profession and for her pragmatic perspective on the study of contact dermatitis; Luis Requena, my constant and greatest inspiration; Begoña Pastor and Jesús Zarallo for their critical review of the manuscript; Kevin and Isabel for their patience and the time I took away from them; and to my parents, without whose support this project would have never seen the light of day.
Please cite this article as: Pastor-Nieto MA, Alcántara-Nicolás F, Melgar-Molero V, Pérez-Mesonero R, Vergara-Sánchez A, Martín-Fuentes A, et al. Conservantes en productos de higiene y cosméticos, medicamentos tópicos y productos de limpieza doméstica en España. Actas Dermosifiliogr. 2017;108:758–770.


![Percentage of leave-on products containing each preservative by topical product category. The percentages refer to the proportion of leave-on products containing a specific preservative with respect to the total number of products in this sector (no. of leave-on products with a preservative X/total no. of products with a preservative X [expressed as a percentage]). DMDM indicates dimethyl dimethylol; MCI, methylchloroisothiazolinone; MI, methylisothiazolinone. Percentage of leave-on products containing each preservative by topical product category. The percentages refer to the proportion of leave-on products containing a specific preservative with respect to the total number of products in this sector (no. of leave-on products with a preservative X/total no. of products with a preservative X [expressed as a percentage]). DMDM indicates dimethyl dimethylol; MCI, methylchloroisothiazolinone; MI, methylisothiazolinone.](https://static.elsevier.es/multimedia/15782190/0000010800000008/v1_201710010015/S1578219017302391/v1_201710010015/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Percentage of leave-on products containing each type of preservative with respect to the total number of topical products in each category. The percentages refer to the proportion of leave-on products containing a specific preservative with respect to the total number of products in this sector (no. of leave-on products with a preservative X/total no. of products with preservative X [expressed as a percentage]). DMDM indicates dimethyl dimethylol; MCI, methylchloroisothiazolinone; MI, methylisothiazolinone. Percentage of leave-on products containing each type of preservative with respect to the total number of topical products in each category. The percentages refer to the proportion of leave-on products containing a specific preservative with respect to the total number of products in this sector (no. of leave-on products with a preservative X/total no. of products with preservative X [expressed as a percentage]). DMDM indicates dimethyl dimethylol; MCI, methylchloroisothiazolinone; MI, methylisothiazolinone.](https://static.elsevier.es/multimedia/15782190/0000010800000008/v1_201710010015/S1578219017302391/v1_201710010015/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)