We describe a woman in her twenty-ninth week of pregnancy with a subpolar lepromatous form of multibacillary leprosy with hyperactive lesions. The clinical presentation was atypical, which interfered with and delayed the diagnosis.
This 36-year-old Paraguayan woman who had been resident in Spain for the previous 10 years, was seen for a 3-month history of widespread pruritic papules. She had been diagnosed with pruritic urticarial papules and plaques of pregnancy and had received treatment, with no clinical or symptomatic improvement.
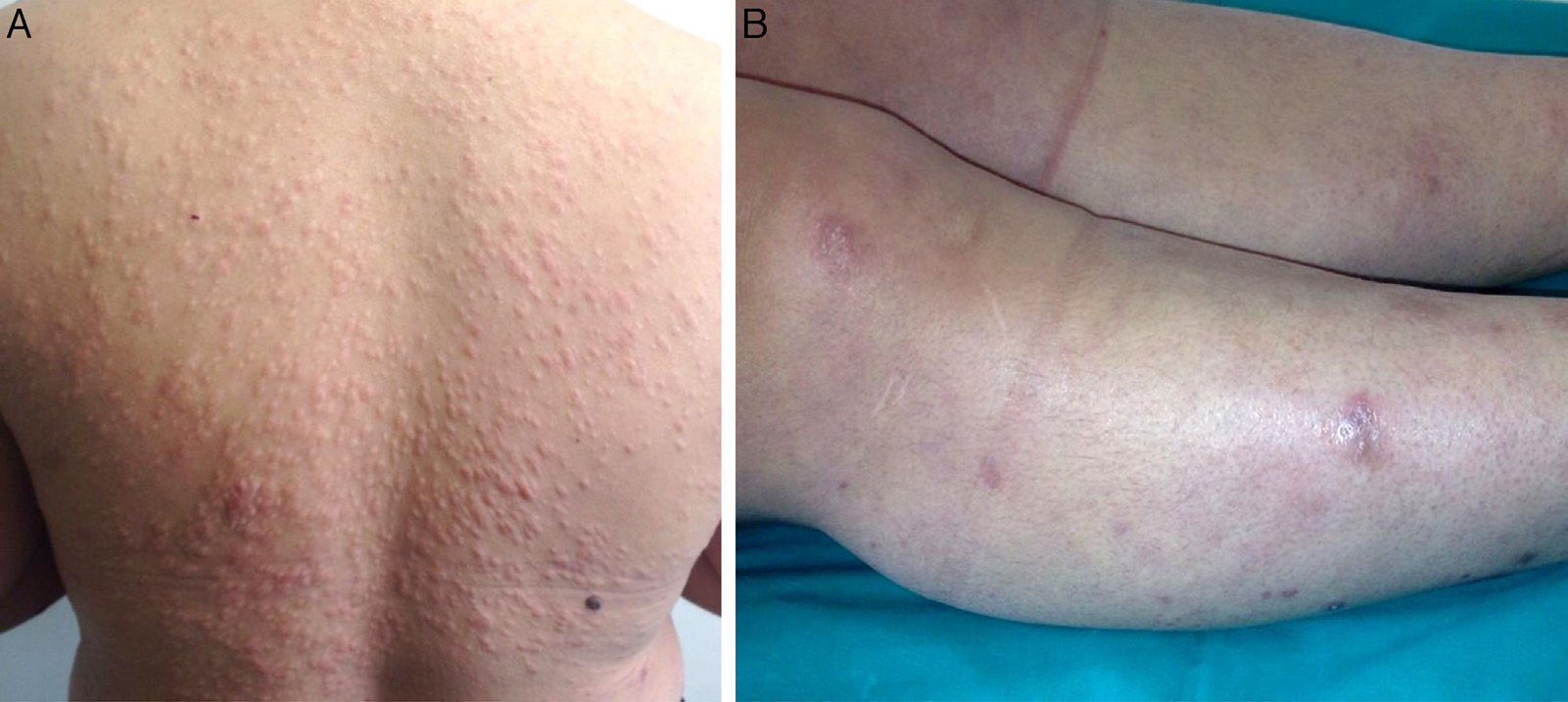
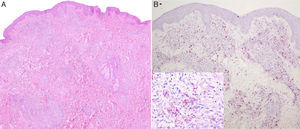
The papules, which measured approximately 5mm, were firm, infiltrated, and of a slightly brownish color. They had first appeared on her back and had then spread to her face, arms, trunk, and lower limbs, where they were larger and more nodular (Fig. 1, A and B). The lesions were not painful and the only symptom was pruritus. The patient did not report general malaise or fever. The rest of the physical examination was normal.
A, Firm infiltrated papules measuring about 5mm in diameter on the patient's back. The lesions were a slightly brownish color or were covered by normal skin. B, Nodules that were firm to palpation and were covered by normal skin. The nodules were on the patient's lower limbs and some measured over 1cm in diameter.
Our differential diagnosis included histiocytic diseases, mastocytosis, metastases, infiltration by a hematologic disease, sarcoidosis, and infection by atypical microorganisms. Full laboratory workup, which included complete blood count, immune studies with measurement of complement levels, protein electrophoresis, hormone profile, and tumor antigens, was normal, and serology for human immunodeficiency virus was negative.
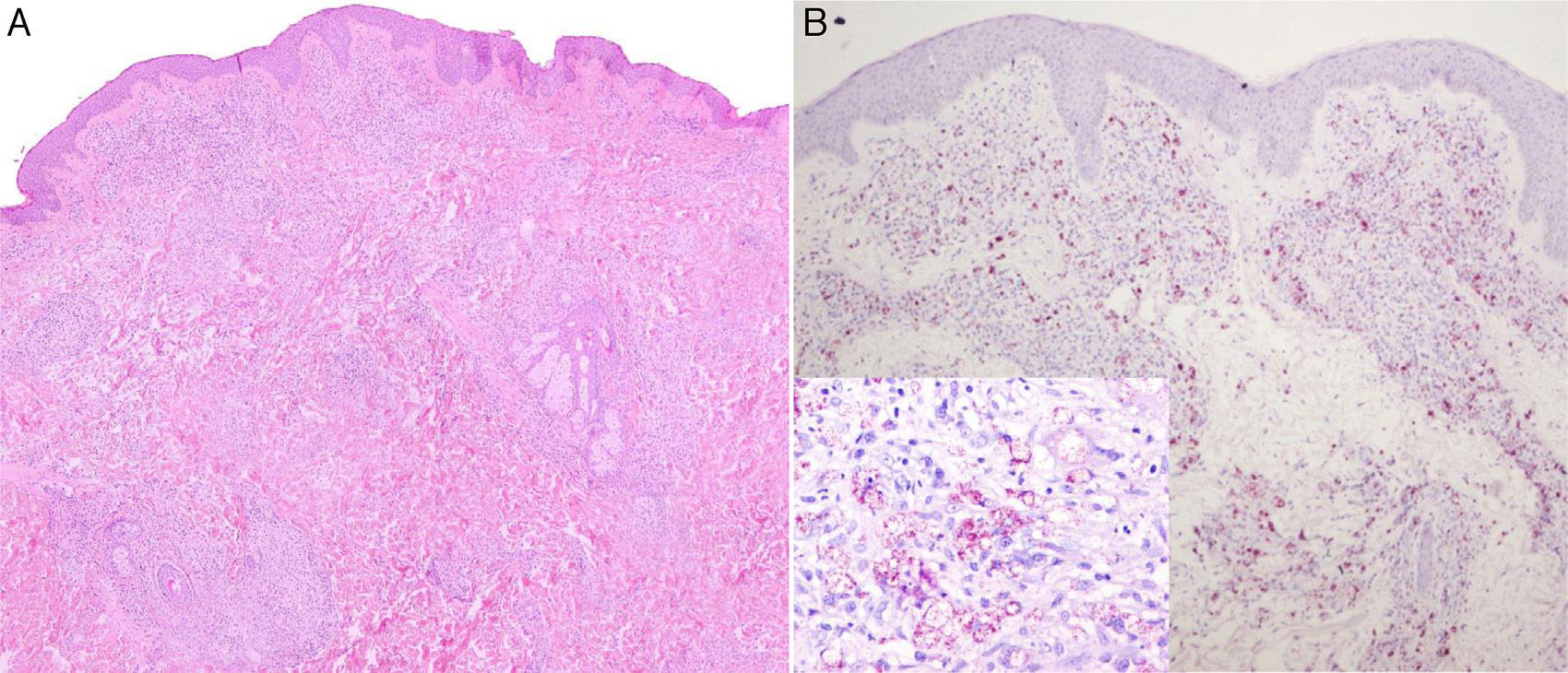
Two biopsies were taken. The first, from a papular lesion on the trunk, showed nodular dermal infiltrates formed of histiocytes with foamy cytoplasm, massively parasitized by bacilli that were positive to staining with Job-Chaco modified Fite-Faraco stain, and a few scattered lymphocytes. This dense dermal infiltrate was separated from the epidermis by an unaffected band of superficial dermis (Fig. 2, A and B). This inflammatory infiltrate was also found to follow the path of the cutaneous nerve endings, despite there being no clinical neurological involvement, as no sensory loss was detected on examination and there were no palpable thickened nerves.
Nodular dermal infiltrates of histiocytes with foamy cytoplasm masively parasitized by bacilli that were positive on Job-Chaco modified Fite-Faraco stain, and scattered lymphocytes. This dense dermal infiltrate was separated from the epidermis by a band of healthy dermis. A, Hematoxylin and eosin, original magnification×4. B, Job-Chaco modified Fite-Faraco stain, original magnification×4.
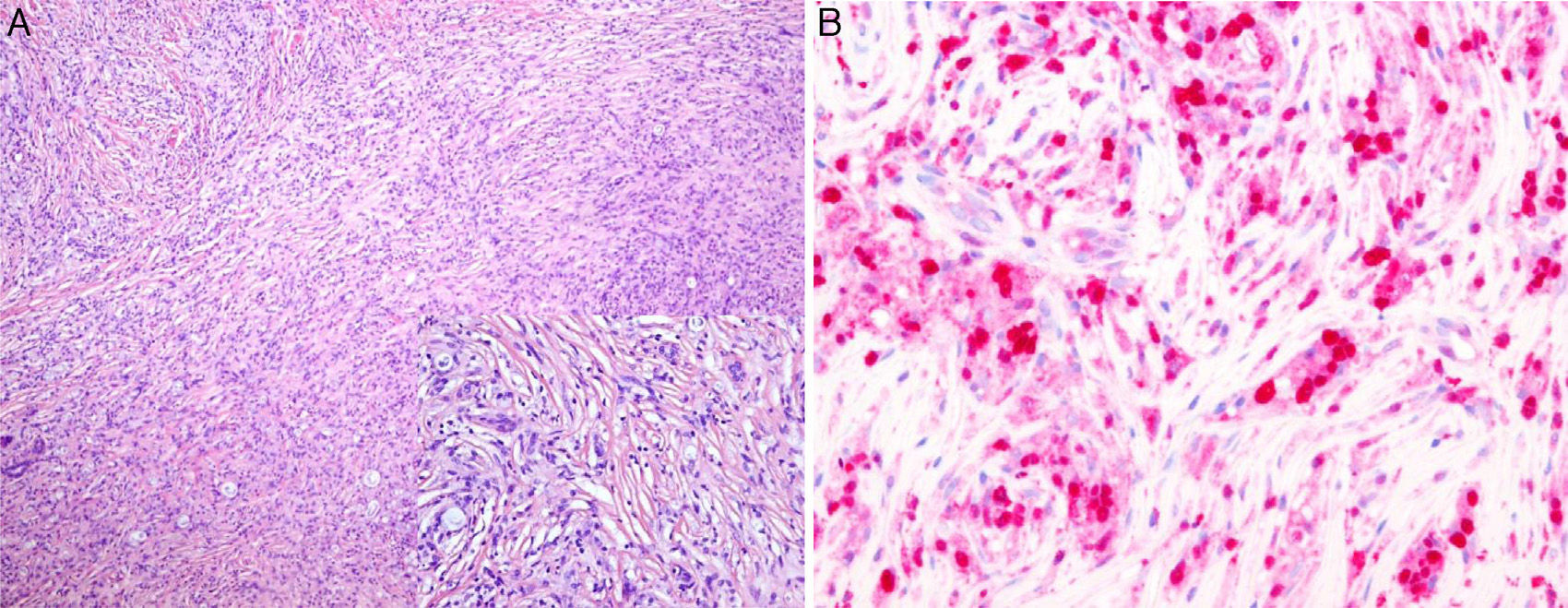
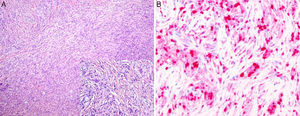
The second biopsy was of a nodular lesion on the calf. In this biopsy, the whole dermis was occupied by a dense infiltrate of interwoven spindle-shaped histioid cells with interspersed macrophages that contained large numbers of bacilli in their cytoplasm. On immunohistochemistry, the histiocytes showed intense and diffuse immunoreactivity to factor XIIIa, but they were negative to S100 (Fig. 3, A and B).
A, Dermis occupied by a dense infiltrate of spindle-shaped histioid cells with interspersed macrophages containing large numbers of bacilli in their cytoplasm. Hematoxylin and eosin, original magnification×10; inset, original magnification×20. B, The histiocytes show an intense and diffuse immune reaction to factor XIIIa. Factor XIIIa stain, original magnification×40.
The patient was diagnosed with multibacillary leprosy. A smear of material from the earlobe revealed innumerable acid-alcohol-fast bacilli (bacillary index, 6+). Treatment was started with the 12-month regimen established by the World Health Organization: dapsone, 100mg, plus clofazimine, 50mg, daily and clofazimine, 300mg, plus rifampicin, 300mg, once a month. After 6 months of treatment, the lesions had become asymptomatic and were difficult to palpate; the lower limbs presented intense residual hyperpigmentation where the nodules had been.
Persons cohabiting with the patient were examined and found to be free of skin lesions at that time. The patient will have to undergo a minimum of 5 years follow-up with smears and measurement of the bacillary index at 6 months and annually.
Leprosy is a chronic infectious-contagious disease caused by Mycobacterium leprae.1,2 Close contact is required as transmission rates are low. Transmission occurs through bacilli released in the nasal exudate or oral droplets and, less frequently, via eroded skin. The incubation period varies, but is typically of 2 to 5 years for the tuberculoid form and of 8 to 12 years for the lepromatous variant.1
Not all infected persons develop the disease. On contagion or later, the individual's immune system is determinant in phenotype of the disease that develops.1,3,4 At the lepromatous pole of the spectrum are patients with a poor immune response to M leprae; as in our case, these patients have multibacillary disease.
The skin lesions in the pure lepromatous form tend to be widespread and symmetrical and include macules, papules, nodules, and diffuse infiltration of the skin. Histopathology typically shows infiltrates in the dermis with a few scattered lymphocytes, and foam cells (Virchow cells) heavily laden with bacilli, which occasionally form aggregates called globi. Bacilli can also be found in the sweat glands, nerves, endothelium, and Schwann cells. This dense dermal infiltrate is separated from the epidermis by a band of healthy dermis, the Unna band or Grenz zone. Macrophages present in the lesions of the lepromatous pole disease show constant and intense expression of protein S100.3,4
In the subpolar or borderline lepromatous (BL) form, there are numerous, poorly defined lesions, but nerve involvement is less prominent. Histology reveals collections of macrophages with foamy cytoplasm and a variable number of lymphocytes, particularly around the small cutaneous nerves. Bacilli are easy to find, although there are fewer globi and more T lymphocytes.3,4
In the present case, the lesions on the trunk showed a notable accumulation of macrophages massively parasitized with bacilli, but also an intense inflammatory infiltrate following the path of the cutaneous nerve endings; this was histologically compatible with BL. However, the lesions on the lower limbs were clinically and histologically distinct and were compatible with hyperactive leprosy lepromatous (LL), a variant also known as histioid leprosy.
Hyperactive LL is a form of presentation of multibacillary leprosy with specific clinical, histopathologic, and bacteriologic features. This can arise de novo, in untreated or long-standing disease, after inadequate treatment, or when resistant microorganism are present. Hyperactive LL is due to microorganisms that survive inactive and that are present in approximately 10% of multibacillary patients.5–7
This rare clinical form of leprosy occurs in patients with hyperactive lesions. Previous lesions, become larger or new lesions appear and become symptomatic. It usually develops over 1 to 3 years as nodules or lepromas preferentially affecting the face, back, buttocks, and limbs.5–7
Histopathology reveals a normal epidermis, with a dermis occupied by a dense infiltrate that mimics a fibrohistiocytic tumor formed of histiocytes that become spindle-shaped. These cells characteristically express factor XIIIa and decrease their expression of protein S100, which may become negative.3,4,7 This was observed in the nodular lesions on the lower limbs of our patient.
The fact that our patient was pregnant when she developed the clinical manifestations of the disease is likely related, as it has been widely reported that leprosy becomes active or recurs during pregnancy or in the puerperium due to alterations and changes in the immune response that occur during this period.2
The children of these women are at relatively high risk of being infected if their mothers have untreated multibacillary leprosy, and although few cases of vertical transmission have been reported, the newborn infant must be followed up for a period, possibly with investigations to detect the presence of M leprae in the nasal mucus. Our patient started treatment during pregnancy, making the risk of contagion very low, and there is no evidence of transmission with breastfeeding, though parents must be educated about the disease and about the need to consult for any suggestive sign.1,2
The incidence of leprosy in Spain is increasing, mainly due to cases in patients arriving from endemic areas. The erroneous idea of its low frequency in Spain means that we often do not include it in the differential diagnosis, making its recognition difficult.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We would like to thank Dr Félix Contreras Rubio, Professor of Histology at Universidad Autónoma de Madrid, and Dr Elena Ruiz Bravo-Burguillos, of the Histology Department of Hospital Universitario La Paz, for their help and collaboration in the diagnosis of the case presented.
Please cite this article as: Sánchez-Orta A, Albízuri Prado MF, González Pessolani T, Sendagorta Cudós E. Lesiones pruriginosas en el embarazo como presentación inusual de una variante poco frecuente de lepra multibacilar. Actas Dermosifiliogr. 2016;107:353–355.