Biologic therapy has represented a major advance in the treatment of moderate to severe psoriasis but its use depends upon the characteristics of the patient and the criteria applied by the dermatologist. The aim of this survey was to determine the criteria employed by dermatologists in the decision to use these drugs.
MethodsA cross-sectional survey was undertaken among Spanish dermatologists with experience in the treatment of moderate to severe psoriasis. The survey comprised 31 items distributed in 5 sections: investigator profile, disease management, treatment of moderate to severe psoriasis, use of biologic drugs, and evaluation of the use of biologic drugs for the treatment of moderate to severe psoriasis.
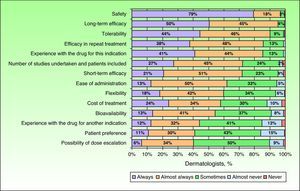
ResultsOne hundred-ninety dermatologists were included in the study. The study participants reported that 31% of patients receiving treatment for moderate to severe psoriasis are treated with biologic drugs. Of those, 28% require a change in treatment at some point due either to lack of activity or the appearance of side effects. Biologic drugs would be administered as monotherapy in 73% of cases. In between 53% and 59% of cases, biologic drugs would be prescribed as continuous treatments. On a scale of 1 to 5, the most valued pharmacological properties by dermatologists were safety (4.8 points; 95% confidence interval [CI], 4.7-4.9), long-term efficacy (4.6 points [4.5-4.7]), and tolerance (4.5 [4.4-4.6]).
ConclusionsDermatologists with experience in the use of biologic drugs employ this treatment option in slightly more than a quarter of cases of moderate to severe psoriasis. In their opinion, the choice of biologic drug should be based on, in order of importance, safety, long-term efficacy, and tolerance.
La terapia biológica ha supuesto un importante avance en el tratamiento de la psoriasis moderada-grave, pero su manejo depende de las características del paciente y del criterio del dermatólogo. El objetivo de esta encuesta fue conocer los criterios utilizados por los dermatólogos para la elección de estos tratamientos.
MétodoEstudio transversal mediante encuesta a dermatólogos con experiencia en psoriasis moderada-grave en España. La encuesta constó de 31 ítems distribuidos en 5 secciones: perfil del investigador, manejo de la enfermedad, tratamiento de la psoriasis moderada-grave, uso de fármacos biológicos y valoración de estos.
ResultadosParticiparon 190 dermatólogos con experiencia en el manejo de fármacos biológicos en psoriasis. En opinión de los participantes, el 31% de los pacientes con psoriasis moderada-grave que está en tratamiento activo utiliza algún fármaco biológico. De estos el 28% en algún momento del tratamiento necesita un cambio de principio activo, motivado principalmente por falta de actividad o aparición de alguna reacción adversa. La administración de los fármacos biológicos sería en monoterapia en el 73% de los casos. Un 53-59% de los dermatólogos administra estos fármacos en tratamiento continuo. Las propiedades farmacológicas mejor valoradas por los dermatólogos fueron: seguridad (4,8 puntos; IC 95%: 4,7-4,9), eficacia a largo plazo (4,6; IC 95% 4,5-4,7) y tolerabilidad (4,5; IC 95%: 4,4-4,6).
ConclusionesLos dermatólogos con experiencia en fármacos biológicos utilizan esta opción terapéutica en algo más de la cuarta parte de los pacientes con psoriasis moderada-grave. En su opinión, el biológico de elección debería ser, por este orden: seguro, eficaz a largo plazo y bien tolerado.
Psoriasis is a chronic erythematous, scaling skin disease that affects between 2% and 3% of white individuals.1 It has a prevalence of 1.4% in the Spanish population.2 The disease has been estimated to account for 8.7% of dermatology consultations,3 and around 25% of patients with a diagnosis of psoriasis have moderate or severe forms of the disease.4 It is usually diagnosed clinically5 and its severity depends on the body surface area (BSA) that is affected.6
Moderate to severe psoriasis is generally treated with systemic drugs and/or phototherapy,7,8 but long-term use can be limited by cumulative toxicity9 and loss of efficacy.10 The introduction of biologic therapies, however, has gone a long way towards overcoming these limitations. Biologic drugs are generally safe and effective,11 have few side effects,12,13 and display little or no interaction with other treatments.14 These drugs are also very versatile, since they can be used alone15 or in combination with other therapies,16 particularly systemic drugs. Nevertheless, treatment of psoriasis should always be individualized.5,17,18
Biologic therapies belong to a group of active proteins that includes monoclonal antibodies, fusion proteins, and recombinant cytokines. In psoriasis, their pharmacology specifically targets the immune mechanisms underlying the pathology of the disease.18 There are currently 4 biologic therapies licensed in Spain for use in patients with psoriasis19: the anti-tumor necrosis factor drugs adalimumab, etanercept, and infliximab, and usterkinumab, a monoclonal antibody against interleukins 12 and 23.18
Good long-term control of psoriasis is essential, particularly in its moderate or severe forms, since it is a highly incapacitating disease that can substantially reduce quality of life in affected patients.20 Dermatology departments are usually responsible for the care of patients with moderate to severe psoriasis.18 Consequently, the experience of specialists in this setting plays a central role in determining the treatment provided. Many clinical practice guidelines are available on the use of biologic drugs in psoriasis.17,18 However, very few studies have addressed the opinions of professionals based on clinical experience.21 Understanding this experience is essential to determining the true picture of how biologic therapies are used to treat moderate to severe psoriasis in Spain.
MethodsStudy DesignA cross-sectional survey was undertaken among dermatology specialists working in hospital outpatient departments and primary or specialist care centers in Spain. Prior to invitation of participants, a list of dermatologists working in public and private settings throughout Spain was prepared. All of the dermatologists included in the study were required to have experience in treating patients with moderate to severe psoriasis as part of their usual clinical caseload, and with the use of biologic therapies for their treatment. Once the complete list had been prepared, a selection was made to include a proportional number of dermatologists from each of Spain's autonomous regions according to the population of each region. In this way, 207 participants were identified and provided with the questionnaire.
Study VariablesData were collected using a questionnaire specifically designed for this study based on a review of the literature. The instrument comprised 31 items distributed in 5 sections (Appendix A): 1) investigator profile (7 items); 2) clinical management of moderate to severe psoriasis (10 items); 3) treatment of moderate to severe psoriasis (6 items); 4) use of biologic therapy in dermatology (5 items); and 5) evaluation of the use of biologic drugs for the treatment of moderate to severe psoriasis (3 items).
The investigator profile included sociodemographic variables (age and gender) and information on professional practice (years of experience, type of health care center). Clinical management of moderate to severe psoriasis was described using variables such as caseload of the dermatologist (number of patients seen each week, percentage of patients with moderate or severe psoriasis, percentage of patients diagnosed in the clinic, and specialty of the diagnosing physician) and follow-up (frequency of follow-up appointments and procedures used to determine severity). Treatment of moderate to severe psoriasis was assessed according to the estimated percentage of patients in active treatment, changes in treatment with biologic therapies (treatment used, type of change, and reason for change), and treatment regimen used (monotherapy or combination therapy, and continuous or intermittent treatment). Specialists were asked to provide their opinions on the use of biologic therapies in moderate to severe psoriasis by evaluating the main attributes of the drugs and the principal factors to be taken into consideration in establishing the treatment regimen. Opinions were provided based on the clinical judgement and experience of the respondents in their daily clinical practice and assessed on a 5-point scale from 1 (never) to 5 (always). A similar scale was used to evaluate possible differences between the use of continuous treatment and intermittent treatment (treatment cycles) (Fig. 1). Although data were collected on the opinion of the dermatologists regarding the drug of choice, it was decided not to present those data here due to the possible bias that could have been introduced.
The biologic drugs considered in the survey were adalimumab, efalizumab (in clinical use at the time the survey was designed), etanercept, and infliximab. Ustekinumab was not included as its marketing approval was considered too recent to allow evaluation of its use in routine clinical practice.19
Prior to the survey, the dermatologists were informed of the aims and methods used and the confidentiality of any data collected. All the respondents provided signed informed consent to their participation in the study.
Statistical AnalysisThe theoretical sample size required to allow analysis of dichotomous variables with a p of 0.5, a precision of 7.5 points, and a statistical significance of P=.05, assuming a nonresponse rate of 15%, was 156 completed questionnaire.
A descriptive analysis of all the study variables was carried out using the statistical package SPSS 17.0 for Windows. Continuous variables were described as means and 95% confidence intervals (CI), along with the number of valid cases (n). Categorical variables were summarized using the total number of cases in each category and the relative frequency compared to the total number of responses.
ResultsOf the 207 questionnaires sent to participants between March and June 2009, a total of 190 were returned completed (92% participation). The dermatologists who responded to the questionnaires were distributed throughout Spain in a manner that was proportional to regional differences in the population of the autonomous communities. Men accounted for 57% of respondents. The mean (95% CI) age was 43 (42-44) years and the mean length of professional experience was 15 (14-16) years. Professional experience of less than 13 years was reported by 45% of the dermatologists surveyed. Ninety-eight percent of the respondents worked in an urban or metropolitan setting while 92% worked in hospitals, although not exclusively (42% also practiced in an outpatient clinic and 60% had a private practice).
The mean number of patients seen weekly by the surveyed dermatologists was 145 (133-157), of which around 8% (7%-9%)—approximately 12 patients—consult for moderate psoriasis and 3% (2%-4%)—approximately 4 patients—consult for severe psoriasis. The majority of patients (60% [57%-63%] of those with moderate psoriasis and 65% [61%-69%] of those with severe psoriasis) have already been diagnosed with psoriasis when they are seen by the dermatologists. The diagnosing physicians are primary care physicians in 50% of these cases and dermatologists in 47%. Follow-up of patients was 3 monthly according to 71% of the dermatologists surveyed, and the main measures of disease severity were the psoriasis area and severity index (PASI), used by 97% of dermatologists, and the BSA affected, used by 78% of dermatologists.
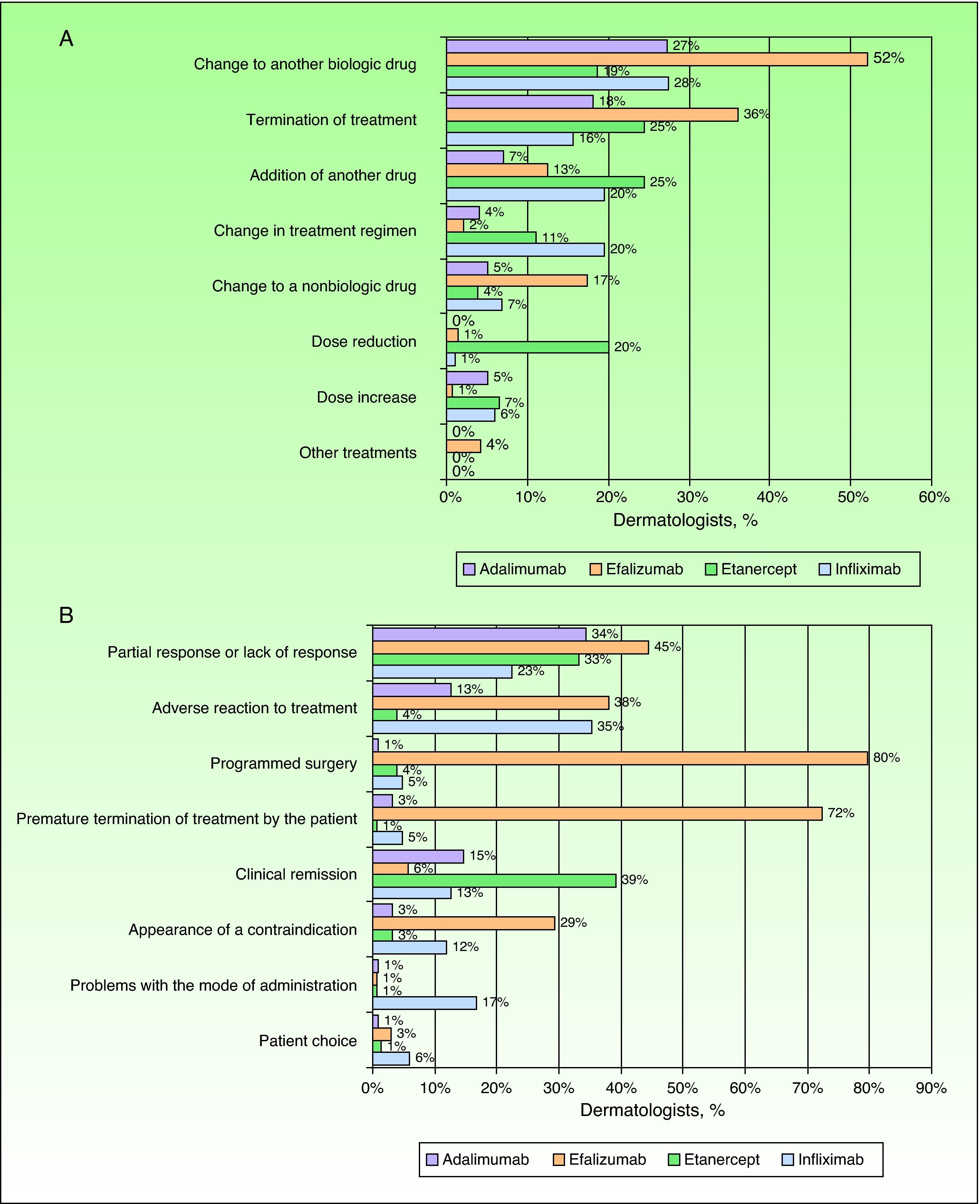
The dermatologists reported that 90% (88%-92%) of patients with moderate to severe psoriasis who attend the clinic are receiving active treatment, and of those, 31% (28%-34%) receive biologic drugs. According to the experience of the surveyed specialists, a change of drug is required in around 28% (25%-31%) of patients receiving biologic therapy. This change would mainly involve substitution of the prescribed therapy to another biologic drug, especially in the case of efalizumab (now withdrawn), for which 52% of specialists had prescribed this type of treatment change (Fig. 2A). Dosage changes were only notable in the case of etanercept, with 20% of dermatologists reporting that they would reduce the dose during treatment. The main reason for changes in biological therapy was inadequate treatment response or the occurrence of adverse events (Fig. 2B). Especially noteworthy were the reasons given for changes in treatment with efalizumab, namely programmed surgery (80%) and premature termination of therapy by the patient (72%). Only 9 dermatologists (5%) mentioned withdrawal of marketing authorization for the drug.
In their day-to-day clinical practice, most of the dermatologists surveyed (73%; between 67% and 79% according to the drug considered) would administer biologic drugs as monotherapy to treat moderate to severe psoriasis. The most common treatment regimen was continuous treatment, with more than 50% of dermatologists (between 53% and 59% according to the drug under consideration) stating that they used it “always” or “almost always”. Intermittent treatment would be used always or almost always with etanercept by 58% of the dermatologists surveyed, whereas less than 20% of the dermatologists would follow such a regimen with the other drugs considered.
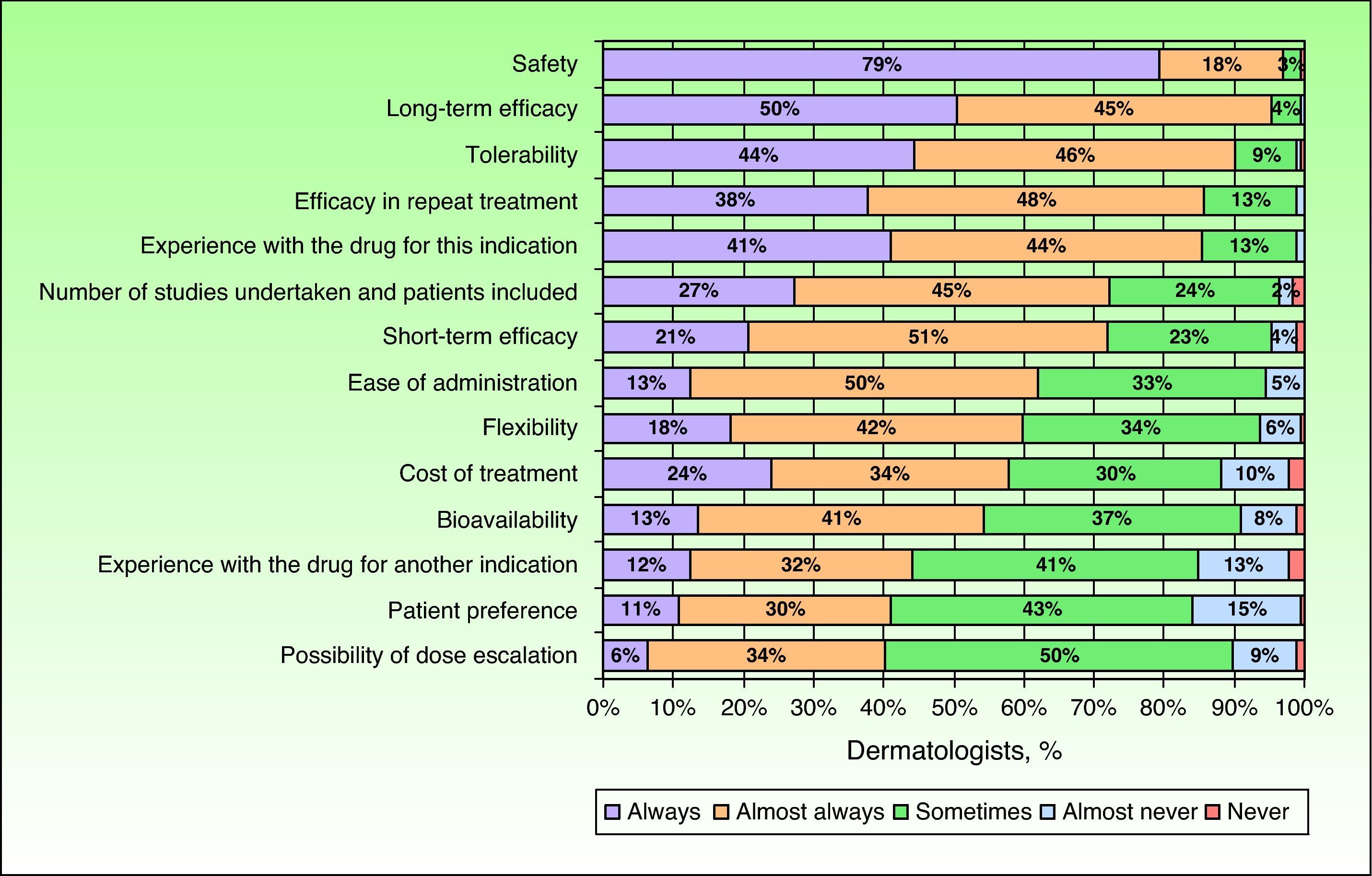
In the opinion of the dermatologists surveyed, an optimal biologic drug should have a good safety profile, long-term efficacy, and good tolerability. These attributes were evaluated as having a mean score on a scale of importance from 0 to 5 of 4.8 (4.7-4.9), 4.6 (4.5-4.7), and 4.5 (4.4-4.6), respectively. These same attributes were also given more consideration when choosing a treatment regimen for patients with moderate to severe psoriasis in clinical practice. Of particular importance for the dermatologists surveyed was the safety of the drug, which was always considered by 79% of the specialists when choosing the most appropriate treatment (Fig. 3).
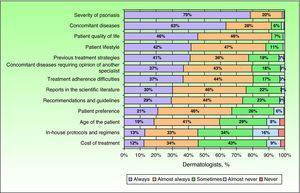
When establishing a treatment regimen, the participating dermatologists stated that, in addition to the properties of the biologic drug, they always considered other factors such as the severity of psoriasis (79%) or the presence of concomitant disease (63%) (Fig. 4). Other factors that were always taken into account by the majority of specialists consulted in such cases were the likelihood of pregnancy (68% and 70% of dermatologists in the case of intermittent and continuous treatment, respectively) and risk of infection (62% and 69% in the case of intermittent and continuous treatment, respectively). No notable differences were observed between the priorities considered by the surveyed dermatologists when it came to choosing a particular therapeutic regimen. In addition, most of the respondents (81%) reported that they always or almost always followed the recommendations included in clinical practice guidelines; they were also influenced by prescription habits (72%) and the specifications of the package insert (71%). In 12% of cases, the dermatologists reported being always or almost always influenced by the web page of the product.
DiscussionThis study used a survey to collect data on the main criteria used by Spanish dermatologists to determine the treatment of choice with biologic drugs for patients with moderate to severe psoriasis. These criteria are based on the experience of specialists in day-to-day clinical practice and are informed by the need to tailor these treatments to each patient.18 According to the results obtained, 11% of patients seen by dermatologists consult for moderate or severe psoriasis. This is a much larger proportion than that reported in the literature, which indicates percentages of 9% for patients with psoriasis and 2% for patients with moderate to severe psoriasis.3,22 This difference could be due to 2 main factors. On the one hand, it may be explained by an increasing incidence of this disease23 combined with the fact that surveyed dermatologists were required to have sufficient experience in the use of biologic drugs, which are employed in moderate or severe forms of the disease.5,18 On the other hand, in agreement with previous studies,22 most of the patients consulting a dermatologist would already have been diagnosed in primary care or by another dermatologist, and this could mean that the patients referred to the dermatologists surveyed would have a more advanced stage of the disease; this would support the findings mentioned above. Nonetheless, considering the methods used, in which dermatologists were asked in a questionnaire to estimate the percentage of patients they saw with moderate to severe psoriasis based on their clinical experience, it cannot be ruled out that they might have overestimated this proportion.
There was a notably high percentage of specialists who reported using PASI or BSA to assess disease severity. It has been suggested that, although these measures are the standard method for assessing the severity of psoriasis in clinical trials,24,25 they are used much less frequently in day-to-day clinical practice.22,26 This increase in the use of PASI and BSA could be related to the higher percentage of patients with moderate to severe disease seen by the participating specialists, or indicate a tendency towards standardization by those professionals due to the large number of studies addressing moderate to severe psoriasis that have been reported in recent years.
According to the surveyed dermatologists, almost all of the patients with moderate to severe psoriasis (90%) who are seen in the clinic are in active treatment. This finding is consistent with clinical practice guidelines on the management of these patients.5 The information provided by dermatologists in this study indicates that the use of biologic therapy in these patients has been increasing in recent years. Based on data from previous studies,26 the use of these drugs in patients with moderate to severe psoriasis in Spain would have increased from 23% to 31%. This proportion, however, would still be lower than that reported in other countries.27 The treatment regimen preferred by the surveyed dermatologists was monotherapy and, wherever possible, continuous treatment. Nevertheless, more than half of the respondents (58%) would also consider intermittent treatment in the case of etanercept.
Unlike with conventional treatments,28 only 28% of patients would require a change in biologic therapy at some point during treatment according to the surveyed dermatologists. The most common reasons for this change would be a lack of treatment response or the appearance of adverse effects, and the solution would generally involve changing to another biologic drug (especially in the case of efalizumab). When the study was designed, efalizumab was still marketed in Spain. Due to safety concerns, however, marketing authorization was withdrawn on February 19, 2009.19 This withdrawal would be the main explanation for the finding that efalizumab had the highest rate of treatment modification involving substitution of another biologic drug.
With the exception of efalizumab, biologic drugs have been shown to have a good safety profile with few adverse effects,21 qualities that are valued highly by dermatologists seeking to improve the quality of life of patients with moderate to severe psoriasis.26 Consistent with this, the main properties considered by the surveyed dermatologists to be important when choosing a biologic drug would be, in order of priority, safety, long-term efficacy, and tolerability. It is not surprising, then, that one of the main criteria used to choose a particular drug was, in addition to the severity of psoriasis, the presence of concomitant disease. It is known that most biologic drugs are a good treatment option in patients with comorbidities.16 In addition, most of the surveyed dermatologists reported that they always took into consideration the possibility of pregnancy in women treated with biologic drugs; while they are not recommended during gestation, these drugs may be used with caution in women of fertile age.29 The use of biologic drugs, however, also requires special precautions such as assessment of the risk of infection,18 an observation that was confirmed by most of the specialists surveyed in this study.
Given the nature of this study, designed as an opinion survey, the data presented here should be interpreted with caution. Differences in the practice settings of the 190 participating dermatologists could explain the high degree of variability in the results obtained. Nevertheless, the results represent an important source of information given that they are based on the professional experience and day-to-day clinical practice of the dermatologists surveyed. In order to obtain more objective responses, methods were used to guarantee that participants were free to choose their responses. All of the questionnaires were completed anonymously and without supervision. This was communicated in the information sheet provided to all of the participating dermatologists and also in the informed consent form which they subsequently signed. In addition, the possibility of regional bias was addressed by ensuring that the participating dermatologists were distributed in a proportion that reflected the general population of the different Spanish regions.30 Furthermore, the questionnaire was completed by specialists working in both hospital and outpatient settings, independently of the funding source (public or private).
The results obtained in this study are of widespread interest, since opinion surveys based on the clinical experience of specialists yield data on real-life decision making that are not reflected in patient records. Nevertheless, it would also be of interest to compare data based on the professional opinion of the specialists with clinical data contained within patient records.
ConclusionsBiologic therapy is undoubtedly an important treatment option for patients with moderate to severe psoriasis. This is confirmed by a large number of reports in the scientific literature. In Spain, according to the opinion of dermatologists with experience in the use of these drugs, slightly more than a quarter of all patients with moderate to severe psoriasis are treated with biologic drugs. Although these findings are derived from an opinion survey and therefore cannot be extrapolated to the general situation, comparison with similar studies indicates that the use of biologic drugs in patients with moderate to severe psoriasis has increased in recent years.
In the opinion of the majority of the specialists consulted, biologic drugs should display, in order of importance, a good safety profile, long-term efficacy, and good tolerability.
FundingThis study was supported by a grant from Wyeth Farma, S.A. (now Pfizer) and received technical and logistic support from IMS Health.
Conflicts of InterestDr Ara has participated in advisory boards for Pfizer, Janssen, and Abbott. He has also received speaker's fees from the following pharmaceutical companies: Abbott, Janssen, MSD, and Pfizer. Dr Pérez reports that she has no conflicts of interest. Dr Ferrando has given presentations for Abbott and Janssen. He has also been involved in studies for Pfizer.
Unvalidated translation of the questionnaire, provided only for purposes of understanding the present study.
- I.
Investigator Profile
- 1.
Age
- 2.
Gender
- 3.
Number of years as a dermatology specialist
- 4.
Spanish autonomous community in which you practice
- 5.
Practice setting: rural, urban, metropolitan
- 6.
Type of center/s in which you practice: hospital, outpatient clinic, private clinic
- 7.
Is the center in which most of your work is undertaken a referral center or are patients referred to other centers according to their symptoms?
- 1.
- II.
Clinical Management of Moderate to Severe Psoriasis
- 1.
Indicate the average number of dermatology patients you see each week in your clinic (indicate the sum of the averages for each center if you work in more than one).
- 2.
Of the total number of patients that you see each week for dermatology consultations, indicate the approximate percentage that consults for moderate psoriasis (2%-10% of BSA affected or PASI score of between 21 and 50) or severe psoriasis (>10% of BSA affected or PASI score of between 51 and 72).
- 3.
Of the total number of patients with a diagnosis of moderate or severe psoriasis, indicate the approximate percentage distribution according to severity.
- 4.
Of the total number of patients with moderate or severe psoriasis, indicate the approximate percentages that are diagnosed in your clinic and that consult having already received a diagnosis.
- 5.
Of the group of patients with moderate or severe psoriasis who consult with an established diagnosis, indicate who has normally made the diagnosis.
- 6.
Indicate the approximate percentage of patients with moderate to severe psoriasis who are receiving specific treatment for their condition.
- 7.
Indicate the percentage of patients with moderate to severe psoriasis who consult after treatment has been prescribed by another physician. Of those patients who are already receiving treatment, what percentage is treated with biologic drugs?
- 8.
Indicate which health care professional is normally responsible for this prescription: primary care physician, specialist, or other.
- 9.
Indicate the frequency of follow-up appointments usually attended by patients with moderate to severe psoriasis: 3 monthly, 6 monthly, yearly, 2 yearly, or other.
- 10.
Indicate the procedures used in your day-to-day clinical practice for follow-up and evaluation of the severity of psoriasis (choose from the following options): BSA, PASI, Physician's Global Assessment, health-related quality of life questionnaires (eg, Dermatology Life Quality Index), none, or others.
- 1.
- III.
Treatment of Moderate to Severe Psoriasis
- 1.
Of the total number of patients who consult for moderate or severe psoriasis, indicate the proportion who receive active treatment.
- 2.
Of the total number of patients with moderate to severe psoriasis who are receiving active treatment, indicate the proportion who are treated with biologic drugs.
- 3.
Of the total number of patients with moderate to severe psoriasis who are treated with biologic drugs, indicate the proportion treated with each of the following drugs: adalimumab, efalizumab, etanercept, and infliximab.
- 4.
Based on your experience as a dermatologist treating patients with moderate or severe psoriasis using biologic drugs:
- 4.1.
What is the estimated proportion of patients in whom a change of treatment has been necessary?
- 4.2.
On the occasions when it has been necessary to change treatment, indicate which of the following changes have been used most frequently: suspension or withdrawal of treatment, change of treatment to another biologic drug, change of treatment to a conventional drug, addition of another drug, increase in dose, change of treatment regimen (frequency of administration), or other.
- 4.3.
When a change in treatment has been required, indicate the main cause: an adverse reaction to treatment, inadequate treatment response, problems with the mode of administration, clinical remission, programmed surgery, premature termination of treatment by the patient, appearance of a contraindication, patient choice, or other.
- 4.1.
- 5.
According to your experience as a dermatologist treating patients with moderate to severe psoriasis using biologic drugs:
- 5.1.
Do you most often prescribe monotherapy or combination therapy?
- 5.2.
Do you most often prescribe an intermittent or continuous treatment regimen?
- 5.1.
- 6.
In your opinion, based on your experience as a dermatologist in the treatment of patients with moderate to severe psoriasis:
- 6.1.
Indicate a maximum of 3 advantages and 3 disadvantages associated with an intermittent treatment regimen.
- 6.2.
Indicate a maximum of 3 advantages and 3 disadvantages associated with a continuous treatment regimen.
- 6.1.
- 1.
- IV.
Use of Biologic Drugs in Dermatology
- 1.
According to your personal opinion and clinical experience in the treatment of patients with moderate to severe psoriasis, which of the following are the main attributes that an optimal biologic drug should have for the treatment of this condition? Short-term efficacy (speed of action), long-term efficacy (sustained response), efficacy in repeat treatment (tolerability), bioavailability, ease of administration, flexibility (option to use either intermittent or continuous regimens), safety, possibility of dose escalation, number of clinical trials undertaken and number of patients included in the trials, patient preference, experience with using the drug for this indication, experience with using the drug for other indications, and cost of treatment.
- 2.
Bearing in mind the limitations of the existing health care system, according to your day-to-day clinical practice with patients who have moderate to severe psoriasis, which of the following attributes do you consider in your practice when establishing treatment with a biologic drug for this condition? Short-term efficacy (speed of action), long-term efficacy (sustained response), efficacy in repeat treatment (tolerability), bioavailability, ease of administration, flexibility (option to use either intermittent or continuous regimens), safety, possibility of dose escalation, number of clinical trials undertaken and number of patients included in the trials, patient preference, experience of using the drug for this indication, experience of using the drug for other indications, and cost of treatment.
- 3.
According to your personal opinion and clinical experience in the treatment of patients with moderate to severe psoriasis, which of the following factors do you usually take into account when prescribing treatment with a biologic drug? Age of the patient, severity of psoriasis (site and extent of psoriasis), concomitant diseases, concomitant diseases that require the opinion of another specialist (eg, a rheumatologist), quality of life of the patient, patient lifestyle (occupation, possibility of pregnancy, alcohol consumption, etc.), difficulty to adhere to the treatment regimen and access to medication, previous treatment strategies, cost of treatment, opinion of the patient, protocols and guidelines used in the center/hospital, recommendations of clinical practice guidelines, and the scientific literature.
- 4.
According to your personal opinion and clinical experience in the treatment of patients with moderate to severe psoriasis, to what extent should the following patient characteristics be taken into consideration when choosing between an intermittent or continuous treatment regimen? Age of the patient, current history of alcoholism, patient quality of life, occupational impact, hygiene and dietary measures, concomitant diseases, concomitant diseases requiring the opinion of another specialist (eg, a rheumatologist), concomitant treatments, disease course, signs of renal or liver failure, adherence to treatment, likelihood of pregnancy, risk of infection, and frequency of travel.
- 5.
According to your personal experience and day-to-day clinical practice, when one or another biologic drug is selected, to what extent do the following factors influence the decision? Opinion of other dermatologists (conferences, internal meetings, etc.), internal departmental or hospital protocols, recommendations in clinical practice guidelines, the package insert of the drug, news in medical circulars and bulletins, information provided by pharmaceutical companies (company representatives, circulars, etc.), usual prescribing habits, and web page of the product.
- 1.
- V.
Evaluation of Biologic Drugs for the Treatment of Moderate to Severe Psoriasis
- 1.
According to your professional experience in the treatment of moderate to severe psoriasis, which of the following attributes would describe a drug of choice? Short-term efficacy (speed of action), long-term efficacy (sustained response), efficacy in repeat treatment (tolerability), bioavailability, ease of administration, flexibility (option to use either intermittent or continuous regimens), safety, risk of adverse events, cost of treatment, possibility of combination therapy, possibility of dose escalation, confidence in the product, and confidence in the pharmaceutical company.
- 2.
Taking into account the attributes of the biologic drugs used in the treatment of moderate to severe psoriasis, in your opinion, compared with the other biologic drugs, is etanercept better, as good, or worse in relation to the following criteria? short-term efficacy (speed of action), long-term efficacy (sustained response), efficacy in repeat treatment (tolerability), bioavailability, ease of administration, flexibility (option to use either intermittent or continuous regimens), safety, risk of adverse events, cost of treatment, possibility of combination therapy, possibility of dose escalation, confidence in the product, and confidence in the pharmaceutical company.
- 3.
In relation to the new biologic drug that is about to be marketed in Spain:
- 3.1.
Were you aware of the upcoming launch of ustekinumab in Spain?
- 3.2.
How did you become aware of this new biologic drug?
- 3.3.
Based on the information you have received about ustekinumab, how does it compare with existing biologic drugs?
- 3.4.
Based on the information you have received about ustekinumab, how valuable would you consider it for the treatment of your patients with moderate to severe psoriasis?
- 3.5.
Based on the information you have received about ustekinumab, do you think you would use it in your daily clinical practice for the treatment of moderate to severe psoriasis?
- 3.6.
Based on the information you have received on ustekinumab, what is the likelihood that it will substitute existing biologic drugs for the treatment of patients with moderate to severe psoriasis in your practice?
- 3.1.
- 1.
Unvalidated translation of the questionnaire, provided only for purposes of understanding the present study.
Please cite this article as: Ara M, et al. Encuentas a dermatólogos sobre terapiaq biológica en pacientes con psoriasis moderada grave en España. Actas Dermosifiliogr.2011;102:706-716.