Penile squamous cell carcinoma (SCC) is uncommon in Europe, where it accounts for approximately 0.7% of all malignant tumors in men. The main risk factors are poor hygiene, lack of circumcision, human papillomavirus (HPV) infection, and certain chronic inflammatory skin diseases. HPV infection is detected in 70% to 100% of all penile in situ SCCs and in 30% to 50% of invasive forms of the disease, mainly basaloid and warty SCCs. In situ tumors can be treated conservatively, but close monitoring is essential as they become invasive in between 1% and 30% of cases. The treatment of choice for penile SCC is surgery. Inguinal lymph node irradiation is no longer recommended as a prophylactic measure, and it appears that selective lymph node biopsy might be useful for reducing the morbidity associated with prophylactic inguinal lymph node dissection. Survival is directly related to lymph node involvement. Improving our knowledge of underlying molecular changes and their associated genotypes will open up new therapeutic pathways.
El carcinoma escamoso de pene (CEP) es una neoplasia infrecuente en Europa, suponiendo un 0,7% de los tumores en varones. La mala higiene, no estar circuncidado, la infección por el virus del papiloma humano (VPH) y algunas dermatosis inflamatorias crónicas son los principales factores de riesgo. El VPH se detecta en un 70-100% de los CEP in situ y en un 30-60% de las formas invasivas, sobre todo en los tumores basaloides y condilomatosos. Los tumores in situ pueden tratarse de forma conservadora, pero requieren un seguimiento estricto, puesto que del 1 al 30% evolucionan a formas invasivas. En los CEP invasivos el tratamiento de elección es la cirugía. La irradiación profiláctica de los ganglios inguinales está actualmente desaconsejada. Parece que el uso de la biopsia selectiva de ganglio centinela podría ser útil para disminuir la morbilidad asociada a la linfadenectomía inguinal profiláctica. La supervivencia se relaciona directamente con la presencia de metástasis ganglionares. El conocimiento de las alteraciones moleculares y genotípicas subyacentes abrirá nuevas vías terapéuticas.
Penile carcinoma is rare in the developed world, and most cases (98%) correspond to squamous cell carcinoma (SCC). The most important advances in penile SCC in recent years have been the identification of risk factors, improved knowledge of the molecular pathways involved in the development of this tumor, and updating of staging criteria. Progress has also been made in the area of treatment, with an increasing tendency towards conservative surgery, whose aim is to minimize the risk of recurrence while preserving sexual and urinary function.
In Europe, penile SCC is most common between the sixth and eighth decades of life, with two-thirds of cases occurring in patients aged over 65 years.1 The global incidence is 0.1 to 0.7 cases per 100 000 males. It is estimated that approximately 4000 new cases are diagnosed each year; this accounts for less than 0.5% of all cancers.1 In Spain, penile SCC accounts for approximately 0.7% of all malignant tumors in men, with an annual incidence of between 0.7 and 1.5 cases per 100 000 males. Rates are similar in other parts of western Europe, but in some parts of the world, such as Uganda and Brazil, they are up to 4 times higher.1,2 This considerable geographic variation in incidence is probably due to socioeconomic and cultural differences.
Risk factorsThe main risk factors for the development of penile SCC are poor hygiene, lack of circumcision, human papillomavirus (HPV) infection, and certain chronic inflammatory skin conditions.
Poor hygiene contributes to the development of this tumor through the accumulation of smegma and other irritants in the balanopreputial sulcus, and is also associated with a higher incidence of bacterial and candida infections.3
Most penile SCCs occur in uncircumcised men.4 Neonatal circumcision exerts a preventive effect,2,4,5 but it is not known whether a similar effect is achieved with circumcision performed later in life. It is also clear that phimosis, which is present in 40% to 85% of penile SCCs, interferes with adequate hygiene of the glans, contributes to chronic inflammation, and favors the development of this tumor.6
A direct relationship has been demonstrated between HPV infection and penile SCC. Both conditions are directly linked to the number of sexual partners and early sexual debut.2 The association with HPV, however, is less common than in cervical cancer, where 95% of patients have this infection. HPV infection is more common in carcinomas in situ (70%-100% of cases) than in invasive forms (30%-60%); its occurrence also varies according to histologic subtype, with prevalence ranging from just 30% in usual carcinomas to between 70% and 100% in basaloid and warty variants.7,8 The most common HPV type involved in the development of penile SCC is HPV-16 (69% of cases), but other high-risk, or oncogenic, types have been isolated; of particular relevance is HPV-18.9
Even though genital warts are caused by low-risk HPV types, a history of this condition is associated with a 3-fold to 5-fold increased risk of developing penile SCC.10 This is possibly in part because genital warts often occur in individuals who engage in sexual risk behaviors, placing them at a greater risk of becoming infected by other HPV types.
Although Buschke-Löwestein tumor (giant condyloma acuminatum) is classified as a warty carcinoma in the literature, it should preferably be considered a separate entity, mainly because it has distinct clinical and pathological features (which resemble those of warts), is associated with HPV-6 and HPV-11 (low-risk types), appears at a relatively young age, and has practically no potential for metastasis.2,11
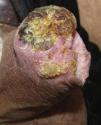
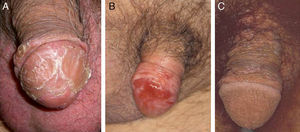
Another risk factor for penile SCC is pseudoepitheliomatous micaceous and keratotic balanitis, which affects the glans of elderly, uncircumcised men. Like Buschke-Löwestein tumor, this lesion is considered to be premalignant or to have low-grade malignant potential. It has not been associated with HPV infection. It presents as a plaque covered with silver micaceous scales (similar to those seen in psoriasis) that can form a thick keratotic layer (Fig. 1A). Histologically, its appearance can range from that of simple epithelial hyperkeratosis and hyperplasia, with minimal cytologic atypia, to a lesion mimicking a warty carcinoma.
Other risk factors for penile SCC are certain chronic inflammatory skin conditions, in particular, lichen sclerosus and its more advanced form, balanitis xerotica obliterans, which is characterized by constrictive fibrosis that affects the entire circumference of the prepuce, preventing its retraction. Penile lichen sclerosus can progress to SCC in 6% of cases, and examination of surgical specimens has shown that up to a third of penile SCCs arise in penile lichen sclerosus lesions.12,13 Accordingly, patients with penile lichen sclerosus should be regularly monitored.
As occurs with other skin cancers, sustained immunosuppression (e.g., in transplant recipients or patients with human immunodeficiency virus) is closely associated with increased risk of penile SCC and worse prognosis. Finally, penile SCC, like cancer of the bladder and the oral cavity, has been associated with tobacco use.2,10,14
Clinical and Histologic FeaturesThere are 2 very distinct clinical and histologic forms of penile SCC, each with different prognostic and therapeutic implications. These are carcinoma in situ and invasive carcinoma.
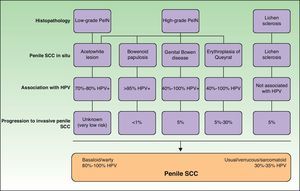
Penile SCC in SituPenile SCC arises from a precursor lesion that can be classified according to the severity and extent of cellular atypia and the presence or absence of HPV infection.5 The clinical appearance of penile SCC in situ is highly variable, ranging from subclinical lesions that can only be seen following the application of acetic acid, to reddish lesions (erythroplasia of Queyrat and genital Bowen disease) white lesions (leukoplakias), and brownish lesions (Bowenoid papulosis).
Subclinical lesions can be visualized by peniscopy after the application of acetic acid.15 These lesions, known as flat penile lesions or acetowhite lesions, are associated with high-risk (hr) HPV infection. They are very common and occur in up to 50% to 70% of male sexual partners of women with cervical intraepithelial neoplasia (CIN) and in up to 10% to 20% of men whose partners do not have CIN. They contain large amounts of viral particles and are highly contagious. Histology tends to show varying degrees of epithelial hyperplasia or dysplasia. The majority of lesions resolve either spontaneously or with treatment within a year or 2, but a small percentage persist and progress to invasive carcinoma.16
Erythroplasia of Queyrat manifests as single or multiple erythematous plaques on the mucosa of the glans or on the inner aspect of the prepuce (Fig. 1B). Genital Bowen disease presents as a single, scaly plaque on keratinized skin, generally on the distal third of the penis.17 Bowenoid papulosis, in turn, affects younger men, in their 30s or 40s. It manifests as multiple, small, brown, well-circumscribed wart-like papules affecting the penis, the glans, the prepuce, or the pubic area (Fig. 1C). It is caused by HPV-16 and is highly contagious, meaning that affected individuals’ sexual partners will have a high risk of developing CIN.
Histology is mandatory in all cases to determine the true nature of penile SCC in situ. Histologic alterations seen in penile SCC in situ are classified according to grades of penile intraepithelial neoplasia (PeIN), using a similar system to that employed in vulvar and cervical cancer (VIN and CIN grades, respectively). The alterations are often found in the epithelium adjacent to penile SCC. A distinction is also made between differentiated PeIN and undifferentiated PeIN.18Differentiated (usual or low-grade) PeIN is characterized by cellular atypia in the basal and suprabasal layers of the epidermis, elongated rete ridges, and conserved architecture in the upper layers.19 It tends to occur in association with lichen sclerosus or epidermal hyperplasia, and can progress to usual or verrucous SCC, or, less frequently, to basaloid or warty SCC.18–21
Undifferentiated (high-grade or Bowenoid) PeIN is correlated with HPV infection and has cellular atypia in at least the lower two-thirds of the epithelium, as well as basaloid cells and abundant mitotic figures.18–21 It usually progresses to basaloid or warty carcinoma but can also give rise to usual SCC; it almost never progresses to verrucous carcinoma.18 Erythroplasia of Queyrat, genital Bowen disease, and Bowenoid papulosis are clinical manifestations of undifferentiated PeIN and they can all progress to invasive penile SCC; the associated risk is 10% to 30%, 5% to 10% and less than 1%, respectively22 (Fig. 2).
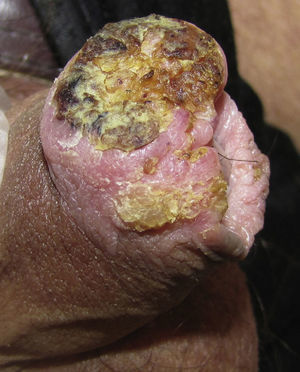
Invasive Penile SCCThe clinical appearance of invasive penile SCC is highly variable, with manifestations ranging from an erythematous plaque or ulcer to an exophytic or verruciform tumor. The lesions, which can measure up to several centimeters in diameter, can have a stony hard or friable consistency and may bleed (Fig. 3). They tend to be solitary lesions and can be located on any part of the penis, although they are more common on the anterior third (glans, balanopreputial sulcus, and/or prepuce). They are found on the shaft of the penis in fewer than 5% of cases. During the clinical evaluation, it is essential to note the number of lesions, their color, morphology, and maximum diameter, and their relationship to other structures (invasion of the external urethral orifice or the corpus spongiosum or cavernosum). It is also important to measure the length of the penis to calculate the approximate length that would remain after a penectomy, should this be necessary.10
Several histologic subtypes have been identified on the basis of architectural and cytologic features. Keratinizing, or usual SCCs are the most common form of invasive penile SCC and account for 50% to 60% of all cases; they generally follow an infiltrative growth pattern and can be well or poorly differentiated (Fig. 4A). Verrucous penile SCC (8%-10% of cases) is architecturally similar to a wart and follows an expansive growth pattern (Fig. 4B). Basaloid penile SCC (4%-6% of cases) is characterized by the presence of nests of clearly basaloid cells with an infiltrating pattern and peripheral palisading23 (Fig. 4C), while warty penile SCC (6%-10% of cases) resembles a wart and has easily identifiable cytopathic changes and larger cells.24 In our experience, some tumors with a usual-type architecture contain a clear basaloid carcinoma component; they are much more common than pure basaloid carcinomas and are related to hrHPV infection. Sarcomatoid penile SCC (1% of cases) is very poorly differentiated and specific immunohistochemical staining for cytokeratins is necessary to demonstrate the true nature of the spindle cells (Fig. 4D). Finally, mixed variants account for 10% to 15% of all forms of invasive penile SCC.25
The histologic subtypes of penile SCC can also be classified by prognosis. Verrucous and warty types have the best prognosis, and carcinoma cuniculatum, a subtype of low-grade SCC, is also associated with good prognosis. Carcinoma cuniculatum has a verruciform architecture characterized by a deeply penetrating, burrowing growth pattern. Basaloid, sarcomatoid, and undifferentiated usual tumors are all associated with a high risk of dissemination. Most of them are poorly differentiated and invade the deep dermis. Intermediate subtypes include usual penile SCCs, several mixed forms, and the pleomorphic variants of warty penile SCCs.25
StagingIn 2009, the American Joint Committee on Cancer (AJCC) proposed a new TNM staging system for penile cancer in which considerable importance was placed on lymph node involvement as a prognostic factor26 (Table 1). In the revised system, the T category is divided into 2 subcategories: T1a, for well differentiated tumors and absence of lymphovascular invasion, and T1b, for tumors that are either poorly differentiated or have lymphovascular invasion. Prostatic invasion has been removed from the T3 category as it is very rare and occurs when many other structures are already affected. The T2 category was not modified but several authors have indicated that prognosis is much worse when there is corpus cavernosum invasion than when there is only corpus spongiosum invasion.26
TNM Classification of Penile Squamous Cell Carcinoma According to the European Association of Urologists Guidelines on Penile Cancer 2009.
| T: Primary Tumor |
| TX: Primary tumor cannot be assessed |
| T0: No evidence of primary tumor |
| Tis: Carcinoma in situ |
| Ta: Noninvasive verrucous carcinoma |
| T1: Tumor invades subepithelial connective tissue |
| T1a: No lymphovascular invasion and the tumor is well differentiated or moderately differentiated (T1-G1/G2) |
| T1b: Lymphovascular invasion and the tumor is poorly differentiated or undifferentiated (T1-G3/G4) |
| T2: Tumor invades corpus spongiosum/corpora cavernosa |
| T3: Tumor invades urethra |
| T4: Tumor invades other adjacent structures |
| N: Regional lymph nodes (p: pathologic classification) |
| NX: Regional lymph nodes cannot be assessed |
| N0: No palpable or visibly enlarged inguinal lymph nodes |
| N1: Palpable mobile unilateral inguinal lymph node |
| N2: Palpable mobile multiple or bilateral inguinal lymph nodes |
| N3: Fixed inguinal nodal mass or pelvic lymphadenopathy unilateral or bilateral |
| M: Distant metastasis |
| M0: No distant metastasis |
| M1: Lymph node metastasis outside the true pelvis in addition to visceral sites |
The revised system also specifies that N1 only refers to unilateral inguinal involvement with mobile lymph nodes. N2 refers to bilateral inguinal involvement with mobile lymph nodes while N3 refers to the presence of 1 or more fixed lymph nodes or metastasis in the pelvic lymph nodes.
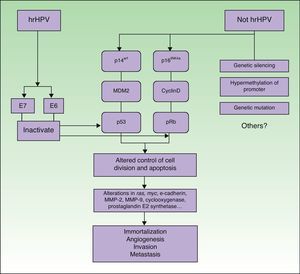
PathogenesisIt seems clear that alterations in different molecular pathways are involved in the etiology and pathogenesis of penile SCC. While little is known about the impact and interrelationship of each of these pathways, it is generally accepted that a proportion of penile SCCs are caused by hrHPV infection while the rest are caused by HPV-independent molecular mechanisms6 (Fig. 5).
Penile SCC caused by hrHPV infection arises from a precursor lesion produced by the virus via a carcinogenic pathway similar to that involved in cervical cancer.27 Nevertheless, tissue-specific and hormonal mechanisms also appear to exert an influence, as penile SCC and cervical cancer are caused by the same infectious agents but differ substantially in terms of incidence and age of onset.
The initiating event is persistent infection of the squamous epithelium by hrHPV, followed by a series of epigenetic alterations that lead to the malignant transformation of the infected cell. hrHPV expresses the oncoproteins E6 and E7, which bind to and inactivate the tumor suppressor gene products p53 and pRb.28–30 E6 and E7 play a key role in inducing and maintaining the transformed phenotype of the infected cell. hrHPV types alter the p14arf/MDM2/p53 and p16INK4a/cyclinD/Rb pathways and interfere with the control of cell division and apoptosis. The functional inactivation of pRB by E7 leads to overexpression of p16INK4a due to the lack of negative feedback; accordingly, p16INK4a overexpression can be used as a marker of HPV infection.31 ProxExC can also be used to indirectly detect HPV infection,32 but this marker has not yet been used in penile SCC.
The pathways involved in HPV-associated penile SCC are the same as those that are altered in penile SCC not caused by HPV infection, with the involvement of the following mechanisms: silencing of tumor suppressor genes, hypermethylation of promoter genes, and overexpression of oncogenes.2,31
The molecular mechanisms involved in more advanced penile SCC are probably the same in both types of tumors (HPV-positive or HPV-negative). Alterations in the expression of the ras and myc genes, E-cadherin, matrix metalloproteinase (MMP) 2 and MMP-9, cyclooxygenase, and prostaglandin E2 synthase have also been identified in penile SCC.33–35 These are probably late events and would therefore be involved in disease progression mechanisms such as angiogenesis, invasion, and metastasis. Some of the above factors are considered to be predictive factors of lymph node metastasis.
Prognostic FactorsInguinal lymph node involvement and the number of affected nodes are the most important prognostic factors of survival in penile SCC. Five-year disease-free survival is as high as 80% when 1 or more superficial, unilateral inguinal lymph nodes are involved (N1), but just 10% to 20% when the involvement is bilateral or pelvic (N2/3), and less than 10% when there is extranodal involvement.36
Perineural invasion, lymphovascular permeation, and grade of differentiation are the most important histologic indicators of the risk of lymph node metastasis and disease-specific death.25,37–40 Other factors that appear to influence prognosis are tumor depth, growth pattern, histologic subtype, and urethral invasion.
The relationship between HPV infection and prognosis is controversial,41,42 and there are also contradictory results regarding whether or not p53 expression is an independent predictor of lymph node metastasis.43,44 Nonetheless, it does appear that strong expression of p53 in T1 tumors is correlated with a higher risk of metastasis and shorter survival.44 High expression levels of ki67 are associated with an increased risk of lymph node metastasis but do not influence survival.45 Studies by Campos et al.33 and Zhu et al.44 have shown that low E-cadherin levels are correlated with a higher risk of lymph node metastasis and worse survival.33,44 Campos et al.33 also showed overexpression of MMP-9 to be a risk factor for tumor recurrence.
TreatmentThe lack of standardized protocols on the management of penile SCC and the absence of data from randomized clinical trials to guide decision-making can be explained by the low incidence of this cancer.
Treatment of the Primary TumorPenile SCC in situ can be treated by simple surgical excision or Mohs micrographic surgery46; both techniques preserve penile function and are associated with low recurrence rates. Other effective treatments include cryotherapy, photodynamic therapy, carbon dioxide or Nd:YAG laser therapy, and topical treatment with 5-fluorouracil 5% or imiquimod 5% cream.47–51 While the risk of penile SCC in situ becoming invasive is relatively low, post-treatment surveillance is recommended. A lack of response or recurrence should alert the clinician to the possibility of disease progression.
The mainstay treatment for invasive penile SCC is surgical resection of the primary tumor with margins of 5 to 10 mm. The goal of treatment is to eliminate the disease and, where possible, to preserve urinary and sexual function. Quality of life and sexual health are also important considerations and should always be discussed with the patient when deciding therapeutic strategies.
There are a number of determining factors when planning treatment, including tumor size and extent of invasion, location of the tumor (glans, prepuce, or shaft), and the experience of the surgeon. In extensive but superficial tumors, treatment options include glans skinning and resurfacing with a partial-thickness skin flap and a combination of laser ablation and surgical reconstruction.52,53 Intralesional chemotherapy (vinblastine, bleomycin, methotrexate) and radiotherapy are reserved for exceptional cases as they are associated with more serious adverse effects. Wide circumcision is the treatment of choice when the prepuce is involved; for more invasive tumors, glansectomy or partial penectomy (depending on the size of the tumor) should be considered.54–57 Total penectomy is an option when the tumor is located on the shaft of the penis or is poorly differentiated.56,57 The first-line treatment in T4 tumors, which are very rare, is neoadjuvant systemic chemotherapy, followed by surgery in patients who respond.
Radiotherapy of the primary tumor is an option in T1 or T2 tumors measuring less than 4 cm in diameter, and is associated with 5-year cure rates of 70% to 90%.58,59 The best results are obtained with brachytherapy or with electron beam radiotherapy, but these treatments are associated with higher rates of local recurrence than partial penectomy. In such cases, salvage surgery can restore local control of the tumor. Radiotherapy-associated complications include urethral stricture (10%-45%), necrosis of the glans (0%-23%), and fibrosis of corpus spongiosum.60
Management of Lymph NodesCareful palpation of inguinal areas in search of potentially metastatic lymph nodes is essential as these areas are most likely to contain the sentinel nodes. As the tumor progresses, it can invade the Buck fascia and the corpus cavernosum, and, via the lymphatic system, progress sequentially to regional superficial and deep inguinal and pelvic nodes, before metastasizing to distant organs. Positron emission tomography with computed tomography is a highly sensitive method that helps to define the stage of disease.61 Ultrasound-guided fine-needle aspiration (FNA) cytology is a fast and simple method for detecting metastasis in palpable inguinal lymph nodes.62 If no tumor cells are detected, the procedure can be repeated; alternatively, the lymph node can be removed or the patient reexamined after 5 weeks of empirical systemic antibiotic therapy. Inguinal lymph node dissection (ILND) is mandatory when tumor cells are detected by FNA cytology or analysis of a lymph node biopsy specimen.
ILND is the treatment of choice in patients with metastatic inguinal lymph nodes. It is, nonetheless, a complex technique that is associated with a 50% rate of complications (range, 24%-87%), the most notable of which are chronic edema in the lower limbs and scrotum, flap necrosis, and infection of the surgical wound; the procedure is associated with death in 1% to 3% of cases.63 Endoscopic ILND has emerged as a promising option for reducing the morbidity associated with this technique.64 Prophylactic bilateral ILDN is associated with high morbidity and is unnecessary in many cases as it does not improve prognosis.There are no noninvasive or minimally invasive techniques for determining lymph node status in patients with moderately or poorly differentiated tumors or tumors classified as T3 or higher without palpable lymph nodes.65 Sentinel lymph node biopsy has been shown to improve survival compared with a watch and wait approach and to reduce morbidity compared with prophylactic ILND; it has a specificity of 100% and a sensitivity of 95%, but is not performed in all centers.66 Algorithms based on histology findings have also been designed to predict the risk of metastasis in penile SCC, but their sensitivity rates do not exceed 80%.13,67–69
Prophylactic radiotherapy in patients with N0 disease is no longer indicated as it does not prevent lymph node metastasis, results in complications, and causes residual fibrosis that complicates follow-up.70 Finally, adjuvant chemotherapy is purely palliative in patients with unresectable metastasis in the inguinal lymph nodes or in distant organs.
Patient SurveillancePatients should undergo strict surveillance for at least 5 years. Those who have undergone conservative surgery should be examined every 3 months for 2 years and every 6 months thereafter. Six-monthly check-ups are sufficient for those in whom more radical surgery is performed,71 and clinical evaluation is considered sufficient for patients with well-differentiated T1 or T2 tumors without lymphovascular invasion or palpable lymph nodes at the time of treatment. Inguinal ultrasound is advisable in all other cases.
The overall risk of local recurrence is under 5%, and in patients who have undergone penile-sparing surgery, most cases of recurrence are seen within the first 2 years. Local recurrence is generally detected by the patient, or by his partner or general practitioner, and therefore does not tend to affect survival.
Patients in whom lymph node palpation was used as a staging tool have a higher risk of lymph node metastasis than those in whom surgical techniques such as prophylactic ILND or sentinel node biopsy were used (9% vs 2.3%). The risk of recurrence in patients treated for lymph node metastasis is 19%.10
PreventionPenile SCC is a preventable disease. Patients with phimosis that prevents adequate exploration and good glans hygiene should be circumcised.
Because HPV infection, particularly HPV-16 infection, is a key factor in the etiology of certain types of penile SCC, HPV vaccines may have beneficial effects if administered to boys before they become sexually active. There are currently 2 HPV vaccines available: a bivalent one against types 16 and 18 and a quadrivalent one against types 16, 18, 6, and 11. However, considering that the incidence of penile SCC is much lower than that of cervical cancer, it is unlikely that routine vaccination of children will be indicated. Furthermore, penile SCC is much more common in developing countries, where it would be very difficult to vaccinate large groups of individuals. While condom use does not offer 100% protection against HPV infection, it has been shown to reduce the risk of transmission and to shorten the time it takes HPV lesions to heal.72
ConclusionsThe creation of multidisciplinary teams formed by urologists, dermatologists, pathologists, and molecular biologists will improve our understanding of the oncogenic mechanisms underlying penile SCC and help to correctly diagnose initial lesions, identify prognostic factors, implement prevention campaigns, and identify molecular targets that will optimize treatment, increase survival, and reduce morbidity.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ferrándiz-Pulido C, de Torres I, García-Patos V. Carcinoma escamoso de pene. Actas Dermosifiliogr.2012;103:478-487.




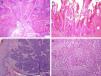

![Histologic subtypes of penile squamous cell carcinoma. A, usual (hematoxylin-eosin [H&E], original magnification ×20. B, verrucous (H&E, original magnification ×20). C, Basaloid (H&E, original magnification ×100). D, Sarcomatoid (H&E, original magnification ×100).](https://static.elsevier.es/multimedia/15782190/0000010300000006/v1_201304241313/S1578219012001990/v1_201304241313/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)