Allergic contact dermatitis due to plants is common. Potentially allergenic plants and plant products are found in many everyday environments, such as the home, the garden, the workplace, and recreational settings. By improving our knowledge of allergenic plant-derived chemical compounds, we will be better positioned to identify novel allergens. We review the most relevant chemical allergens that contribute to plant allergic contact dermatitis and propose a clinical classification system based on 5 major families of chemical sensitizers: α-methylene-γ-butyrolactones, quinones, phenol derivatives, terpenes, and miscellaneous structures (disulfides, isothiocyanates, and polyacetylenic derivates). We also describe the different clinical pictures of plant allergic contact dermatitis and review currently available patch test materials. A better understanding of the specific allergens involved in plant allergic contact dermatitis will help to predict cross-reactivity between different plant species or families.
La dermatitis alérgica por contacto debida a plantas es común. Se pueden encontrar plantas y productos vegetales potencialmente alergénicos en muchos entornos habituales como el hogar, el jardín, el lugar de trabajo y ambientes recreativos. Mejorando nuestro conocimiento de los compuestos químicos alergénicos derivados de plantas estaremos en una mejor posición para identificar alérgenos nuevos. Revisamos los alérgenos químicos que contribuyen de manera más relevante a la dermatitis alérgica por contacto por plantas y proponemos un sistema de clasificación clínica basado en 5 principales familias de sensibilizadores químicos: α−metilen-γ-butirolactonas, quinonas, derivados fenólicos, terpenos y estructuras misceláneas (disulfuros, isotiocianatos y derivados poliacetilénicos). Describimos también los diferentes cuadros clínicos de dermatitis alérgica por contacto por plantas y revisamos los materiales de pruebas epicutáneas actualmente disponibles. Un mejor entendimiento de los alérgenos específicos involucrados en la dermatitis alérgica por contacto por plantas ayudará a predecir reacciones cruzadas entre diferentes especies o familias de plantas.
Plants are living organisms belonging to the kingdom of Plantae, which includes at least 300000 species of trees, herbs, bushes, grasses, vines, ferns, mosses and green algae. Although most plants are harmless on contact with the skin or ingestion, the possibility of mild to serious adverse events is well recognized. Several cutaneous adverse reactions to plants such as nonimmunological contact urticaria, irritant contact dermatitis, allergic contact dermatitis, phototoxic contact dermatitis and photoallergic contact dermatitis have been reported.
Allergic contact dermatitis is one of the most frequent cutaneous side effects in individuals exposed to plants. Study of the pathogenic mechanisms implicated in the development of allergic contact dermatitis (ACD) to plants may be challenging because of the large number of species, the diversity of chemical compounds that they contain, and the simultaneous contact with more than one allergen.
A wide range of plants and plant allergens responsible for ACD have been identified and the different contact dermatitis triggers have often been classified according botanical criteria. These classifications are useful but do not allow prediction of cross-reactivity among different species or families and require a botanical knowledge often cumbersome to physicians, dermatologists, and allergologists. A possible classification of plant contact dermatitis according to the main chemical structures acting as allergens could be useful and may help prevent future exposures to the responsible agent or its cross-reacting compounds present in other species or families.
Our main objective is to review ACD to plants from a chemical point of view. The responsible plants are classified according their chemical allergens. Several families of chemical sensitizers causing different types of contact and photocontact allergic dermatitis are reviewed (Table 1).
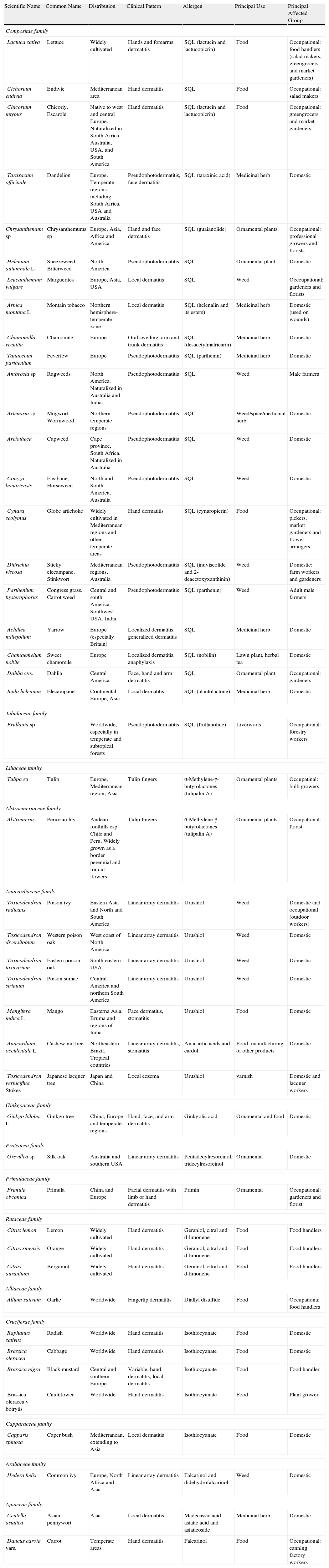
Main Plants Responsible for Allergic Contact Dermatitis.
| Scientific Name | Common Name | Distribution | Clinical Pattern | Allergen | Principal Use | Principal Affected Group |
| Compositae family | ||||||
| Lactuca sativa | Lettuce | Widely cultivated | Hands and forearms dermatitis | SQL (lactucin and lactucopicrin) | Food | Occupational: food handlers (salad makers, greengrocers and market gardeners) |
| Cichorium endivia | Endivie | Mediterranean area | Hand dermatitis | SQL | Food | Occupational: salad makers |
| Chicorium intybus | Chicoriy, Escarole | Native to west and central Europe. Naturalized in South Africa, Australia, USA, and South America | Hand dermatitis | SQL (lactucin and lactucopicrin) | Food | Occupational: greengrocers and market gardeners |
| Taraxacum officinale | Dandelion | Europe. Temperate regions including South Africa, USA and Australia | Pseudophotodermatitis, face dermatitis | SQL (taraxinic acid) | Medicinal herb | Domestic |
| Chrysanthemum sp | Chrysanthemums sp | Europe, Asia, Africa and America | Hand and face dermatitis | SQL (guaianolide) | Ornamental plants | Occupational: professional growers and florists |
| Helenium autumnale L | Sneezeweed, Bitterweed | North America | Pseudophotodermatitis | SQL | Ornamental plant | Domestic |
| Leucanthemum vulgare | Marguerites | Europe, Asia, USA | Local dermatitis | SQL | Weed | Occcupational: gardeners and florists |
| Arnica montana L | Montain tobacco | Northern hemisphere-temperate zone | Local dermatitis | SQL (helenalin and its esters) | Medicinal herb | Domestic (used on wounds) |
| Chamomilla recutita | Chamomile | Europe | Oral swelling, arm and trunk dermatitis | SQL (desacetylmatricarin) | Medicinal herb | Domestic |
| Tanacetum parthenium | Feverfew | Europe | Pseudophotodermatitis | SQL (parthenin) | Medicinal herb | Domestic |
| Ambrosia sp | Ragweeds | North America. Naturalized in Australia and India. | Pseudophotodermatitis | SQL | Weed | Male farmers |
| Artemisia sp | Mugwort, Wormwood | Northern temperate regions | Pseudophotodermatitis | SQL | Weed/spice/medicinal herb | Domestic |
| Arctotheca | Capweed | Cape province, South Africa. Naturalized in Australia | Pseudophotodermatitis | SQL | Weed | Domestic |
| Conyza bonariensis | Fleabane, Horseweed | North and South America, Australia | Pseudophotodermatitis | SQL | Weed | Domestic |
| Cynara scolymus | Globe artichoke | Widely cultivated in Mediterranean regions and other temperate areas | Hand dermatitis | SQL (cynaropicrin) | Food | Occupational: pickers, market gardeners and flower arrangers |
| Dittrichia viscosa | Sticky elecampane, Stinkwort | Mediterranean regions, Australia | Pseudophotodermatitis | SQL (inuviscolide and 2-deacetoxyxanthinin) | Weed | Domestic: farm workers and gardeners |
| Parthenium hysterophorus | Congress grass. Carrot weed | Central and south America. Southwest USA. India | Pseudophotodermatitis | SQL (parthenin) | Weed | Adult male farmers |
| Achillea millefolium | Yarrow | Europe (especially Britain) | Localized dermatitis, generalized dermatitis | SQL | Medicinal herb | Domestic |
| Chamaemelum nobile | Sweet chamomile | Europe | Localized dermatitis, anaphylaxis | SQL (nobilin) | Lawn plant, herbal tea | Domestic |
| Dahlia cvs. | Dahlia | Central America | Face, hand and arm dermatitis | SQL | Ornamental plant | Occupational: gardeners |
| Inula helenium | Elecampane | Continental Europe, Asia | Local dermatitis | SQL (alantolactone) | Medicinal herb | Domestic |
| Jubulaceae family | ||||||
| Frullania sp | Worldwide, especially in temperate and subtopical forests | Pseudophotodermatitis | SQL (frullanolide) | Liverworts | Occupational: forestry workers | |
| Liliaceae family | ||||||
| Tulipa sp | Tulip | Europe, Mediterranean region; Asia | Tulip fingers | α-Methylene-γ-butyrolactones (tulipalin A) | Ornamental plants | Occupatinal: bulb growers |
| Alstroemeriaceae family | ||||||
| Alstromeria | Peruvian lily | Andean foothills esp Chile and Peru. Widely grown as a border perennial and for cut flowers | Tulip fingers | α-Methylene-γ-butyrolactones (tulipalin A) | Ornamental plants | Occupational: florist |
| Anacardiaceae family | ||||||
| Toxicodendron radicans | Poison ivy | Eastern Asia and North and South America | Linear array dermatitis | Urushiol | Weed | Domestic and occupational (outdoor workers) |
| Toxicodendron diversilobum | Western poison oak | West coast of North America | Linear array dermatitis | Urushiol | Weed | Domestic |
| Toxicodendron toxicarium | Eastern poison oak | South-eastern USA | Linear array dermatitis | Urushiol | Weed | Domestic |
| Toxicodendron striatum | Poison sumac | Central America and northern South America | Linear array dermatitis | Urushiol | Weed | Domestic |
| Mangifera indica L | Mango | Easterna Asia, Bruma and regions of India | Face dermatitis, stomatitis | Urushiol | Food | Domestic |
| Anacardium occidentale L | Cashew nut tree | Northeastern Brazil. Tropical countries | Linear array dermatitis, stomatitis | Anacardic acids and cardol | Food, manufacturing of other products | Domestic |
| Toxicodendron verniciflua Stokes | Japanese lacquer tree | Japan and China | Local eczema | Urushiol | varnish | Domestic and lacquer workers |
| Ginkgoaceae family | ||||||
| Ginkgo biloba L | Ginkgo tree | China, Europe and temperate regions | Hand, face, and arm dermatitis | Ginkgolic acid | Ornamental and food | Domestic |
| Proteacea family | ||||||
| Grevillea sp | Silk oak | Australia and southern USA | Linear array dermatitis | Pentadecylresorcinol, tridecylresorcinol | Ornamental | Domestic |
| Primulaceae family | ||||||
| Primula obconica | Primula | China and Europe | Facial dermatitis with limb or hand dermatitis | Primin | Ornamental | Occupational: gardeners and florist |
| Rutaceae family | ||||||
| Citrus lemon | Lemon | Widely cultivated | Hand dermatitis | Geraniol, citral and d-limonene | Food | Food handlers |
| Citrus sinensis | Orange | Widely cultivated | Hand dermatitis | Geraniol, citral and d-limonene | Food | Food handlers |
| Citrus aurantium | Bergamot | Widely cultivated | Hand dermatitis | Geraniol, citral and d-limonene | Food | Food handlers |
| Alliaceae family | ||||||
| Allium sativum | Garlic | Worldwide | Fingertip dermatitis | Diallyl disulfide | Food | Occupationa: food handlers |
| Cruciferae family | ||||||
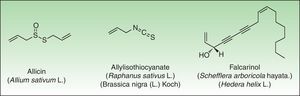
| Raphanus sativus | Radish | Worldwide | Hand dermatitis | Isothiocyanate | Food | Domestic |
| Brassica oleracea | Cabbage | Worldwide | Hand dermatitis | Isothiocyanate | Food | Domestic |
| Brassica nigra | Black mustard | Central and southern Europe | Variable, hand dermatitis, local dermatitis | Isothiocyanate | Food | Food handler |
| Brassica oleracea v botrytis | Cauliflower | Worldwide | Hand dermatitis | Isothiocyanate | Food | Plant grower |
| Capparaceae family | ||||||
| Capparis spinosa | Caper bush | Mediterranean, extending to Asia | Local dermatitis | Isothiocyanate | Food | Domestic |
| Araliaceae family | ||||||
| Hedera helix | Common ivy | Europe, North Africa and Asia | Linear array dermatitis | Falcarinol and didehydrofalcarinol | Weed | Domestic |
| Apiaceae family | ||||||
| Centella asiatica | Asian pennywort | Asia | Local dermatitis | Madecassic acid, asiatic acid and asiaticoside | Medicinal herb | Domestic |
| Daucus carota vars. | Carrot | Temperate areas | Hand dermatitis | Falcarinol | Food | Occupational: canning factory workers |
Abbreviation: SQL, sesquiterpene lactones.
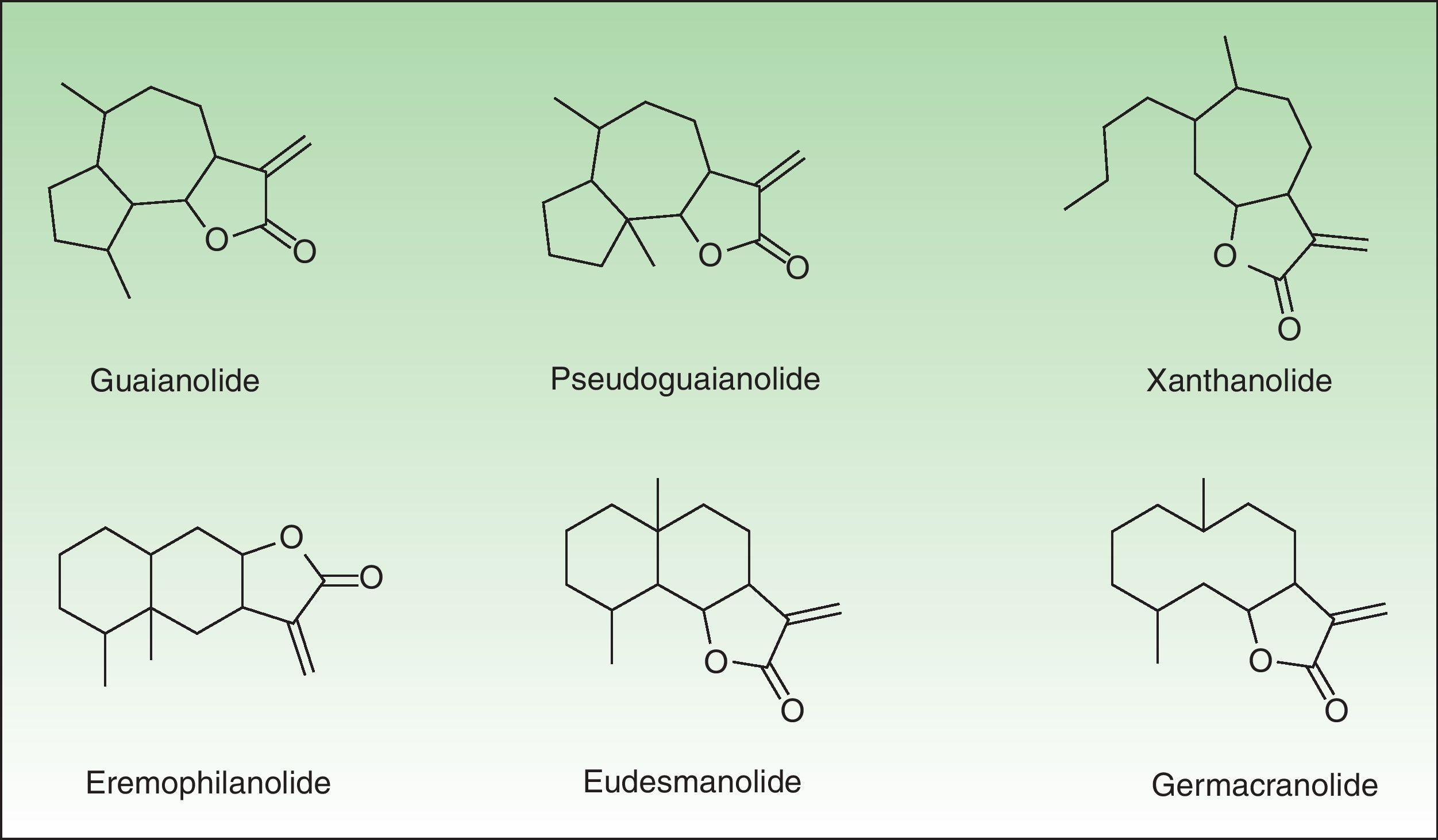
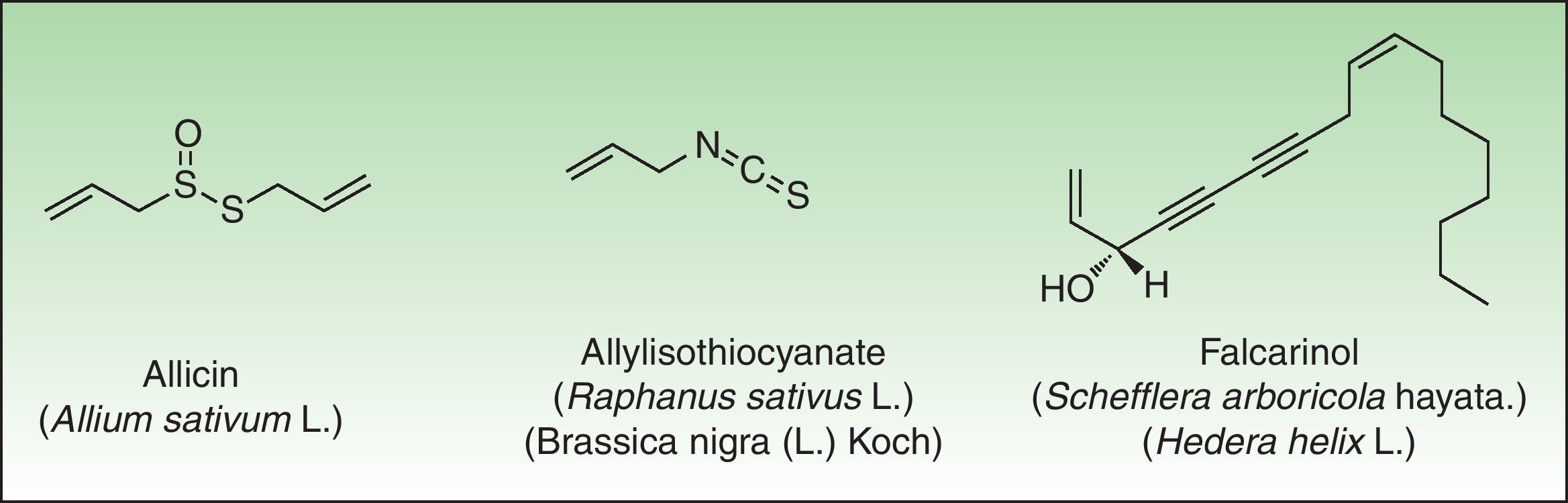
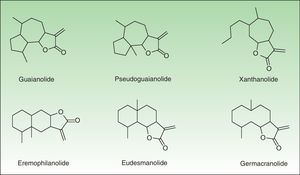
The α-methylene-γ-butyrolactones are part of a large and varied group of bioactive natural products widely present in plants. Their high chemical reactivity arises because of an α,β-unsaturated carbonyl system which allows the formation of covalent adducts with nucleophilic residues on diverse biomolecules. Most of these chemicals are of the sesquiterpene type (15-carbon-atom molecule) with an α-methylene group directed exocyclically to the γ-lactone ring. They are classified into 6 main groups of sesquiterpene lactone (SQL) structures: guaianolide, pseudoguaianolide, xanthonolide, ermophilanolide, eudesmanolide, and germacranolide (Fig. 1). They are the predominant allergens in the Compositae family as well as in other related plant families such as Jubulaceae, Magnoliaceae, Winteraceae, and Lauraceae.1 The α-methylene-γ-butyrolactones can also be found as more simple structures in the Liliaceae (tulipaline A) and Alstroemeraceae families.
Asteraceae or Compositae FamilyThe Asteraceae/Compositae family—also referred to as the aster, daisy, or sunflower family—comprises the second largest family of flowering plants in the world. It includes more than 22750 currently accepted species, spread across 1620 genera and 12 subfamilies. Over 200 species are important causes of contact dermatitis worldwide.2,3 This family presents a variety of edible plants, such as lettuce, chicory, dandelion, salsify, sunflower, scorzonera, artichoke and yacon. Many members are decorative flowers, such as chrysanthemums, gerbera, calendula, dendranthema, dahlias, and heleniums while many others are considered as common weeds. Species such as arnica, chamomile or feverfew are also used medicinally. Asteraceae/Compositae can be recognized by its inflorescence, consisting of many tiny flowers clustered to form a flower head and subtended by an involucre.
SQLs are the predominant allergens although other compounds such as epoxythymol-diesters and polyacetylenes may also be involved.4 SQLs are usually lipophilic, and are often located in the liquid-soluble part (oleoresin fraction) of the leaf, stem flower, and possibly in polen.2,4,5 Although the mechanism of sensitization is unknown, several possibilities have been proposed: pollen or dry debris airborne transportation, direct and/or indirect contact with parts of the plant, and allergen inhalation or ingestion.4,6
Some peculiar cutaneous eruptions caused by the Asteraceae/Compositae family have been described. The pseudophotodermatitis pattern occurs almost exclusively in middle-aged men with a history of outdoor exposure but is rare in children and women, although some studies have suggested an equal sex distribution.6,7 Exposed areas of the face, V of the neck, hands, and forearms are typically involved. In addition, areas not clearly exposed to sunlight such as retroauricular regions (Wilkinson triangle), eyelids, nasolabial folds, and the area beneath the chin are also involved, allowing its differentiation from a true photo-related dermatitis.7 In hot regions, during summer months dry dead plant material contributes to this airborne pattern of dermatitis. In the US, many Compositae weeds, including ragweed (Ambrosia species), induce this clinical pattern often called ragweed dermatitis8 or weed dermatitis in regions where other composite weeds predominate, such as Ambrosia, Artemisia, Helenium, and Iva species.9,10 A similar pattern was described in Australia where it is known as bush dermatitis because it is caused by species such as Arctotheca, Cassini, Conyza, Cynara, and Dittrichi11,12 and also in India where it is called parthenium dermatitis, where the culprit plant is Parthenium hysterophorus L13,14 Although these environmental conditions are not normally encountered in the temperate regions of Europe, some isolated reports of airborne dermatitis (ragweed dermatitis) induced by feverfew (Tanacetum parthenium [L] Sch. Bip.), chicory and lettuce, liverworts of the genus Frullania, and chrysanthemums1,6,9 have exceptionally been reported.
A second special clinical pattern has been described as atopic eczema-like eruption. Compositae allergy may be manifested as a generalized eczematous eruption with primarily flexural involvement. Although it most commonly occurs in elderly men in rural areas, cases in children have also been reported.4,15 An erythrodermic exfoliative pattern classically induced by the weed Parthenium hysterophorus L was also described. This plant was accidentally introduced to the west coast of India in contaminated seed wheat. Several thousands of cases of allergic contact dermatitis occurred, some of them fatal.16
Hand eczema is more frequent in women working in gardening after contact with weeds. A palmar distribution often predominates.17 Hand dermatitis is also associated with handling lettuce.18,19 Dermatitis confined to one or more localized areas such as facial dermatitis secondary to steaming chamomile tea or hand and arm dermatitis from herbal compresses20 has rarely been reported. Oral swelling, perianal pruritus and dermatitis of the trunk and arms were described in a sensitized patient who drank chamomile tea.20 Stomatitis and throat swelling have occurred in some patients after eating lettuce and endivia.21 In one single patient erythema multiforme was reported to develop after patch testing.22
A rare chronic and disabling eccematous photodermatosis known as chronic actinic dermatitis (CAD), photosensitive dermatitis, or actinic reticuloid, has been linked to the ACD to compositae. A high incidence of ACD to one or more allergens has been found among patients with CAD,23 and sesquiterpene lactones are commonly identified as allergens. It is thought that about 20% to 75% of patients sensitized to Asteracea (Compositae) have some degree of photosensitivity. Thus, the progression of ACD to sesquiterpene lactones to CAD has been postulated, but its origin is still unknown.
Patch TestNo ideal patch testing material to study Compositae-induced dermatitis exists. The Compositae family includes more than 1350 SQLs and almost 50% of them are potential contact allergens.2 SQLs with slight differences in molecular structure, may cause true cross-reactions, while identical SQLs from different species may be responsible of false cross-reactions. SQLs are not exclusive to the Compositae family and frequently show cross-reactivity with other plant families. Because the concentration of SQLs shows seasonal and geographic variations and as cross-reactions between different species are not complete, when a patient is not tested with the relevant plants, the diagnosis may be easily overlooked.
Fresh plants or plant extracts should not be used for patch testing because they may cause severe contact reactions and can also elicit active sensitization. False positive irritant reactions or false negative results caused by low content of allergens (degradation products) can also occur.
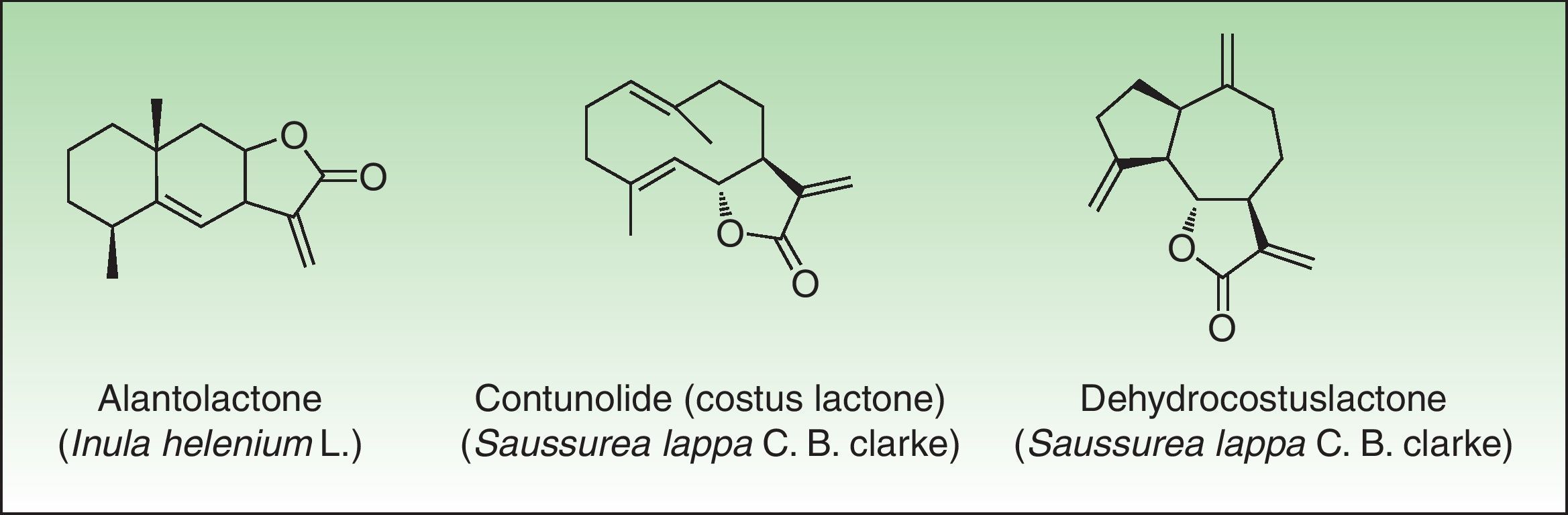
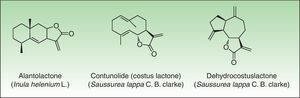
In 1986, Benezra and Epstein developed the sesquiterpene lactone mix (SL mix) a nonsensitizing and seldom irritant mixture of 3 of the 6 main structural groups of sesquiterpene lactones (alantolactone, costunolide and dehydrocostuslactone of eremophilanolide, germacranolide, and guaianolide group) (Fig. 2). This mixture can enable identification of about 60% of SQL sensitization cases24 and was included in the European baseline series.17 Other studies19,25–27 reported different percentages, which can partly be explained by phytogeographical variations and the diversity of SQL structures.28 An alternative preparation, the Compositae mix, based on a mixture of plant extracts (arnica, yarrow, tansy, German chamomile, and feverfew), detects a higher proportion of the cases; however, it may be irritant and cause patch test sensitization.29
Dandelion and feverfew extracts, together or individually, also appear to be more useful than the SL mix.30,31 Other blends have been proposed, such as a mixture of Achillea millefolium L, Chamaemelum nobile All, Helianthus anuus L, Tagetes minuta L, and Tanacetum vulgare L as well as extracts mixtures of arnica, German chamomile, feverfew, tansy, and yarrow.27
Jubulaceae Family (Liverworts)Liverworts are nonvascular plants with a bryophyte life cycle. They comprise 4 genera: Frullania accounts for nearly 800 species and Jubula for 15, whereas Steerea and Neohattoria are both montypic. These plants are often epiphytes growing on trunks and branches of trees or shrubs. They are found throughout the world and are common in tropical and subtropical regions but rare in frigid climates. They are often resistant to desiccation and direct light. Jubula species are largely confined to damp shaded rock surfaces.
At least 11 species of Frullania have been described as responsible for allergic contact dermatitis.32Frullania dilatata (L) Dum and Frulanina tamarisci (L) Dum have been frequently implicated in Europe. Cases of allergic contact dermatitis were also described in British Columbia (Canada), the Pacific North-West of the United States, and in the South-West of France.
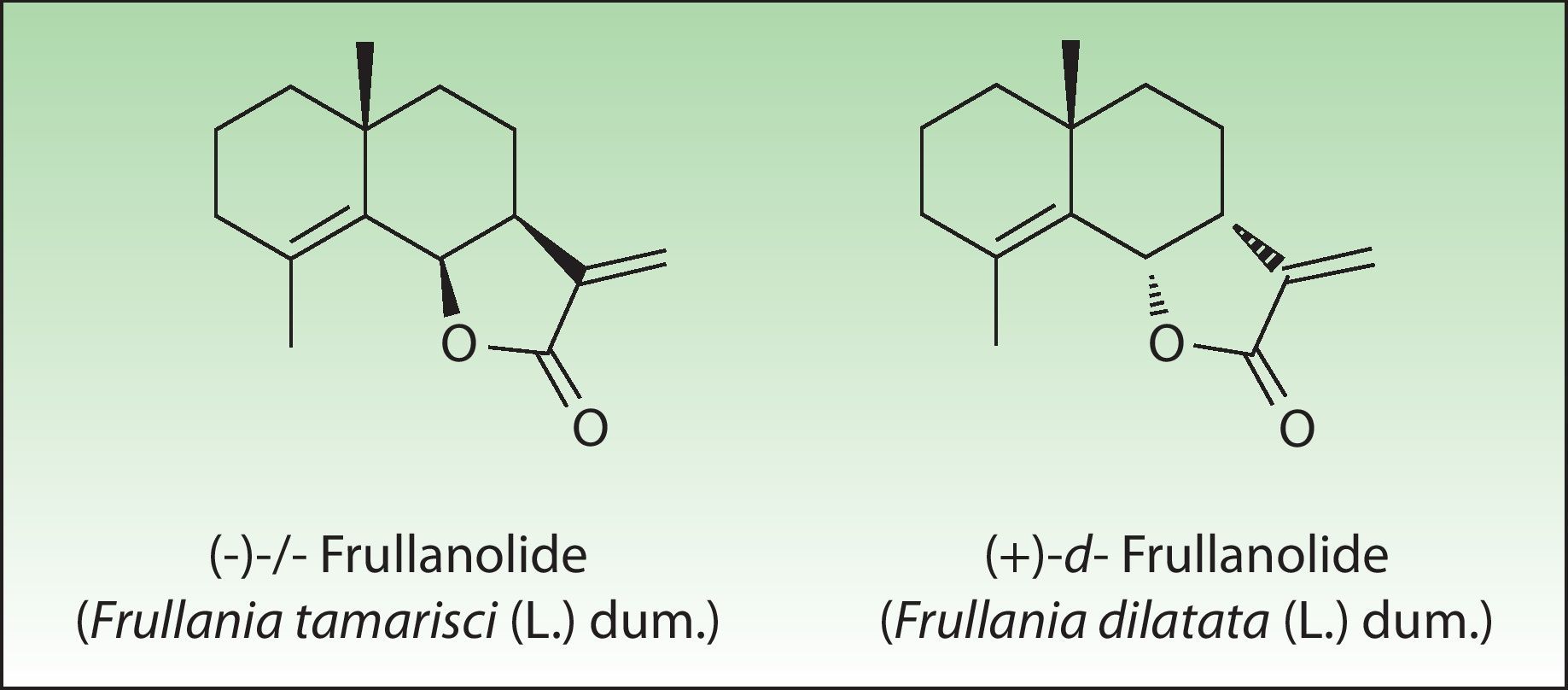
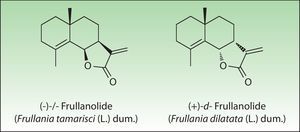
The most important allergen is frullanolide,33–35 an α-methylene-γ-butyrolactone sesquiterpene from the eudesmanolide structural group. Other α-methylene-γ-butyrolactones have been reported as sensitizers such as oxyfrullanolide 5, cis-b-cycloscotunolide 6, and eremofrullanolide 7.35–37Frullania dilatata (L) Dum contains exclusively the d- or (+)-enantiomer of frullanolide while Frullania tamarisci (L) Dum exclusively yields the l- or (−)-enantiomer.36 Both enantiomers are sensitizers, and in guinea pigs the stereospecificity of sensitization to one enantiomer has been demonstrated36,37 (Fig. 3).
Sensitization to Frullania can occur by direct handling of trees or by airborne contact through volatilization of its small particles, especially during warm weather or by the action of chainsaws.34,35 Forest workers often suffer from woodcutter's eczema and domestic allergy can present in individuals who use lobe-leaved trees as firewood. Exposed areas of skin are typically affected, with an airborne pattern on the face and the V of the neck. This clinical pattern can mimic a photosensitivity pattern but it involves areas with little photoexposure such as the eyelids and nasolabial folds. The hands may become edematous, with involvement of finger webs but often sparing of the palms. Exceptional involvement of the genitalia and intertriginous folds has also been reported.38
Patch TestTesting the patient with small pieces of Frullania is associated with a high risk of active sensitization. The SQL mix only detects about 1% of allergic reactions to Frullania. The frullanolide mix, a racemic mixture of (+)- and (−)-frullanolide at 0.01%, 0.033% and 0.1% in petrolatum39 is a safe and nonsensitizing test, but only shows 0.35% of positive reactions.
Although testing the patient with small pieces of Frullania is associated with a high risk of active sensitization, it could be used if there is high clinical suspicion. Frullanolide mix, a racemic mixture of (+)- and (−)-frullanolide at 0.01%, 0.033% and 0.1% in petrolatum39 has been used in several clinical trials proving to be a safe and nonsensitizing test, but with a lower sensitivity than the SQL mix. Therefore the allergen of choice in these cases is SQL mix, even though it only detects 1% of the allergic reactions to Frullania.
Cross-reactions with botanically unrelated plants that contain similar allergens, eg Laurus nobilis L,35Magnolia grandiflora L,40 and several Compositae may also occur.32,41,42
LiliaceaeThe tulip genus which comprises 109 species of showy flowers is the most important genus of the Liliaceae family. A number of species and many hybrid cultivars are grown in gardens, used as pot plants, or displayed as freshly cut flowers. Most cultivars of tulip are derived from Tulipa gesneriana. Professional bulb growers, bulb collectors, sorters, and packers are particularly at risk of contact dermatitis.
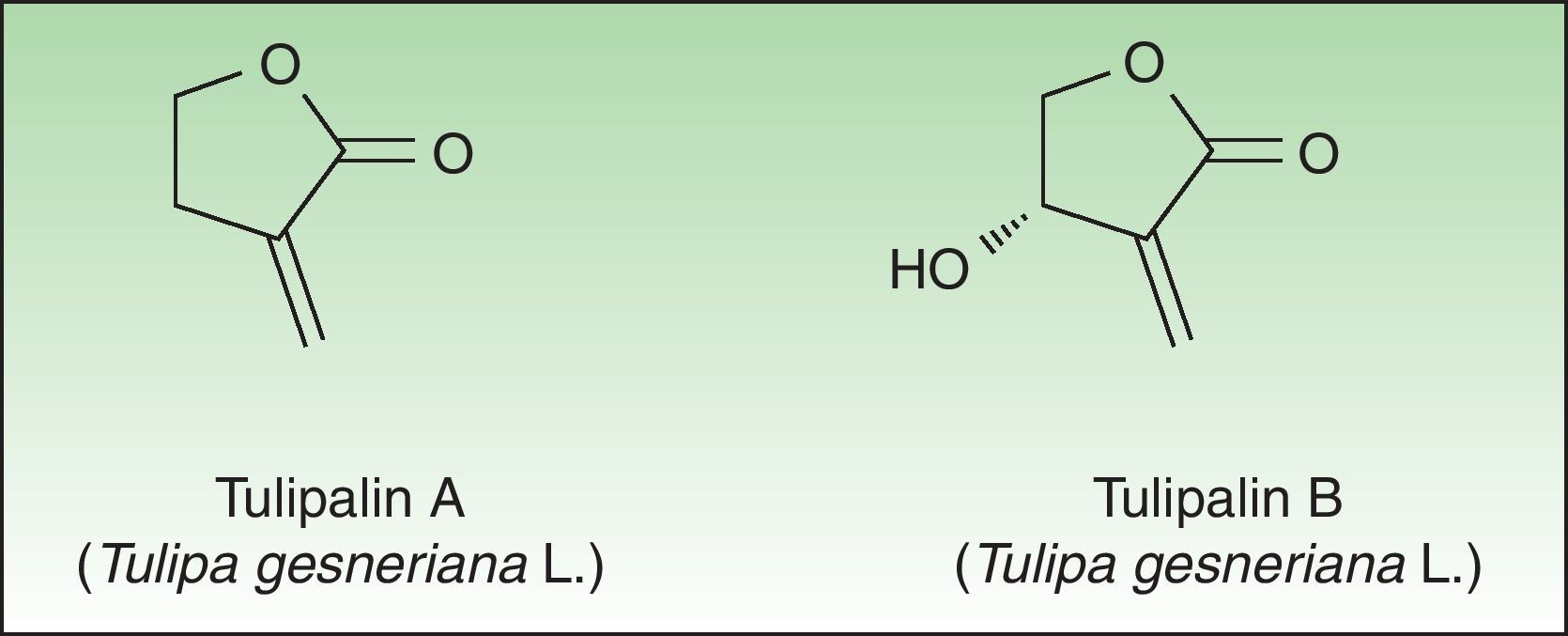
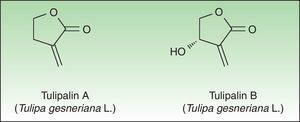
Tulip bulbs contain several glucosides including tuliposides A and B, which are weakly allergenic but are rapidly hydrolysed to tulipalin A and B. Tulipalin A appears to be the main sensitizer in clinical practice43 although tulipalin B also causes sensitization in guinea pigs44 (Fig. 4). Flowers, leaves and stems possess higher concentrations of tuliposide A than the bulbs; but sensitivity can develop from contact with any portion of the plant, although most of the cases occur in bulb growers who are continuously handling the bulbs. Contact with airborne allergens, although infrequent has also been reported.45
The most common presentation is a combined allergic and irritant hand dermatitis known as tulip fingers. It is characterized by painful, subungual and periungual erythematosquamous, sometimes erosive lesions involving the fingertips, particularly the first and second fingers of the dominant hand.46 There may be fissures, suppuration, and hyperkeratosis. The dermatitis may extend to the adjacent skin and rarely may involve distant areas or even give rise to a diffuse dermatitis.45,47 The dust in tulip sheds may induce conjunctivitis, rhinitis and asthma. Reaginic responses to tulip antigens can also occur.47
AlstroemeriaceaeThese species have a cluster of tuberous roots and produce leafy stems, bearing one to several brightly colored flowers. Several species are found in special collections and only 2 species are widely distributed and produced: Alstroemeria aurantiaca D Don, with orange flowers, which grows as a border plant, particularly in cottage gardens, and Alstroemeria ligtu (Peruvian lilies) which give rise to numerous colors, mostly in shades of lilac and pink, with darker veins and mottling on the petals (Fig. 5). They grow in Holland, South America, USA and Australia. Tulipalin A, the same allergen of the Liliaceae family, is the main responsible sensitizer and is found mainly in the petals and stem. The rate of sensitization for tulipalin A can exceed 50% in workers on Alstroemeria plantations.48
Dermatitis most commonly affects florist who strip off the leaves manually and are therefore exposed to the broken plant with a high risk of allergic contact dermatitis. The lesions, in contrast to tulip finger dermatitis, can be more severe and tend to involve the fingertips of all digits of both hands. Occasionally, the clinical pattern can be very similar to that produced by the garlic clove (but in a bilateral and symmetric distribution).49 Workers who cut the flowers often show palm eczema.50–55 Airborne dermatitis56 and post-inflammatory depigmentation have rarely been reported.57 False positive responses due to a primary irritant reactions may also occur.58
Patch testing Liliaceae and AlstroemeriaceaePatch testing with short ether extract of the leaves and petals as well as direct testing with them is not recommended because of the high risk of active sensitization.58 Since pure tulip allergen is not readily available, patch tests may be performed with outermost bulb scales, after removal of the brown skin. To obviate dependence on seasonal supplies, extracts may be prepared from bulbs which have been tested for allergenicity.59
Several ways of performing patch testing have been recommended. A 96% ethanol extract of tulip bulbs or an extract prepared by shaking fresh bulbs for 90min in acetone (80% in water) using 1% of this extract in 70% ethanol was recommended by Hjorth et al.59 A methanol extract of the fresh petal or leaf/stem material in petrolatum at a 0.001% concentration was recommended by Hausen et al.58 A 6-tuliposide A at 0.01% or an α-methylene-γ-butyrolactone at 0.0001% in ethanol was proposed by Santucci et al.51
Patients with allergic contact dermatitis to Alstroemeria generally cross-react with tulip extracts.57,60 No cross-reactions between the sesquiterpene lactones and tulipalin A have been reported (even though both contain an α-methylene-γ-butyrolactone in the lactone ring61).
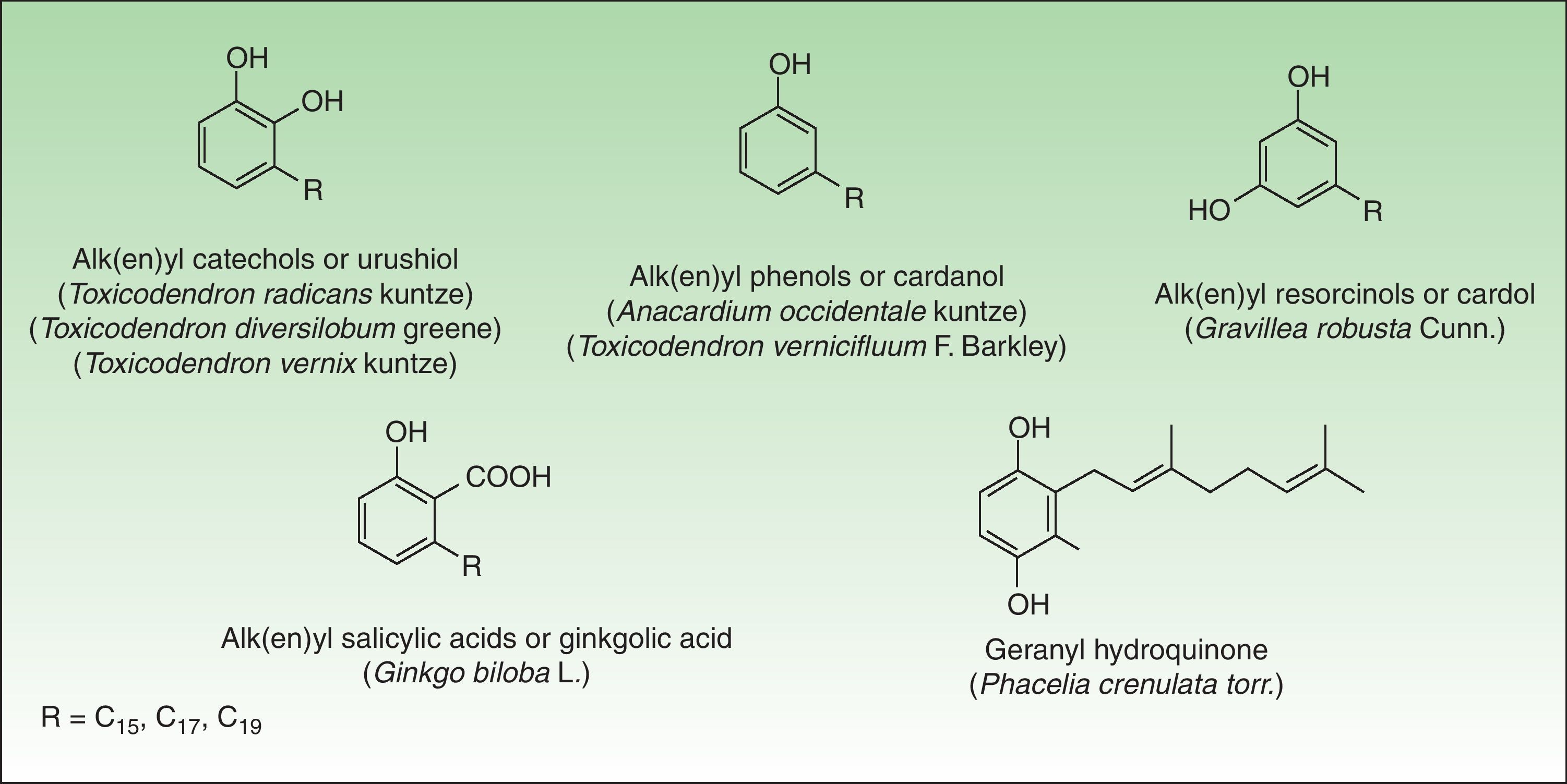
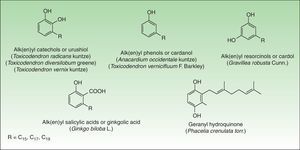
Phenol and DerivatesThis is a group of mono- or dihydroxybenzene derivatives of phenol, catechol, resorcinol, or salicylic acid with a long alkyl or alkenyl carbon side chain. Mixtures of catechols with alk(en)yl side chains are also known as urushiol and are the main allergens in the toxicodendron genus. Phenols, resorcinols, and salicylic acid with alk(en)yl side chains are called cardanol, grevillol, and ginkgolic acid, respectively and are the main allergic contact sensitizers in the Anacardiaceae, Gingkgoaceae and Proteaceae family respectively. They are very good examples of prohaptens, that is, nonelectrophilic substances which have to undergo a chemical transformation such as oxidation to yield the active substance. Thus, many phenolic derivatives are metabolized into ortho-quinones or para-quinones, which are strong electrophiles62 (Fig. 6).
AnacardiaceaeThe Anacardiaceae family also known as the cashew family or the sumac family is a very broad family of flowering plants. This family includes about 70 genera and 650 species of evergreen deciduous trees, shrubs, and woody vines. These plants are native to tropical and subtropical areas of the world, but some species are also found in temperate regions. Members of the Anacardiaceae plant family that are important in dermatology include: the Toxicoendron genus, (common poison ivy, poison oak, poison sumac), Mangifera indica L (mango), Toxicodendron vernicifluum (lacquer tree), and Anacardium occidentale L (cashew nut).
Toxicodendron GenusToxicodendron is the most allergenic genus of this family. It includes poison ivy (Toxicodendron radicans Kuntze and subspecies such as T radicans Kuntze var, rydbergii Erskine), poison oak (Toxicodendron diversilobum Greene and Toxicodendron toxicarium Gillis), and poison sumac (Toxicodendron striatum Kuntze, T vernix Kuntze) complexes. These genera are the main causes of allergic contact dermatitis in the United States and approximately 50%–70% of the population seems to be sensitized.63
Occupational contact dermatitis to poison ivy is an important cause of occupational disability and represents a significant cost for the health system in the United States. Wilderness, rural and outdoor activities as well as outdoor occupations related to agriculture, forestry, and fire fighting, bring with them a high risk of sensitization.64
Poison ivy dermatitis is generally unknown in Europe. The plants can be recognized by their compound leaves, with 3 or more smaller leaflets. Flowers and fruit arise in the angle between the leaf and the twig. The leaf stalk also leaves a “U”- or “V”-shaped scar after it falls off.65
The pentadecylcatechols, consisting of 1, 2-dihydroxy benzenes with a 15-atom side chain at position 3 (3-pentadecylcatechol), also called urushiols, are the sensitizers in Toxicodendron plants. Urushiols are colorless or slightly64 yellow in their natural state, but they oxidize, polymerize and turn black when exposed to air. Urushiol is found in the stems, roots, leaves, and skin of the fruits of these plants and also on fomites, where it retains its antigenic potential in the dry state indefinitely.66 Young leaves contain greater amounts of urushiol than the older ones.67
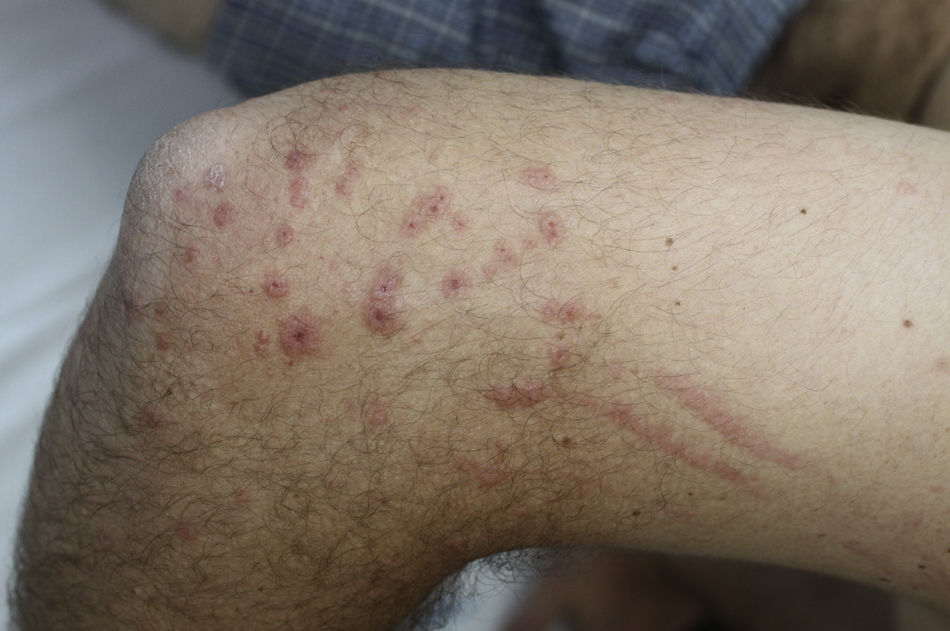
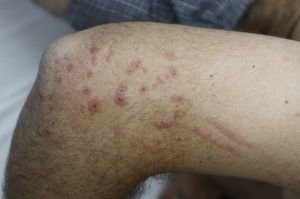
Contact dermatitis primarily results from direct contact with the oleoresin obtained from a portion of a bruised or injured plant. However, contamination and re-exposure to the antigen may occur, with the most important reservoirs being the fingernails, clothing, animals, garden tools, or firewood.10,68 Contact dermatitis usually appears within 2–4 days; however it may appear as late as 2 weeks after the moment of contact. It is characterized by intense pruritus and an erythematous reaction followed by the appearance of multiple papules and vesiculo-bullous lesions in a linear distribution (Fig. 7). The face, neck, and genitalia are commonly affected and usually show intense edema. The dermatitis has a self-limiting course, lasting approximately 1–2 weeks, but it can last up to 6 weeks.64
Other clinical presentations include erythema multiforme-like reactions,69 exanthematous and urticarial eruptions, and even renal damage that may occur after accidental, medicinal or alimentary ingestion.70,71 Stomatitis and anal itch have been described after chewing the leaves or secondary to hyposensitization treatments. Black spot poison ivy dermatitis is a rare and very special condition, consisting of asymptomatic black lesions on the skin that cannot be washed off. These lesions are followed by subsequent pruritic papules and have been mistaken for melanoma in several cases.72
Patch TestPlant oleoresin diluted 1:10 in acetone may be used for patch testing. The pure synthetic antigen, 3-pentadecylcatechol, may also be used in various dilutions, but is not readily available and probably does not contain all the antigenic materials of the poison ivy oleoresin.73
Mango (Mangifera indicaL) is a fruit found in eastern Asia, Burma and regions of India. It has been widely cultivated throughout the world, particularly in Hawaii, Florida, Mexico, and Central America.
The responsible allergens are 3 resorcinols derivatives: heptadecadienylresorcinol, heptadecenylresorcinol and pentadecylresorcinol.74 They are found in the stems, leaves, and peel, but not in fruit juice, which may be drunk by sensitized persons. Allergic contact dermatitis occurs after touching the fruit with the peel intact. Dermatitis can be limited to vesicles at the commissures of the mouth but it usually affects the entire perioral area, and it may affect the buccal mucosa and face. The hands can carry the allergen to the eyes and neck, and these eruptions sometimes become generalized. Some rare cases of systemic contact dermatitis have also been reported after ingestion by people previously sensitized by contact.75 Climbing the tree can result in allergic dermatitis due to the content of urushiol in the leaves and bark of the tree. Symptoms of wheezing have also been reported, presumably due to an immediate hypersensitivity reaction.
Patch test: Leaves and bark but not fruit extracts could be used to demonstrate contact allergy. There is no standardized procedure and a possible active sensitization should be taken into account. Cross hypersensitivity between the allergens of mango and the Anacardiaceae and Gingkoaceae families has been reported.74
Cashew nut tree (Anacardium occidentaleL) is a small tree native to northeastern Brazil but it is now cultivated in most tropical countries. The tree produces a kidney shaped fruit (cashew nut) that grows at the end of the accessory fruit called the cashew apple. The cashew nuts are composed of an internal kernel and a double-layered outer shell. Between the 2 layers of the shell irritant and allergenic oil is found. The nuts are used in the food industry as a substitute for almonds. The oil obtained from the nut shells (cashew nut shell liquid) is used in manufacturing various products. Friction dust is used for brake linings and clutch facings, epoxy resins, paints, varnishes, and foundry core oil as well as insecticides to control mosquito larvae and schistosomiasis vectors.
Anacardic acid (a mixture of 2-carboxyl-3-alkyl phenols) and cardol (a mixture of 5-alkyl resorcinols such as 5-pentadecadienylresorcinol), are the most important allergens.36 Any part of the cashew tree, except the roasted nut, can induce dermatitis76 although allergic contact dermatitis is generally caused by direct contact with the oil. Topical contact with the oil can induce the same type of reaction seen in individuals exposed to poison ivy.77 Oral ingestion can lead to stomatitis and pruritus ani78 and even to widespread dermatitis with flexural accentuation, typically distributed on the extremities, groin, and buttocks. This eruption occurs generally from 1 to 3 days after ingestion of raw cashew nuts contaminated with allergenic oil.79 Dermatitis resulting from sensitized children handling and playing with the nuts has been reported.80 Improperly shelled cashew nuts caused an outbreak of perioral and more generalized dermatitis in Pennsylvania.81
Patch test: There is no standardized patch test recommendation for cashew nut oil, although a 30% concentration has been occasionally used.82 The occurrence of cross-sensitivity between the urushiols of the poison ivy (Toxicodendron radicans Kuntze) and the cashew nut allergen has long been recognized.83,84
Toxicodendron vernicifluaStokes (Japanese lacquer tree) is an indigenous tree of Japan and China. Its trunk yields a milky juice, which on contact with air darkens and becomes a durable lacquer used as varnish on furniture, floors, tea pots, rifle stocks, canes, wooden toilet seats, and ornaments. All plant parts as well as lacquered objects can trigger an allergic phytodermatitis, even after many years.85 The allergens are also urushiols, but they differ from poison ivy's by the positions of the double bonds, with the 15-carbon side chain at positions 8, 11 and 13 instead of at positions 8, 11 and 14 in poison ivy's urushiols.86
Clinical features are variable depending on the mode and sites of contact, and the degree of sensitivity. The most common is an inflammatory reaction and erythema at the site of contact with the varnish; however more extensive persistent and severe cases have been described.87 Dermatitis of the face induced by burning logs from lacquer trees has also been reported.88
Patch test: Previous experience is scarce and there is no special protocol. General recommendation for plants should be followed. Patients allergic to poison ivy usually cross-react to Japanese lacquer tree urushiol.86
Ginkgoaceae FamilyGinkgo biloba L, the ginkgo tree, is the only representative of this family and is regarded as one of the world's oldest surviving tree species. Originating in southeastern China 200 million years ago, it was introduced to Europe during the early 18th century, and it now grows in temperate regions throughout the world. Contact dermatitis from the ginkgo tree is not due to its leaves but to its malodorous fruit exclusively borne in female trees.
The alkylphenols (anacardic or ginkgolic acids, cardols, and cardanols) have been identified as the potential allergens. Ginkgolic acid 1, the most important allergen, is found mainly in the fruit, but it can also be detected in Ginkgo leaves.89 Allergic reactions seem to occur only with open fruit extruding pulp. Handling the intact fruit is not harmful to sensitized persons.90
Contact dermatitis occurs especially in East Asians, who enjoy eating the nuts (seed kernels), and manipulate the seeds to remove the fleshy pulp and obtain the kernels. Pruritic, edematous and erythematous plaques with papules or vesicles appear on the hands, face, arms, and anywhere the seed pulp is spread. Ingestion may also cause stomatitis and anal pruritus.91 While reactions are uncommon in the West, a small epidemic occurred among a group of schoolgirls who stomped upon fallen fruit. They developed a streaky, vesicular dermatitis on their legs, similar to that of poison ivy contact dermatitis.92
Patch TestGinkgo fruit pulp 1–10 in acetone is recommended for patch testing91 as it avoids irritation at this concentration, whereas the fruit pulp itself is strongly irritant.92,93
Proteaceae FamilyThe Proteaceae family comprises 1050 species in 62 genera and is found predominantly in the tropics. The most important genus of this family is the genus Grevillea which is made up of approximately 360 species, most native to Australia, where they range from low shrubs to forest trees. A number of different Grevillea species and hybrids, especially of the eugrevillea section, appear to cause allergic contact dermatitis.94 The most important species are Grevillea robusta Cunn, also known as silky oak or silver oak and Grevillea x Robyn Gordon, a natural hybrid between Grevillea banksii R Br and Grevillea bipinnatifida R Br which is perhaps the most important plant in this genus from a human health perspective and probably represents the most common cause of plant-induced dermatitis in Australia.95
The allergens appear to differ between the species and their hybrids. The allergens of G robusta are 5-tridecylresorcinol (grevillol), 5-pentadecylresorcinol and 5-pentadecenylyresorcinol. Grevillea banksii R Br shows the resorcinol derivative pentadecylresorcinol. Other long carbon chain alkenyl resorcinols include the haptens in Grevillea Robyn Gordon.96
Contact with the wild and cultivated tree, and especially with the flowers, as well as with objects made with its wood, induces a bullous eruption similar to that caused by poison ivy and poison oak (Toxicodendron sp).97 Grevillea poisoning was described among men who cut the fallen trees and developed a severe bullous contact reaction.98
Patch TestThe 0.1% ethanol extract of the Grevillea flower in petrolatum has been used for patch testing. Because the allergens of Grevillea are related chemically to those of the Toxicodendron genus, cross-sensitivity can be seen. Patients who are known to be sensitive to Toxicodendron should avoid Grevillea species contact and vice versa.
QuinonesThis is a group of (poly)cyclic organic compounds with at least one 6-membered unsaturated ring to which 2 oxygen atoms are bound as carbonyl groups (C=O). They represent oxidized forms of ortho- or para-diphenol. They are strong electrophilic substances that easily react with the nucleophilic groups of proteins, acting in this case as strong haptens.
The most important allergens are benzoquinones or naphthoquinones. At high concentrations, they are primary irritants. They are the main sensitizers present in tropical woods as well as in some families of plants, of which the Primulaceae family is the most important.
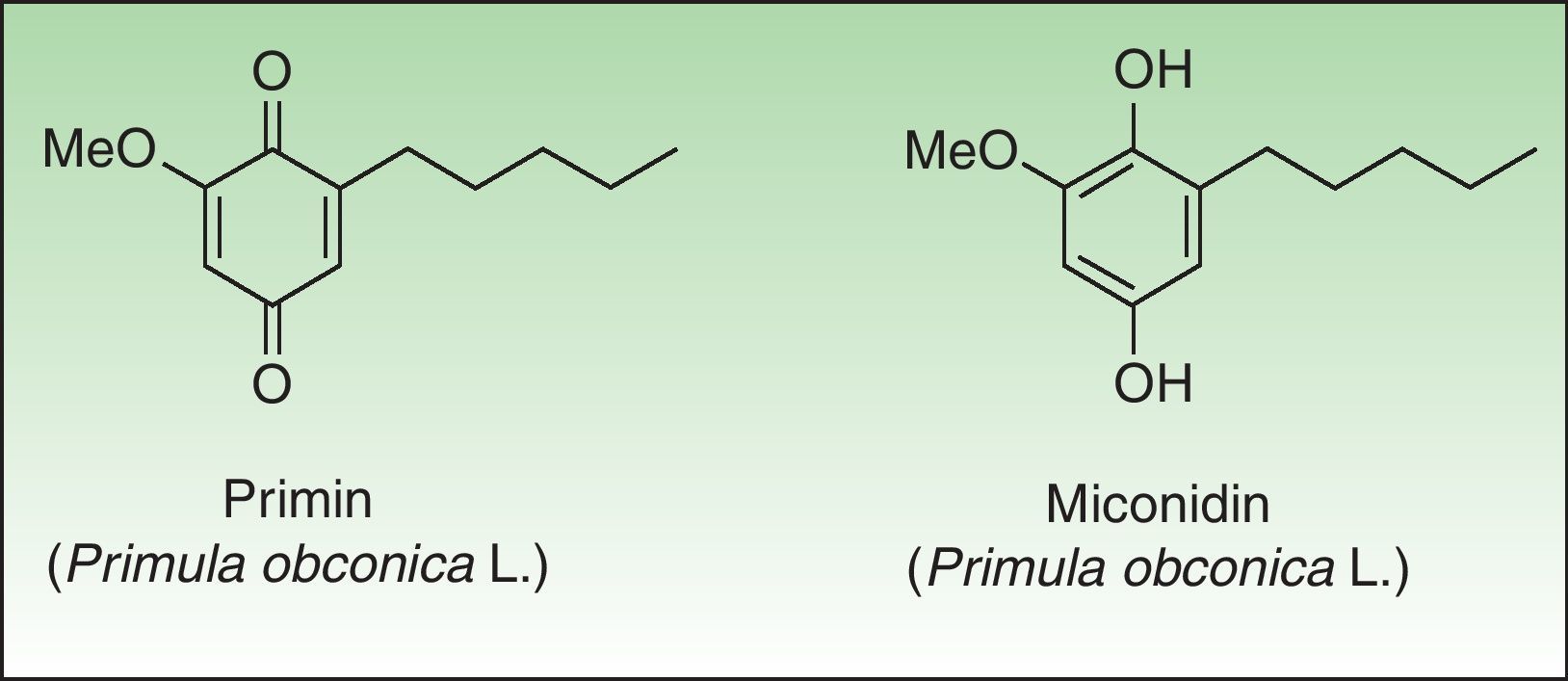
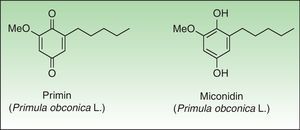
PrimulaceaePrimulaceae is a broad family of flowering plants with about 1000 species in 24 genera. Despite the large number of specimens and cosmopolitan distribution only Primula sp presents a common dermatological hazard. Primula was exported from China to England in the year 1880. Their beautiful flowers, long flowering period, and their ease of care led to a quick introduction into Europe although success was more limited in America. For a number of years Primula obconica Hanse was the most common cause of plant-induced contact dermatitis in Europe; however since the introduction of primin-free P obconica Hanse into the European market, its incidence has declined.99 Other species of Primula are reported to be allergenic, such as P auricula L, P denticulata Sm and P vulgaris Huds, and vary widely in their allergen content.100–102
The primin (2-methoxy-6-n-pentyl-p-benzoquinone) is the major allergen of primrose dermatitis, a quinone formed by oxidation of its biosynthetic precursor miconidin (Fig. 8); however other allergens such as primetin (a flavone),103 and miconidin (2-methoxy-6-pentyl-1,4-dihydroxybenzene)104 have also been suggested. Primin is contained in the terminal cells of the microscopic glandular hairs on the surface of the leaves, stems, and flowers. The allergen content of the plant varies considerably, depending on season, the number hours of sunshine, the method of cultivation, and the horticultural variety, which explains the striking seasonal variation in its allergenicity, greatest in summer and lower in the winter months.
Sensitization to P obconica Hance can occur by direct contact with plants or by indirect contact, e.g. via hand-shakes, door handles, or paper fibers. Primin can be released through pollen or small dust particles, causing an airborne allergic contact dermatitis in some sensitized patients.105
Dermatitis most commonly occurs in elderly women and a variety of cutaneous reactions have been described: facial dermatitis, alone or combined with limb dermatitis and hand dermatitis.42,106,107 Eruptions vary from linear to erythematous papules, vesicles and bullae, and to lesions resembling erythema-multiforme and lichen planus.108–113 A few cases of airborne dermatitis111,114,115 and facial eruption mimicking herpes simplex116 as well as one case presenting as vitiligo117 have also been reported.
In order to reduce the incidence of allergic primin contact dermatitis, a primin-free Primula obconica Hance was developed.118 Although its introduction reduced significantly the cases related to cultivars, it still remains as an important contact allergen, justifying its maintenance in the standard series.
Patch TestPrimin 0.01% in petrolatum is included in the European baseline series.
TerpenesThe terpenes represent a large class of hydrocarbon compounds, produced by a wide variety of plants, particularly conifers. They are the main components of most of the essential oils.
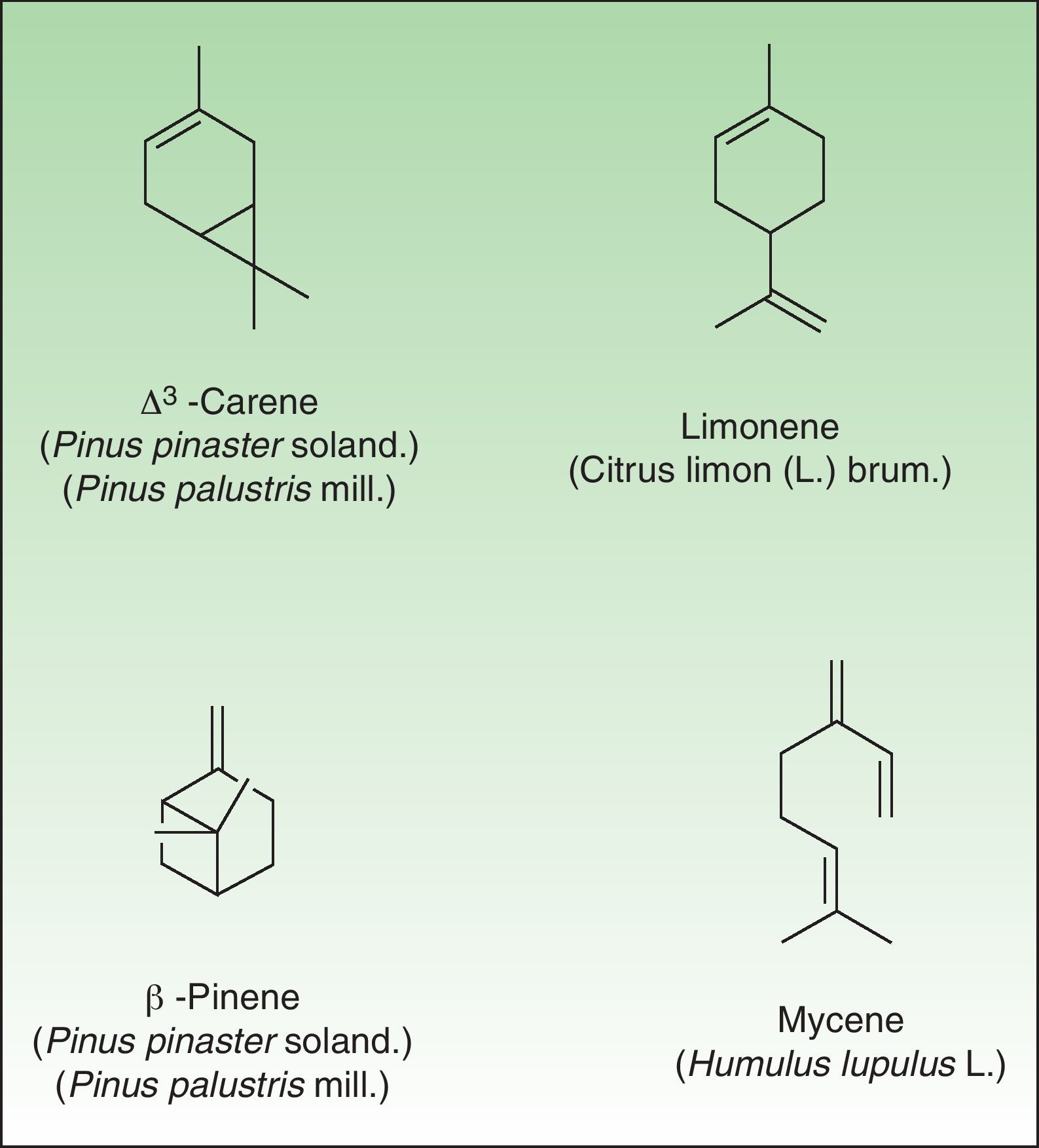
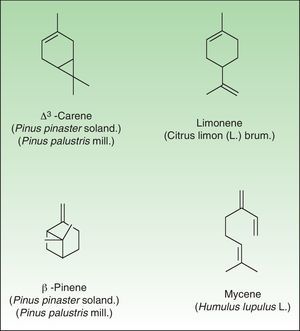
The terpenes are composed biosynthetically of units of isoprene, which has the molecular formula C5H8. Chemically, terpenes are multiples of isoprene units, (C5H8)n where n is the number of linked units. The terpenes are classified by the number of isoprene units included in the complete molecule: hemiterpenes (a single isoprene unit), monoterpenes (2 isoprene units, e.g. limonene, pinene and 3-carene), sesquiterpenes (3 isoprene units), diterpenes (4 isoprene units), and so on. Terpenes by themselves are not sensitizers because they lack the electrophilic properties necessary to bind covalently to skin proteins. They act as prehaptens and need to undergo chemical transformation in order to become true haptens.
Turpentine, a variable mixture of numerous terpenoid compounds, is one of the most important terpene representatives. Turpentine is obtained from the oleoresin of various pine species. Turpentine can easily auto oxidize into hydroperoxides (3-carene, α-pinene and β-pinene) which later yield ketones and aldehydes, both of which are excellent electrophilic substances. Another important example of sensitizing terpene is colophony. Colophony is the residue left after distilling off the volatile oil from the oleoresin obtained from Pinus sylvestris L and other species (Fig. 9).
RutaceaeThis family includes 900 species in 150 genera which are found in tropical and temperate regions especially in southern Africa and Australia. It is an important cause of phototoxic reactions although some cases of ACD to the citrus genus have also been reported.
Citrus GenusTwelve species are native to southern China, south-east Asia and Indo-Malaysia; however, with the aim of achieving larger fruits, with more juice, better taste and lack of seeds, numerous other species have emerged from various artificial crosses. The most common species are Citrus sinensis L (orange), Citrus aurantium L (Bergamot), and Citrus Limon L Burm. Fil. (lemon). Besides its use in the food industry, citrus is used for aromatic oils in the flowers and fur, in perfumery, and in the manufacture of soaps and cosmetics.
Allergic contact dermatitis to citrus fruits is unusual and hand dermatitis has been described in food handlers.119 The potential sensitizers include geraniol, citral and the hydroperoxide derivative of d-limonene,120 which are usually included in fragrances. The mechanism of sensitization is currently an object of research.
Patch TestOxidized limonene is available as a commercial allergen for patch testing at 3% in petrolatum (from Chemotechnique). A multicenter study led by Ann Therese Karlberg from Gothenborg, Sweden, with oxidized limonene and linalol is currently being performed. A recommended patch test is still unavailable.
Miscellaneous StructuresNatural products such as disulfides (Alliaceae), isothiocyanates (Cruciferae) and polyacetylenic derivates (Araliaceae, Apiaceae) are other types of natural products containing reactive functional groups which have been associated with skin allergies (Fig. 10).
DisulfidesAlliaceaeAlliaceae family members are widely distributed in temperate, subtropical and warm regions throughout the world. These species can be recognized easily by their characteristic garlic smell as well as their soft, fleshy leaves. The genus Allium is the most important genus in dermatology and includes many important vegetables such as garlic and onion. Garlic is the main species involved in cases of allergic contact dermatitis. Because of its multiple antibacterial, choleretic, spasmolytic, and in particular proven anti-atherosclerotic effect, the plant has been cultivated since ancient times in China, India and Egypt, and its healing effects are used as folk medicine nowadays in many countries.
All parts of the plant, when damaged, release the potentially irritating and allergenic sap. Diallyl disulfide, allicin, and allyl propyl disulfide have been identified as the principal low molecular weight allergens.121 Allergic contact dermatitis is generally considered as being caused by diallyl disulfide.122
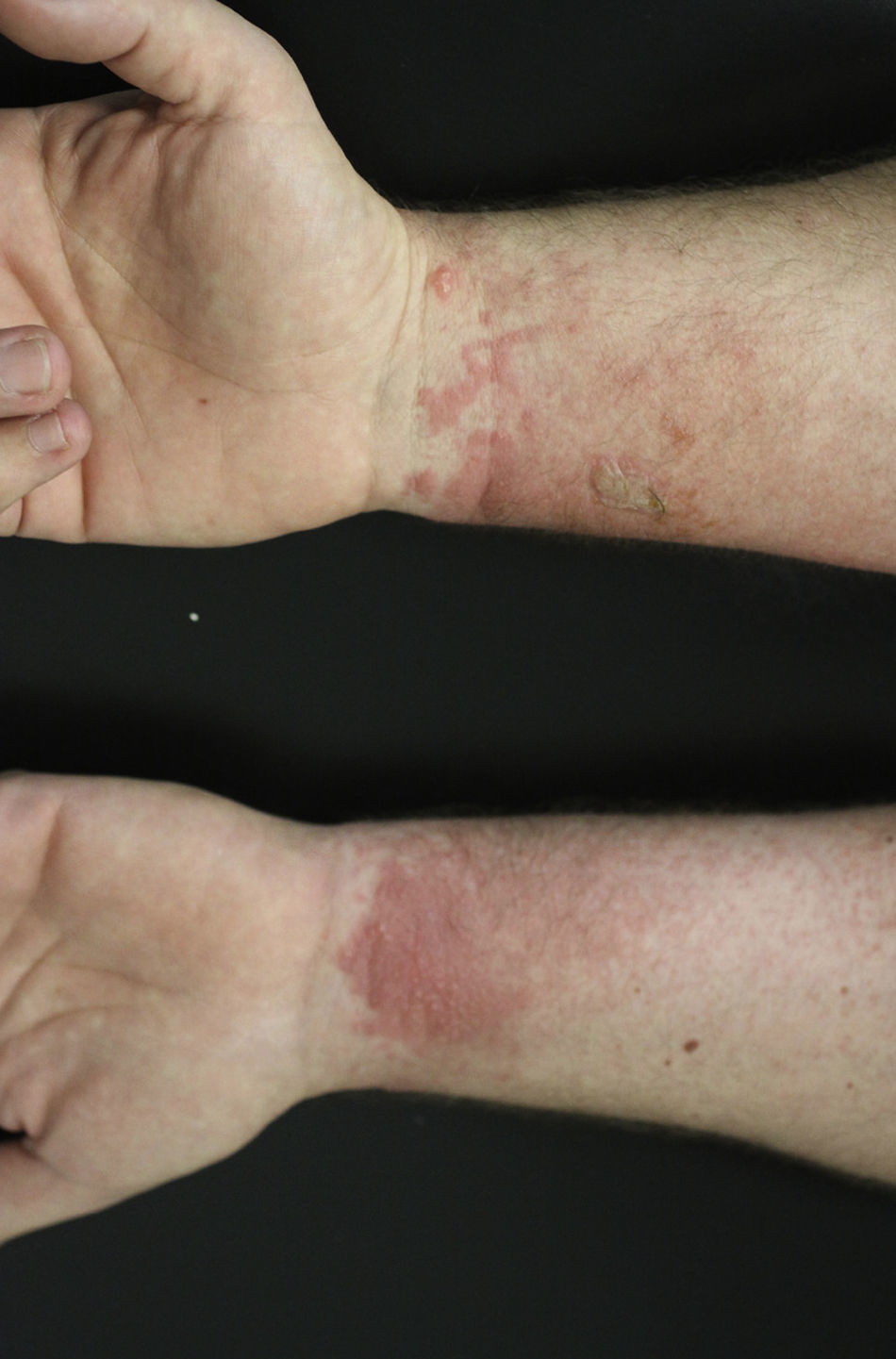
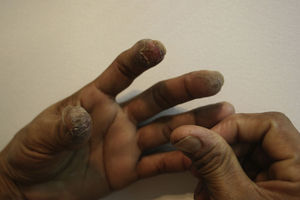
Occupational dermatitis has been frequently reported in the literature. The typical occupational gesture of cutting the bulb, which is held between the thumb and first/second finger of the nondominant hand, while the knife is held in the dominant hand leads to the characteristic distribution of hyperkeratotic eczema on the tips of the first 2 or 3 fingers of the nondominant hand and thumb of the dominant hand123–127 (Fig. 11). The hypersensitivity not always presents with this classical asymmetrical pulpitis, but frequently also as hand eczema (desquamation, fissuring and hyperkeratosis) involving the palm.
Lesions at different locations from those of typical hand eczema have also been described, reflecting a different pattern of exposure, especially after use for medicinal purposes.128 Because of its sensitizing potential and the high degree of irritation (especially under occlusion), the use of garlic as a topical medicinal agent should be discouraged.
Other less typical presentations attributed to garlic include a pemphigus vulgaris-like eruption,123 cheilitis,122 a garlic induced systemic contact dermatitis,126,129 cases of photodermatitis,130 and an occupational airborne ACD from garlic with concurrent type I allergy.131
Allergic contact dermatitis to onion is less widely reported. However, patients with allergic contact dermatitis to onion can be cross-reactive to garlic.132
Patch TestPatch test has been performed either with 50% garlic/onion in arachis oil133 or diallyl disulfide 5% pet134 but actually 1% in petrolatum (Chemotechnique diagnostics®, Malmö, Sweden) is the recommended concentration to be used.
IsocyanatesIsocyanates are a family of highly reactive, low molecular weight compounds identified by the number of NCO (nitrogen–carbon–oxygen) groups they contain. Mono-isocyanates contain 1 group; di-isocyanates (used in polyurethane) contain 2, and so on.
Mono-isothiocyanates, otherwise known as mustard-oils because they are characteristic of and were indeed first isolated from mustard plants (Cruciferae family), occur in a wide variety of higher plants, but above all in the mustard family and its tropical counterpart, the caper family.
Several species of this family yield in their leaves, fruits, seeds and root extracts glucosidic precursors (glucosinolates), which, through enzymatic hydrolysis by an enzyme named myrosinase, produce the isothiocyanates. Naturally occurring isothiocyanates can be either saturated or unsaturated.135 Instauration of the β,γ carbons to the isothiocyanate nitrogen (allyl and benzyl isothiocyanate) may make the thiocayante more immunologically reactive. Compounds with instauration at the β,α positions, such as phenylisothiocyanate, are probably less immunologically reactive and have a greater potential for irritation.
Cruciferae (Cabbage or Mustard Family, Brassicaceae) and Capparaceae FamilyThe Brassicacea family contains about 3200 species in 375 genera. It includes several important vegetables, such as cabbage (Brassica oleracea L), cauliflower (B oleracea L v botrytis), radish (Raphanus sativus L), horse radish (Armoracia rusticana P Gaertn, B Mey, and Scherb), turnip (Brassica campestris L) and mustrard (Brassica nigra [L] WDJ Koch). The Capparaceae, regarded as the tropical relative of the mustard family, comprises some 650 species of small trees and shrubs in 30 genera. The most important representative of this family is Capparis spinosa L, or caper bush, a shrub with grayish oval leaves and showy white-pink flowers with several prominent stamens. It is better known for the edible bud and fruit which are usually consumed pickled.
Several species contain thioglucosides, which are broken down enzymatically, in the presence of water, to isothiocyanates, potent irritants, which appear to be also the most important allergens. Contact dermatitis from these plants can be both irritant and allergic,136,137 with a high potential irritant capacity. Allergic contact dermatitis is rare.
Allergic contact dermatitis is more commonly seen in food handlers. Raphanus sativus L induced an acute vesiculo-bullous dermatitis of both palms in a waitress who chopped them.135 Cabbages provoked occupational contact dermatitis138 and cabbage juice can produce positive patch test reactions in patients with hand dermatitis suspected to have been caused by vegetables.139Brassica nigra induced an allergic contact dermatitis due to mustard powder in a salad maker137 and Brassica oleracea v. Botrytis (cauliflower) induced a hand eczema in a plant grower.140Capparis spinosa L induced an acute vesiculo-bullous allergic contact dermatitis in a woman who applied a wet compress of the minced leaves and fruits to her arm.141
Patch test: Isothiocyanates present in these plants are usually primary irritants, and they can be tested at a concentration of 0.05% in petrolatum. As the irritancy of mustard oil tends to overshadow its allergic potential, a positive patch test with mustard can be difficult to interpret.135
Polyacetylenic DerivatesThe polyacetylenes are a group of chemically reactive and biologically active compounds with a carbon-carbon triple bond or alkynyl functional group. They can be found in 7 families of plants, and the majority has been isolated from the closely related plant families Araliaceae and Apiaceae. The polyacetylenic derivatives of the Araliaceae and Apiaceae are mostly the aliphatic C17-polyacetylenes of the falcarinol-type, derived from oleic acid.142 They act as very reactive alkylating agents towards, for example, thiol groups in proteins, forming hapten-protein complexes (antigens).142,143
AraliaceaeThis family of some 700 species in 55 genera consists mostly of trees and shrubs, but includes some twiners. Most species occur in tropical regions, particularly in Indo-Malaysia and tropical America. Other species are native to temperate regions, and some species have become widely distributed by horticulture and as houseplants.
Perhaps the best known temperate species is Hedera helix L, common ivy. The plants are native to Europe, North Africa, and Asia but are now grown in many other countries. Three main subspecies are found in Europe, namely helix (English ivy), canariensis (Canary Island ivy), and poetarum. They are a family of evergreen climbing plants usually found scrambling over rocks and through hedges or climbing up trees or walls (Fig. 12).
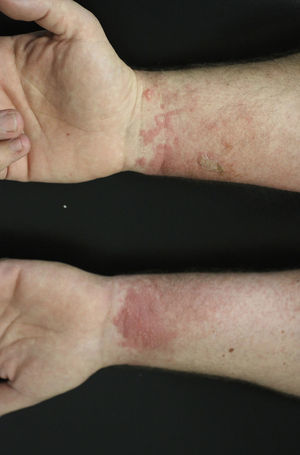
Falcarinol (heptadeca-1,9-diene-4,6-diyne-3-ol) and didehydrofalcarinol are the most important allergens. They are detected in stalks, leaves, and roots and may cause both irritant and allergic contact dermatitis. Falcarinol seems to be the stronger sensitizer and concentration and the ratio didehydrofalcarinol:falcarinol seems to vary considerably, depending on climate and other conditions of growth.144Sensitization can occur through contact with plants on climbing trees covered by them or by pruning or handling the plants in an occupational setting. Any site in contact with the plant may be affected and dermatitis commonly begins with an itching and erythema that in a few days develops into a vesicular eczema (Fig. 13).145 The most frequently affected sites are hands and forearms followed by involvement of the face and neck; however, involvement of trunk and legs as well as widespread dermatitis has also been reported.145,146 Urticarial-like dermatitis has also been reported.147 Immediate, possibly allergic, reactions from common ivy causing rhinitis and asthma have rarely been reported, The majority of mucosal symptoms in gardeners are probably irritant, perhaps caused by hederin.148–150
Patch TestTesting the plant material as is as well as extracts of these carries the risk of false positive reactions. Falcarinol 0.03% and didehydrofalcarinol 0.3% in petrolatum can be used.
ApiaceaeAlso known as Umbelliferae, this family of 2850 species in 275 genera is of cosmopolitan distribution but is found mainly in north temperate regions. Most of the species of dermatological importance are similar in floral structure, with cluster of flowers on stalks of roughly equal length arising from a single point as an umbrella structure. Most of the members of this family have been shown to be rich in psoralenes and therefore produced phototoxic reactions. Some ornamental plants and weeds as well as edible plants of this family have also been related to allergic contact dermatitis. Two important species in dermatology are Centella asiatica and Daucus carota vars.
Centella asiaticaCentella asiatica, formerly named Hydrocotyle asiatica (L) Urban, is a small herbaceous annual plant of the subfamily Mackinlayoideae of the family Apiaceae. It is native to India, Sri Lanka, northern Australia, Indonesia, and other parts of Asia. The fresh and dried leaves as well as the stems of this species have been widely used topically in many medical and dermatological conditions for example on scars and keloids, in plastic surgery, scleroderma, phlebology, slow-healing wounds and chronic leg ulcers among others.151–155
Several commercial topical preparations have been formulated including Blastoestimulin, Centelas, Collaven, Emdecassol, Madecassol, Marticassol, and Trofolastin. Contact allergy to the Centella asiatica extract demonstrated by patch testing has been reported with the topical use of Madecassol,156 Centelase,157–159 and Blastoestimulina.160–164 The potential allergens are madecassic acid, asiatic acid and, asiaticoside, 3 triterpenic acids of high molecular weight and low sensitizing capacity.165
Daucus carota varsDaucus carota, the carrot, is a root vegetable of usually orange color, though purple, red, white, and yellow varieties exist. In early use, carrots were grown for their aromatic leaves and seeds, but they are now cultivated mainly for food.
Allergic contact dermatitis from carrot is well recognized, particularly affecting employees working in canning factories.139,166–168 Leaves and fruits contain falcarinol, an allergen also present in celery (Apium graveolens L) and common ivy (Hedera helix L).168 Hand or fingertip dermatitis is the typical clinical pattern, but involvement of the face has also been reported.169
Patch test: If the oleoresin of carrots is not available for testing, patch testing may be performed with the external surface of an unpeeled raw carrot or with the surface of a slice of carrot.
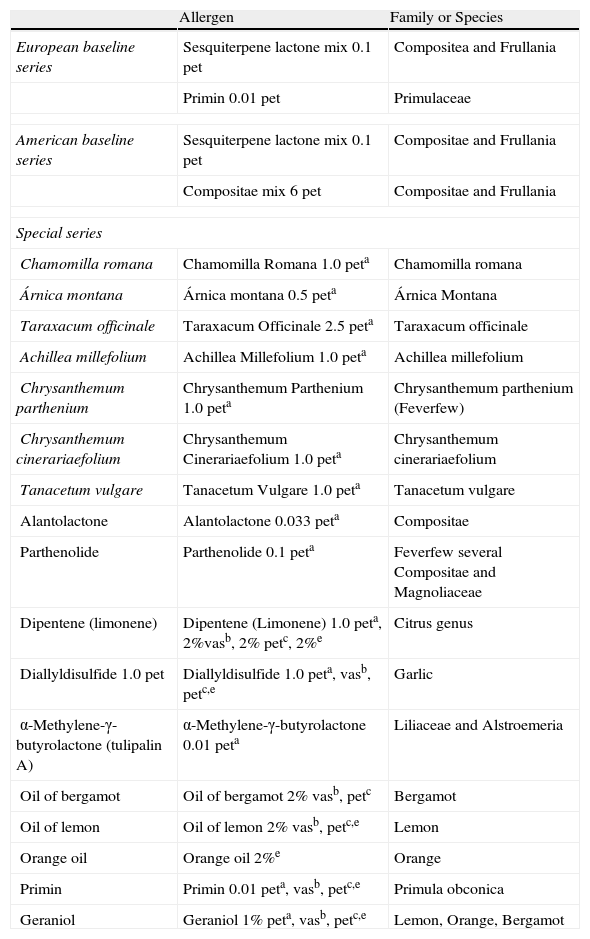
Available Resources to Patch Test PlantsCurrently we have a limited availability of commercial test materials for patch testing plants.
The European baseline series includes sesquiterpene lactone mix at 0.1% in petrolatum for Compositae and Frullania and primin at 0.01% in petrolatum for Primulaceae. In contrast, the American baseline series includes the sesquiterpene lactone mix at 0.1% and the Compositae mix at 6% in petrolatum for Compositae and Frullania.
Special plant series for testing Compositae, Frullania, Liliaceae, Alstroemeriacea, Alliaceae and the citrus genus can be purchased from different European and American suppliers. Table 2 summarizes the currently available allergens. Commercial patch test material for Anacardiaceae, Cruciferae, Capparaceae, Ginkgoaceae, Araliaceae, and Apiaceae are not available.
Available Allergens for Plant Patch Testing.
| Allergen | Family or Species | |
| European baseline series | Sesquiterpene lactone mix 0.1 pet | Compositea and Frullania |
| Primin 0.01 pet | Primulaceae | |
| American baseline series | Sesquiterpene lactone mix 0.1 pet | Compositae and Frullania |
| Compositae mix 6 pet | Compositae and Frullania | |
| Special series | ||
| Chamomilla romana | Chamomilla Romana 1.0 peta | Chamomilla romana |
| Árnica montana | Árnica montana 0.5 peta | Árnica Montana |
| Taraxacum officinale | Taraxacum Officinale 2.5 peta | Taraxacum officinale |
| Achillea millefolium | Achillea Millefolium 1.0 peta | Achillea millefolium |
| Chrysanthemum parthenium | Chrysanthemum Parthenium 1.0 peta | Chrysanthemum parthenium (Feverfew) |
| Chrysanthemum cinerariaefolium | Chrysanthemum Cinerariaefolium 1.0 peta | Chrysanthemum cinerariaefolium |
| Tanacetum vulgare | Tanacetum Vulgare 1.0 peta | Tanacetum vulgare |
| Alantolactone | Alantolactone 0.033 peta | Compositae |
| Parthenolide | Parthenolide 0.1 peta | Feverfew several Compositae and Magnoliaceae |
| Dipentene (limonene) | Dipentene (Limonene) 1.0 peta, 2%vasb, 2% petc, 2%e | Citrus genus |
| Diallyldisulfide 1.0 pet | Diallyldisulfide 1.0 peta, vasb, petc,e | Garlic |
| α-Methylene-γ-butyrolactone (tulipalin A) | α-Methylene-γ-butyrolactone 0.01 peta | Liliaceae and Alstroemeria |
| Oil of bergamot | Oil of bergamot 2% vasb, petc | Bergamot |
| Oil of lemon | Oil of lemon 2% vasb, petc,e | Lemon |
| Orange oil | Orange oil 2%e | Orange |
| Primin | Primin 0.01 peta, vasb, petc,e | Primula obconica |
| Geraniol | Geraniol 1% peta, vasb, petc,e | Lemon, Orange, Bergamot |
aChemotetechnique.
bMarti Tor.
cAllergEAZE.
dTROLAB.
We can confirm that often the study of a specific plant-induced contact dermatitis or photocontact dermatitis needs an accurate research into the published literature. The previous experience developed by our colleagues will help us to avoid unnecessary steps and allow more useful research protocols to be drawn up.
Conclusions and Further ResearchThis review aimed to cover some of the essential aspects of the allergic contact dermatitis induced by plants. The intention of the chemical approach presented was to allow a more rational understanding of the presently confusing cross-reactions between apparently unrelated genera. Plant contact dermatitis tends to be underestimated and misdiagnosed. We lack definitive patch test material and often diagnosis requires an individualized approach using our own material. Plant-induced contact and photocontact dermatitis can be severe and the basis for the development of other inflammatory skin diseases such as chronic actinic dermatosis. Recognition of the problem will help to find the trigger and allow preventive measures to be taken.
Conflict of InterestThe authors declare that they have no conflict of interest.
Please cite this article as: E. Rozas-Muñoz, J.P. Lepoittevin, R.M. Pujol, A. Giménez-Arnau. Allergic Contact Dermatitis to Plants: Understanding the Chemistry will Help our Diagnostic Approach. Actas Dermosifiliogr.2012;103:456-477.