The eighth edition of the staging manual of the American Joint Committee on Cancer incorporates important changes in the classification of skin cancers. Coming 40 years after the first edition, the latest manual preserves its specific system for Merkel cell carcinoma and takes into account recent publications on the prognosis of squamous cell carcinoma by defining a completely new T category for this neoplasm. Staging for squamous cell carcinoma considers head and neck tumors (excluding the eyelid) and does not offer solutions for other sites except the vulva, penis, and perianal region. Regarding melanoma, use of the mitotic index has been eliminated and the prognosis of the primary tumor is based on Breslow thickness and ulceration. In addition, thickness is now recorded to an accuracy of 0.1mm, and the T0 concept has been introduced to define those metastatic melanomas in which the primary tumor has regressed completely. In this new edition, changes have also been made to the N category of all the skin cancer staging systems, and M1d has been added to the M category for melanoma to refer to metastatic involvement of the central nervous system, which, up to now, had been included in the M1c category. This new system will need to be validated with patient series to determine if it adequately satisfies the objective of tumor risk stratification.
Cuarenta años después de la publicación de la primera edición de la estadificación del American Joint Committee on Cancer, la octava edición se publica con cambios relevantes en el cáncer de piel. En el cáncer cutáneo no melanoma el American Joint Committee on Cancer mantiene un enfoque específico para el carcinoma de células de Merkel y tiene en cuenta algunos trabajos publicados recientemente sobre el pronóstico del carcinoma epidermoide cutáneo en la definición de una categoría T completamente nueva para este tumor. Por otra parte, la estadificación se contempla para el carcinoma epidermoide cutáneo de cabeza y cuello (excluyendo el párpado) y en otras localizaciones; únicamente ofrece soluciones para la estratificación de tumores de vulva, pene y región perineal. En relación con el melanoma, el valor del índice mitótico desaparece y el pronóstico del tumor primario se define basándose en el espesor de Breslow y la ulceración. Además, el espesor pasa a registrarse con una precisión de 0,1mm y aparece el concepto de T0 para los melanomas metastásicos en los que el primario ha regresado completamente. Existen diferencias en la categoría N de todos los sistemas de estadificación de cáncer cutáneo en esta nueva edición, y en relación con la categoría M, en el melanoma aparece la categoría M1d para hacer referencia a la afectación metastásica del SNC, que hasta el momento se incluía dentro de la categoría M1c. Será necesario validar este nuevo sistema con series de pacientes para valorar si efectivamente cumple con el objetivo de estratificar por riesgo los tumores de una manera adecuada.
Forty years after the publication by the American Joint Committee on Cancer (AJCC) of the first edition of its TNM staging manual1 the eight version has been published with substantial changes in the case of skin cancer.2 In nonmelanoma skin cancer (NMSC), the new manual preserves a specific system for Merkel cell carcinoma (MCC) and takes into account certain studies on the prognosis of squamous cell carcinoma (SCC) published in recent years, although SCC and other forms of NMSC (with the exception of MSC) still use the same system. That is, SCC and basal cell carcinoma are staged in the same way. In the case of melanoma, the mitotic index is now longer used as a modifier of the T category, which is stratified only by the Breslow thickness and the presence of ulceration.
Nonmelanoma Skin CancerSince the first edition of the manual in 1977,1 major progress has been made in assessing NMSC prognosis, leading to progressive changes in successive editions up until the current edition, published in 20172 and to be implemented in clinical practice on January 1, 2018. In reality, up to the sixth edition of the AJCC TNM staging manual,2 and despite several relevant studies in the 1990s,4–19 the changes in the T category of NMSC have been very subtle. With small differences between some of the systems, basically the tumor was considered T1 if its greater dimension measured less than 2cm, T2 if it measured between 2 and 5cm, T3 if it measured more than 5cm, and T4 if bone, muscle, or cartilage invasion was present.1,11–14 Studies were published that recognized the influence of different factors on SCC prognosis.15–23 Some of these studies explicitly highlighted the need for an improvement in the staging system.18,21
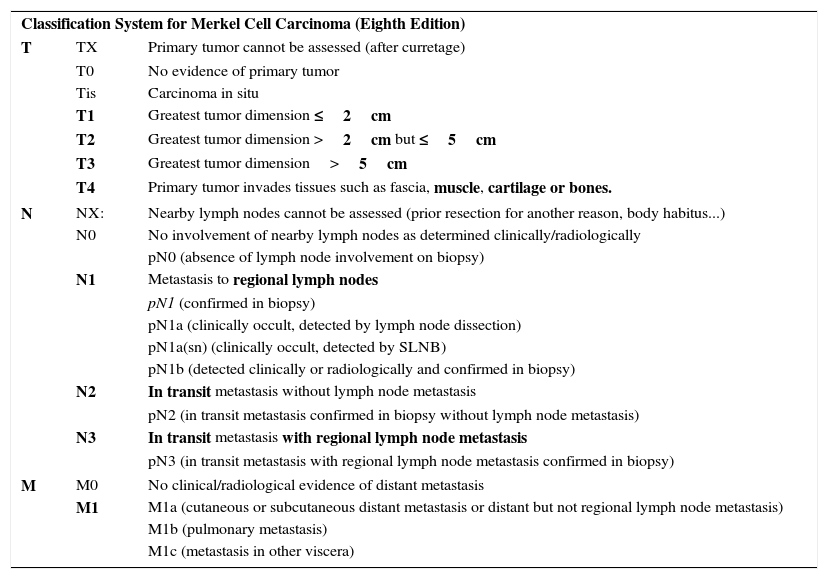
Merkel Cell CarcinomaIn 2010, the seventh edition of the AJCC TNM staging manual incorporated significant novelties in NMSC classification.24 For the first time, a separate staging system was introduced for Merkel cell carcinoma (MCC), which until then had been classified with the same staging system as other NMSC. This means that all forms of NMSC other than MCC should be classified according to the criteria for SCC. TNM classification for MCC has been modified in the most recent edition2 in light of a study that assessed clinical outcomes in a large series of patients.25 In reality, T categorization has not been changed, but there are significant changes in N categorization and stratification of stages I-IV.2,25 Furthermore, clinical and pathologic staging are separated out in this eight edition of the manual.2,25 In the T category, MCC is classified as T1 if it measures 2cm or less in the largest dimension, T2 if it measures between 2 and 5cm in the largest dimension, T3 if it measures more than 5cm, and T4 if it invades deep structures (bone, muscle, fascia, or cartilage).2,25
Within the N category, a tumor is considered N1 if metastasis to the regional lymph nodes is present, N2 if in transit metastasis is present without metastasis to the regional lymph nodes, and N3 if both in transit and lymph node metastases are present. With regards the corresponding pathologic staging within the N category, a distinction is made between the N1a subtype (clinically occult, detected by lymph node dissection), N1a(sn) subtype (clinically occult, detected by selective lymph node biopsy), and N1b subtype (detected clinically and radiologically and confirmed by biopsy). In the case of the M category, M1a is used for skin or distant subcutaneous involvement (not in transit metastasis, which would be covered within the N category) or involvement of distant lymph nodes (not regional ones); category M1b refers to lung involvement and M1c to involvement of viscera other than the kidney. If microscopic confirmation is obtained, the tumor is considered pM1, with its corresponding subclassification. Table 1 summarizes the current staging system for MCC.
Eighth Edition of the AJCC TMN Staging Manual: Merkel Cell Carcinoma.
| Classification System for Merkel Cell Carcinoma (Eighth Edition) | ||
| T | TX | Primary tumor cannot be assessed (after curretage) |
| T0 | No evidence of primary tumor | |
| Tis | Carcinoma in situ | |
| T1 | Greatest tumor dimension ≤2cm | |
| T2 | Greatest tumor dimension >2cm but ≤5cm | |
| T3 | Greatest tumor dimension>5cm | |
| T4 | Primary tumor invades tissues such as fascia, muscle, cartilage or bones. | |
| N | NX: | Nearby lymph nodes cannot be assessed (prior resection for another reason, body habitus...) |
| N0 | No involvement of nearby lymph nodes as determined clinically/radiologically | |
| pN0 (absence of lymph node involvement on biopsy) | ||
| N1 | Metastasis to regional lymph nodes | |
| pN1 (confirmed in biopsy) | ||
| pN1a (clinically occult, detected by lymph node dissection) | ||
| pN1a(sn) (clinically occult, detected by SLNB) | ||
| pN1b (detected clinically or radiologically and confirmed in biopsy) | ||
| N2 | In transit metastasis without lymph node metastasis | |
| pN2 (in transit metastasis confirmed in biopsy without lymph node metastasis) | ||
| N3 | In transit metastasis with regional lymph node metastasis | |
| pN3 (in transit metastasis with regional lymph node metastasis confirmed in biopsy) | ||
| M | M0 | No clinical/radiological evidence of distant metastasis |
| M1 | M1a (cutaneous or subcutaneous distant metastasis or distant but not regional lymph node metastasis) | |
| M1b (pulmonary metastasis) | ||
| M1c (metastasis in other viscera) | ||
| AJCC TMN Staging System for Merkel Cell Carcinoma (Eighth Edition) | |||
| Tis | N0 | M0 | Stage 0 |
| T1 | N0 | M0 | Stage I |
| T2-3 | N0 | M0 | Stage IIA |
| T4 | N0 | M0 | Stage IIB |
| T1-4 | N1a(sn) or N1a | M0 | Stage IIIA |
| T0 | N1b | M0 | Stage IIIB |
| T1-T4 | N1b-N3 | M0 | Stage IIIC |
| T0-4 | N0-N3 | M1 | Stage IV |
We have taken into account pathologic staging. In boldface, aspects most relevant to staging.
Abbreviation: SLNB, sentinel lymph node biopsy
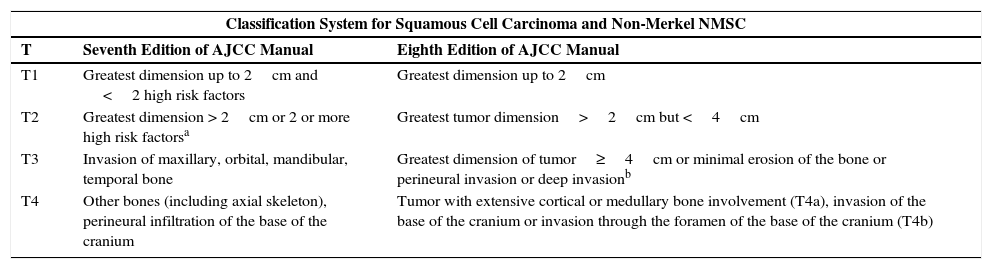
In the case of the staging system for SCC, the seventh edition included a series of high risk factors that permitted modification of the T category of a primary tumor regardless of the horizontal size (this represented an important advance compared with previous editions) (Table 2).24 Specifically, the manual recognized poor degree of histological differentiation, perineural infiltration, vertical depth of more than 2mm, Clark levels iv and v, and the involvement of the ear or lower lip, along with the horizontal size of the tumor as risk factors defining the T category of the primary tumor: a) a tumor measuring <2cm with <2 risk factors was T1; b) a tumor measuring >2cm or measuring <2cm with 2 or more risk factors was considered T2; c) T3 or T4 corresponded to bone invasion: T3 corresponded to invasion of the maxillary, mandibular, orbital, or temporal bones, while T4 corresponded to invasion of other bones or perineural infiltration of the base of the cranium.24 However, not all risk factors were included and, as validation studies were performed, deficiencies emerged. The main criticism was that the T2 category encompassed a range of heterogeneous patients in terms of risk and prognosis.26–31 The seventh edition of the staging manual seemed to focus on the poor prognosis of SCC in T2 tumors.32,33 In fact, in a cohort of 1818 patients, only 6 cases of T3-T4 tumors were detected and most adverse outcomes occurred in tumors classified as T2 (72% of local relapses, 82% of lymph node metastases, and 67% of disease-specific deaths).32
Comparison of the Seventh and Eighth Editions of the American Joint Committee on Cancer (AJCC) Staging Manual: Squamous Cell Carcinoma and Nonmelanoma Skin Cancer (NMSC) Other Than Merkel Cell Carcinoma Differences in the 4 T Categories.
| Classification System for Squamous Cell Carcinoma and Non-Merkel NMSC | ||
| T | Seventh Edition of AJCC Manual | Eighth Edition of AJCC Manual |
| T1 | Greatest dimension up to 2cm and <2 high risk factors | Greatest dimension up to 2cm |
| T2 | Greatest dimension > 2cm or 2 or more high risk factorsa | Greatest tumor dimension>2cm but <4cm |
| T3 | Invasion of maxillary, orbital, mandibular, temporal bone | Greatest dimension of tumor≥4cm or minimal erosion of the bone or perineural invasion or deep invasionb |
| T4 | Other bones (including axial skeleton), perineural infiltration of the base of the cranium | Tumor with extensive cortical or medullary bone involvement (T4a), invasion of the base of the cranium or invasion through the foramen of the base of the cranium (T4b) |
Thickness greater than 2mm, poor degree of differentiation, perineural infiltration, involvement of the ear or lower lip, Clark level iv-v invasion.
Deep invasion defined as thickness greater than 6mm or invasion deeper than subcutaneous fat. For a tumor to be classified as T3, perineural invasion should be present in nerves greater than 0.1mm, deeper than the dermis, or clinical and radiological involvement of affected nerves without involvement or invasion of the base of the cranium.
The eight edition of the AJCC2 seems to have taken into account some studies that had focused, on the one hand, on assessing the influence of thickness on prognosis,21 and on the other the influence of thickness of the involved nerve branch that infiltrates the tumor and the depth of invasion of the SCC32 for stratification of the T category (Table 2). N stratification is based on the system shared with other head and neck tumors. Moreover, for the first time, SCC of the head and neck is given its own independent section (in previous editions, NMSC referred in general to any site) and includes SCC of the lower lip given its relationship with UV radiation. However, it excludes a site on the eyelid and all tumors located outside the head and neck. Although for the special sites of the eyelid, vulva, penis, and perineal region, the AJCC manual offers solutions, it does not define any system for classification of tumors located outside the area of the head and neck, and so does not cover these particular sites. This is clearly a drawback and means, for example, that an SCC on the dorsum of the hand cannot be classified according to the eighth edition of the AJCC TNM staging manual. On the other hand, forms of NMSC other than SCC and MCC follow the same criteria as SCC (as was the case in previous editions).
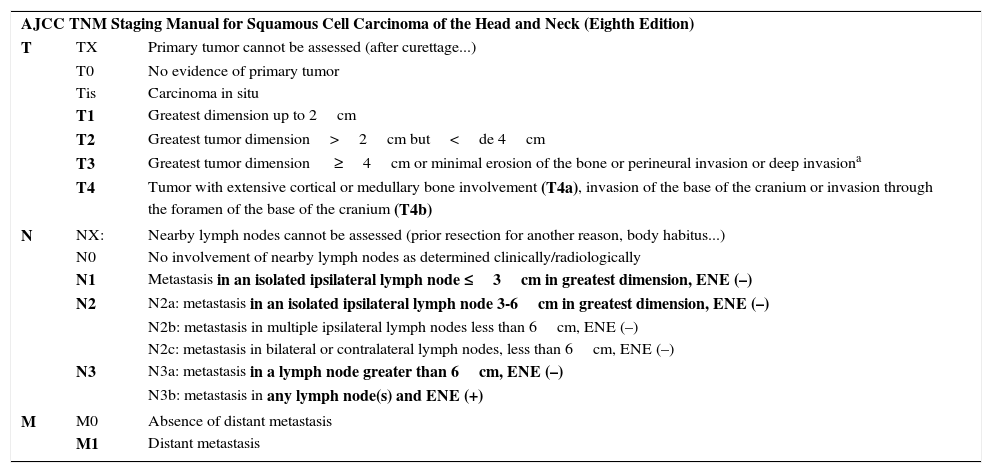
Regarding the T category, the eighth edition of the AJCC considers a tumor measuring less than 2cm in greatest dimension as T1, one measuring 2 to 4cm as T2, and one greater than 4cm as T3. So far, this category is similar to editions prior to 2010. However, a tumor is also T3 if it has a thickness greater than 6cm, if it shows perineural infiltration of nerve branches of more than 0.1cm, if invasion extends beyond the fatty tissue, or if it shows minimal bone erosion. The tumor is classified as T4 if it invades the bone more extensively, as well as if it invades the base or foramen of the cranium.2 Thus, tumors with poor prognosis should tend to be categorized as T3. In view of the study by O’Brien et al,34 the expert panel of the AJCC considered staging patients also in accordance with progressive lymph node involvement. The N category is based on a system shared with other tumors of the head and neck. A tumor is considered N1 if it involves an isolated ipsilateral lymph node, is less than 3cm in diameter, and is without extracapsular extension, N3 if there is metastasis to a major lymph node and it is more than 6cm in diameter or any size if there is extracapsular extension. N2 is used for intermediate situations between N1 and N3 (Table 3). On the other hand, involvement of other organs is defined as M1 (without establishing any subgroups). Table 3 summarizes classification of SCC of the head and neck according to the latest edition of the TNM AJCC staging manual.
Eighth Edition of the TNM Staging Manual of the AJCC for Cutaneous Epidermal Carcinoma (SCC) of the Head and Neck and Nonmelanoma Skin Cancer Other Than Merkel Cell Carcinoma of the Head and Neck.
| AJCC TNM Staging Manual for Squamous Cell Carcinoma of the Head and Neck (Eighth Edition) | ||
| T | TX | Primary tumor cannot be assessed (after curettage...) |
| T0 | No evidence of primary tumor | |
| Tis | Carcinoma in situ | |
| T1 | Greatest dimension up to 2cm | |
| T2 | Greatest tumor dimension>2cm but<de 4cm | |
| T3 | Greatest tumor dimension ≥4cm or minimal erosion of the bone or perineural invasion or deep invasiona | |
| T4 | Tumor with extensive cortical or medullary bone involvement (T4a), invasion of the base of the cranium or invasion through the foramen of the base of the cranium (T4b) | |
| N | NX: | Nearby lymph nodes cannot be assessed (prior resection for another reason, body habitus...) |
| N0 | No involvement of nearby lymph nodes as determined clinically/radiologically | |
| N1 | Metastasis in an isolated ipsilateral lymph node ≤3cm in greatest dimension, ENE (–) | |
| N2 | N2a: metastasis in an isolated ipsilateral lymph node 3-6cm in greatest dimension, ENE (–) | |
| N2b: metastasis in multiple ipsilateral lymph nodes less than 6cm, ENE (–) | ||
| N2c: metastasis in bilateral or contralateral lymph nodes, less than 6cm, ENE (–) | ||
| N3 | N3a: metastasis in a lymph node greater than 6cm, ENE (–) | |
| N3b: metastasis in any lymph node(s) and ENE (+) | ||
| M | M0 | Absence of distant metastasis |
| M1 | Distant metastasis | |
| AJCC TNM Staging System for SCC of Head and Neck (Eighth Edition) | |||
| T1 | N0 | M0 | Stage i |
| T2 | N0 | M0 | Stage ii |
| T3 | N0, N1 | M0 | Stage iii |
| T1 | N1 | M0 | Stage iii |
| T2 | N1 | M0 | Stage iii |
| T1-T3 | N2 | M0 | Stage iv |
| Any T | N3 | M0 | Stage iv |
| T4 | Any N | M0 | Stage iv |
| Any T | Any N | M1 | Stage iv |
Sites on the lower lip are included, eyelid carcinoma is excluded. Tumors of the vulva, pens, perineal region, and other sites other than the head and neck are excluded.
In boldface, aspects most relevant to staging.
Abbreviations: ENE extranodal or extracapsular extension defined as extension through the lymph node capsule in the surrounding connective tissue with or without stromal reaction; SCC squamous cell carcinoma; SLNB sentinel lymph node biopsy.
Deep invasion defined as thickness greater than 6mm or invasion deeper than subcutaneous fat. For a tumor to be T3, perineural invasion should be present in nerves greater than 0.1mm, deeper than the dermis, or clinical and radiological involvement of affected nerves without involvement or invasion of the base of the cranium.
The following criticisms could be made: 1) Omission of certain risk factors; 2) lack of a specific staging system for basal cell carcinoma or for other forms of NMSC (excluding MCC, which has its own staging system since 2010); 3) exclusion of sites other than the head and neck, which means that some cases cannot be staged according to the eighth edition of TNM of the AJCC. However, the system seems to have improved significantly compared with previous editions and at least considers relevant factors that had been ignored in the past, although validation series would be necessary for confirmation.
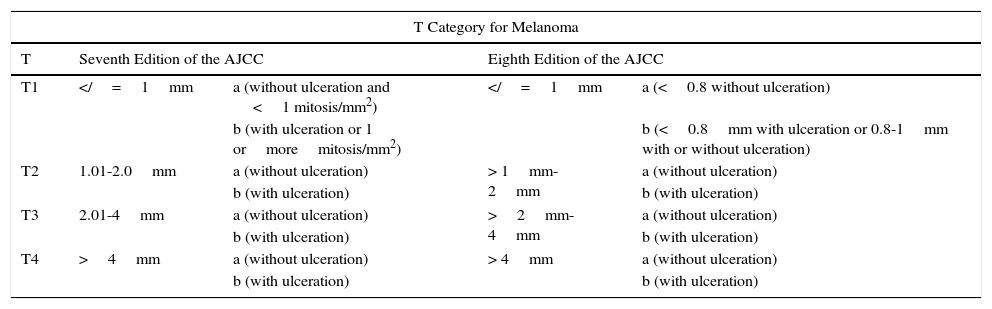
MelanomaMelanoma has also undergone some important changes. In the case of the T category, the discrepancy resides in stratification of the thinner melanomas (Table 4). The changes throughout the different staging systems for melanoma have been more noteworthy than in the case of NMSC (due mainly to series of patients studied more extensively with more robust methodology and increasing evidence concerning risk factors that is not available for other forms of skin cancer). Since the appearance of the original article by Alexander Breslow in 1970,35 support for the impact of melanoma thickness (in mm, measured from the granular layer) has become increasingly solid. Although initially the intention was not to displace but rather complement melanoma prognosis according to degree of Clark invasion,35 from the first edition of the AJCC TNM staging manual, the so-called Breslow index already figured with a similar weight to Clark invasion,1,11,12 and this was maintained until the sixth and seventh editions removed reference to Clark invasion.24,36 Ulceration, another of the classic prognostic factors in melanoma, whose influence is known since before introduction of the Breslow index,37 is another of the parameters that has been retained in the successive staging systems.1,3,11–14,24
Comparison of the Seventh and Eighth Editions of the American Joint Committee on Cancer (AJCC) Staging Manual for Melanoma.
| T Category for Melanoma | ||||
|---|---|---|---|---|
| T | Seventh Edition of the AJCC | Eighth Edition of the AJCC | ||
| T1 | =1mm | a (without ulceration and <1 mitosis/mm2) | =1mm | a (<0.8 without ulceration) |
| b (with ulceration or 1 ormoremitosis/mm2) | b (<0.8mm with ulceration or 0.8-1mm with or without ulceration) | |||
| T2 | 1.01-2.0mm | a (without ulceration) | > 1mm-2mm | a (without ulceration) |
| b (with ulceration) | b (with ulceration) | |||
| T3 | 2.01-4mm | a (without ulceration) | >2mm-4mm | a (without ulceration) |
| b (with ulceration) | b (with ulceration) | |||
| T4 | >4mm | a (without ulceration) | > 4mm | a (without ulceration) |
| b (with ulceration) | b (with ulceration) | |||
Differences are established for the T1 category. The mitotic index disappears from the T category in the eighth edition and stratification is based exclusively on the Breslow index and presence or absence of ulceration
Later on, in the seventh edition, mitosis was introduced as a useful system for stratifying prognosis in melanomas of less than 1mm thick,38 and then went on to replace the level of Clark invasion for stratification of these fine melanomas.24,36 In T1 tumors, ulceration, presence of mitosis, and thickness were the factors that had greatest impact on patient survival.36 However, the influence of mitosis on the prognosis of melanoma was important not only in tumors of less than 1mm thick (T1) but also in general in localized melanomas.39–41 Studies emerged that demonstrated its prognostic value in melanomas more than 1mm thick and as predictive factors of positive sentinel lymph node biopsy,42 thus reducing its specificity in the prognosis of fine melanomas. On the other hand, certain variability between observers was noted along with a low reproducibility of the mitotic index. This made it necessary for 3 to 5 serial sections to be taken for the assessment of mitosis to be reliable,43 and it was therefore not considered a good parameter for making therapeutic decisions.44 In fact, some multivariate analyses were unable to demonstrate its true prognostic relevance.45,46
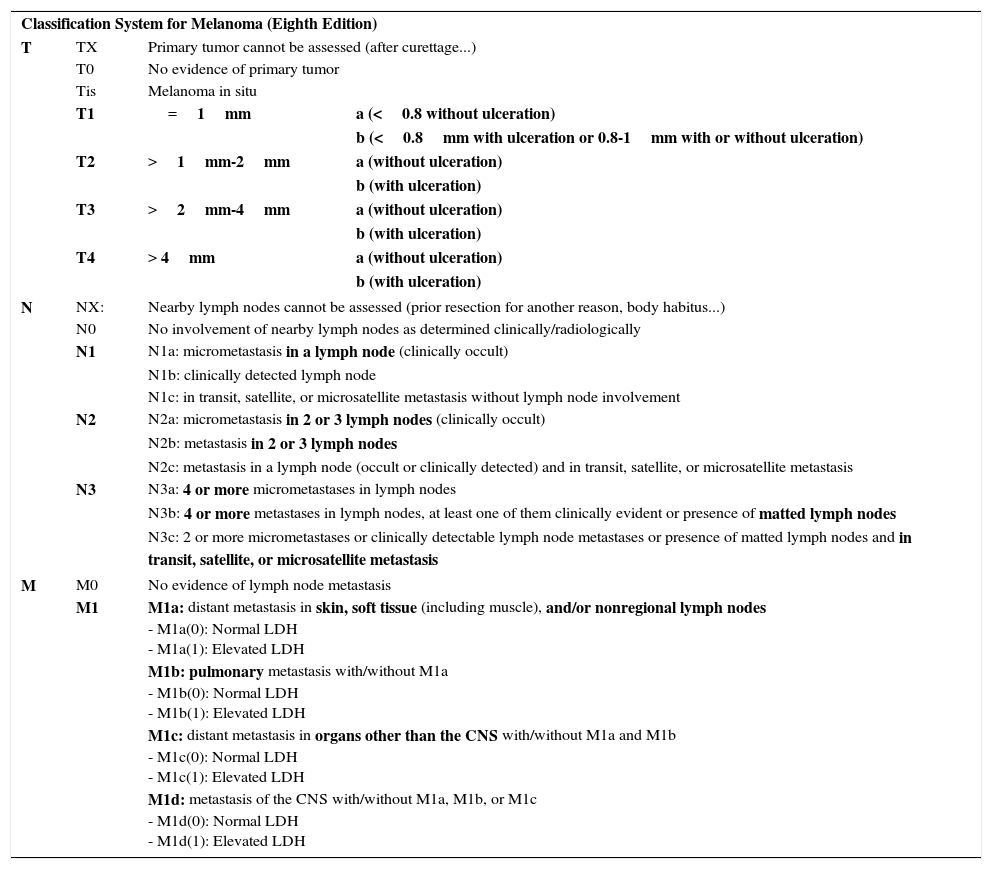
In the eight edition of AJCC staging manual, once again, the main points of contention lie in fine tumors.2 Mitosis is no longer used to stratify T1 tumors and the focus is exclusively on the thickness and presence of ulceration.2 Thus, a tumor is classified as T1a if it measures less than 0.8mm thick and is not ulcerated and T1b if it measures less than 0.8mm thick and is ulcerated or measures 0.8-1mm thick, regardless of whether ulceration is present or not.2 Measures of thickness should be recorded with a precision of 0.1mm (instead of 0.01mm, as proposed in the seventh edition). Tumors of up to 1mm thick can be measured with a precision of 0.01mm if this turns out to be more practical, but the value should be rounded up or down such that the thickness is recorded to one decimal place.2 For the T category, the eighth edition of AJCC staging manual also includes the concept of T0, which is applied in those cases in which melanoma has fully regressed. T0 is not used for cases of partial regression of the primary tumor and starts with distant involvement,2 that is, for cases of metastatic melanoma and primary unknown tumor.
There are also changes regarding stratification within the N and M categories, as well as in the staging itself. Thus, an N1c tumor is defined by the presence of in transit, satellite, or microsatellite metastases without lymph node involvement (lesions which were N2c in the previous edition) and N2c is now reserved for the presence of metastases in a lymph node associated with in transit metastases. The N3 category is also stratified into N3a, N3b, and N3c (unlike in the previous edition).2 The M category is also modified, and M1d is now used to refer to melanomas that develop metastases in the central nervous system; until now these were included in the M1c category. LDH levels are used for stratification of the M category, although this does not affect staging.2 There are no substantial changes up to stage IIC or in stage IV although there are some changes in stage III, which is now more complex.2Table 5 summarizes the characteristics of TNM staging according to the latest edition of the AJCC manual.
Eighth Edition of the AJCC TNM Staging Manual for Melanoma (Cutaneous).
| Classification System for Melanoma (Eighth Edition) | |||
| T | TX | Primary tumor cannot be assessed (after curettage...) | |
| T0 | No evidence of primary tumor | ||
| Tis | Melanoma in situ | ||
| T1 | =1mm | a (<0.8 without ulceration) | |
| b (<0.8mm with ulceration or 0.8-1mm with or without ulceration) | |||
| T2 | >1mm-2mm | a (without ulceration) | |
| b (with ulceration) | |||
| T3 | >2mm-4mm | a (without ulceration) | |
| b (with ulceration) | |||
| T4 | > 4mm | a (without ulceration) | |
| b (with ulceration) | |||
| N | NX: | Nearby lymph nodes cannot be assessed (prior resection for another reason, body habitus...) | |
| N0 | No involvement of nearby lymph nodes as determined clinically/radiologically | ||
| N1 | N1a: micrometastasis in a lymph node (clinically occult) | ||
| N1b: clinically detected lymph node | |||
| N1c: in transit, satellite, or microsatellite metastasis without lymph node involvement | |||
| N2 | N2a: micrometastasis in 2 or 3 lymph nodes (clinically occult) | ||
| N2b: metastasis in 2 or 3 lymph nodes | |||
| N2c: metastasis in a lymph node (occult or clinically detected) and in transit, satellite, or microsatellite metastasis | |||
| N3 | N3a: 4 or more micrometastases in lymph nodes | ||
| N3b: 4 or more metastases in lymph nodes, at least one of them clinically evident or presence of matted lymph nodes | |||
| N3c: 2 or more micrometastases or clinically detectable lymph node metastases or presence of matted lymph nodes and in transit, satellite, or microsatellite metastasis | |||
| M | M0 | No evidence of lymph node metastasis | |
| M1 | M1a: distant metastasis in skin, soft tissue (including muscle), and/or nonregional lymph nodes - M1a(0): Normal LDH - M1a(1): Elevated LDH | ||
| M1b: pulmonary metastasis with/without M1a - M1b(0): Normal LDH - M1b(1): Elevated LDH | |||
| M1c: distant metastasis in organs other than the CNS with/without M1a and M1b - M1c(0): Normal LDH - M1c(1): Elevated LDH | |||
| M1d: metastasis of the CNS with/without M1a, M1b, or M1c - M1d(0): Normal LDH - M1d(1): Elevated LDH | |||
| AJCC TNM Staging System for Melanoma (Eighth Edition) | |||
| Tis | N0 | M0 | Stage 0 |
| T1a-T1b | N0 | M0 | Stage IA |
| T2a | N0 | M0 | Stage IB |
| T2b-T3a | N0 | M0 | Stage IIA |
| T3b-T4a | N0 | M0 | Stage IIB |
| T4b | N0 | M0 | Stage IIC |
| T0 | N1b/1c | M0 | Stage IIIB |
| T0 | N2b/2c, N3b/3c | M0 | Stage IIIC |
| T1a/b-T2a | N1a, N2a | M0 | Stage IIIA |
| T1a/b-T2a | N1b/1c, N2b | M0 | Stage IIIB |
| T2b/T3a | N1a-N2b | M0 | Stage IIIB |
| T1a/T3a | N2c/N3a,b,c | M0 | Stage IIIC |
| T3b/T4a | N1-N3 | M0 | Stage IIIC |
| T4b | N1a-N2c | M0 | Stage IIIC |
| T4b | N3a/b/c | M0 | Stage IIID |
| Any T, Tis | Any N | M1 | Stage iv |
In boldface, aspects most relevant to staging.
The most recent edition of the AJCC staging manual for melanoma places greatest relevance on Breslow thickness and ulceration. Changes are made to the N and M category, as well as staging. Series will be needed to validate whether the predictive capacity of this new staging system is better than previous ones.
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that patient data do not appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
J.C. received partial funding for this study through Project GRS1342/A/16 and the Intensification program of the Castile-Leon Regional Health Authority (INT/M/16/17).
Please cite this article as: Cañueto J, Román-Curto C. Los nuevos sistemas de estadificación del AJCC incorporan novedades en el cáncer cutáneo. Actas Dermosifiliogr. 2017;108:818–826.