The treatment of hair loss is an important part of clinical dermatology given the prevalence of the problem and great impact on patients’ quality of life. Many new treatments have been introduced in recent years. This review summarizes the main ones in 4 groups: a) For androgenetic alopecia, we discuss new excipients for oral minoxidil, dutasteride, and finasteride as well as new forms of application; prostaglandin agonists and antagonists; low-level laser therapy; and regenerative medicine with Wnt signaling activators and stem cell therapy. b) For alopecia areata, Janus kinase inhibitors are reviewed. c) For frontal fibrosing alopecia, we discuss the use of antiandrogens and, for some patients, pioglitazone. d) Finally, we mention new robotic devices for hair transplant procedures and techniques for optimal follicular unit extraction.
La tricología ocupa un área importante dentro de la práctica asistencial de los dermatólogos por la frecuencia de las diferentes tricosis y por el gran impacto que producen en la calidad de vida de los pacientes. Durante los últimos años hemos comprobado la incorporación de muchas novedades terapéuticas en tricología. El objetivo de la presente revisión es resumir de una forma práctica las principales novedades terapéuticas tricológicas, agrupándolas en 4 apartados: a) alopecia androgénica: nuevos excipientes de minoxidil, dutasterida y finasterida oral y nuevas formas de aplicación de estos antiandrógenos, agonistas y antagonistas de las prostaglandinas, láser de baja potencia y medicina regenerativa —activadores de la vía Wnt y terapia con células madre—; b) alopecia areata: fármacos anti-JAK; c) alopecia frontal fibrosante: antiandrógenos y, en algunos pacientes, pioglitazonas, y d) trasplante capilar: nuevos dispositivos tecnológicos y nuevas técnicas de extracción para optimizar la reserva de unidades foliculares.
Trichology is an important field within dermatologic practice. The objective of this review article is to provide a practical synopsis of the main therapeutic novelties in trichology, grouping them into 4 areas: androgenetic alopecia (AGA), alopecia areata (AA), scarring alopecia, and hair transplant.
The study methods consisted of the review of articles included in the Pubmed and Medline databases and in the clinicaltrials.gov clinical trials register between 2013 and 2016, and of the preliminary results of therapies presented at international trichology conferences. The authors of this review also describe their personal experience with the different therapies.
Androgenetic AlopeciaNew treatment options have recently been introduced for men and women with AGA. Additionally, novel uses of minoxidil and antiandrogens have also been described.
MinoxidilA recently published meta-analysis has shown the efficacy of minoxidil versus placebo in the treatment of AGA.1 The authors state that the use of minoxidil is significantly limited by poor compliance due to cosmetic issues, which may be mitigated by the new minoxidil formulations. A Cochrane review article has been published on interventions for female AGA. That review included 47 studies and 5,290 patients and concluded that the application of topical minoxidil at concentrations between 2% and 5% is effective and safe, and is superior to other treatments such as the antiandrogens and low-level laser therapy (LLLT).2 A number of studies looking at the new formulations of minoxidil have shown improved efficacy and better tolerance of foam presentations versus the hydroalcoholic solution in both men3 and women4 with AGA, due to the lower concentration of propylene glycol, which enhances its cosmetic acceptability. The possibility of using niosomes to improve the topical absorption of minoxidil is also being investigated.5 Another novelty is the development of nanoxidil, an active substance with a similar structure to minoxidil, but with a lower molecular weight; it should therefore present better penetration and absorption, although no robust scientific studies have yet been published to support this. Although anecdotal, papers have been published that suggest low-dose oral minoxidil (0.25mg/d) may be safe and effective in AGA (Sinclair, personal communication), permanent chemotherapy-induced alopecia,6 and in moniletrix.7 If these results are confirmed, the use of low doses of oral minoxidil could be a very interesting option in patients with AGA.
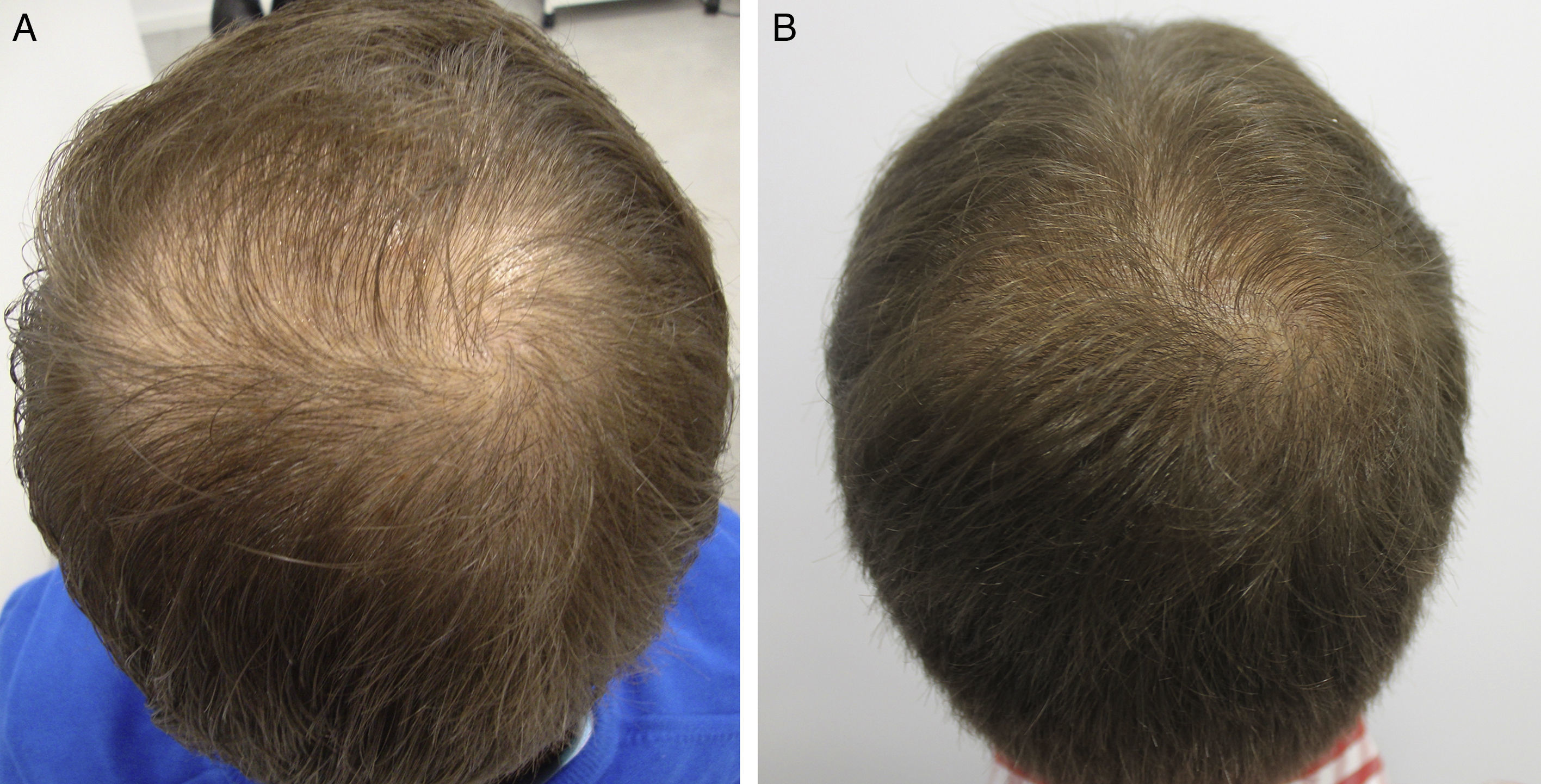
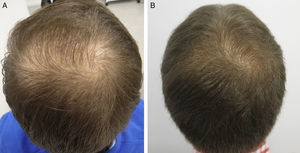
AntiandrogensA number of publications with high levels of evidence support the safety of finasteride and dutasteride in men and women with AGA, because of both the low risk of adverse sexual effects (very similar to placebo),8,9 and the inexistent increase in the risk of cancer.10,11 Regarding one of the most relevant novelties in AGA in recent years, the authors draw attention to the appearance of the drug dutasteride as a safe and effective option in AGA, both in men and in women.12–14 This dual 5α-reductase inhibitor, with a longer half-life than finasteride, has been shown to be more effective that finasteride in predominantly frontal male AGA without presenting additional adverse effects.14–17 With respect to new methods of administration of the antiandrogens, the study by Moftah et al.18 stands out because it demonstrates the usefulness and safety of microinjections of dutasteride in female AGA. Those authors described the treatment of 86 women versus 40 controls, finding a 63% improvement in hair density in the active treatment group versus 17% of the control group (P<.05), with no side effects. The local injection of dutasteride into the scalp after nerve block may be a useful therapeutic option in male and female AGA either in monotherapy (Fig. 1) or as a complement to traditional treatment. Our working group has performed a study in 5 men with AGA, observing the efficacy of local injections of dutasteride every 3 months, with no side effects or alterations of hormone levels in the blood (in press – data not published). As an alternative to the oral administration of antiandrogens, several articles describe the inhibitory activity of topical 0.25% finasteride on follicular 5-alfa-reductase, with lower levels of systemic absorption than oral finasteride.19,20 Studies of the clinical efficacy of topical 0.5% finasteride performed by Milani et al. are still to be published (personal communication); those authors have shown an improvement in male AGA at 6 months in 70% of patients treated in monotherapy.
ProstaglandinsAmong the prostaglandins (PG), the PGF2 analogs latanoprost and bimatoprost are known to stimulate hair growth by prolonging the anagen phase.21,22 However, the concentration of these drugs necessary to improve hairy density in AGA is very high (0.1% latanoprost),22 leading to limitations due to their high cost. Recent research is looking at other drugs that block the PGD2 receptor (GPR44), which has an inhibitory effect on hair growth and which is known to be elevated in the scalp of patients with AGA.23–25 Setipiprant (KITH-105) is a orally administered GPR44 receptor inhibitor that is in the clinical trial phase for asthma, but could have a potential application in AGA.26 A phase II clinical trial is underway to evaluate the use of oral setipiprant in comparison with placebo and with finasteride, 1mg/d, in men aged 18 to 41 years with AGA (NCT02781311).
Physical TherapiesSeveral methodologically robust articles have demonstrated the usefulness of LLLT in both male and female AGA.27–30 The periodic stimulation of hair follicles with LLLT favors the conversion of follicles in telogen to follicles in anagen, and the transformation of vellous follicles into terminal follicles due to the induced perifollicular inflammation, which potentiates follicular growth through activation of stem cells in the bulge. Furthermore, local blood flow is increased and inflammatory mediators and VEGF (vascular endothelial growth factor) are released, favoring the growth of pluripotent stem cells and thus stimulating hair growth and thickening.27–30 In the opinion of the authors, the optimal LLLT protocol for men and women with AGA still remains to be defined, although this therapy appears to be an interesting option with a mechanism of action that differs from traditional therapies. Very interesting articles have been published recently on another physical therapy, microneedling,31,32 a technique that consists of creating microperforations in the scalp in order to produce controlled damage and the release of endogenous growth factors, which stimulate hair growth.
Regenerative Medicine TherapiesIn the final group we have included regenerative medicine therapies designed to stimulate follicular stem cells.
- a)
Three new studies,33–35 one of which is a meta-analysis,34 have been published on the potential usefulness of platelet-rich plasma (PRP) in male and female AGA, showing PRP to achieve increased hair density and number of follicles in anagen with virtually no adverse effects. In the opinion of the authors, this treatment has an excellent safety profile, but its efficacy profile is variable, with a very good response in some patients but more modest in others.
- b)
The initial results have now been published on the usefulness and safety of topical activators of the Wnt/β-catenin pathway in AGA: methyl vanillate,36 valproic acid,37 and SM04554.38 The Wnt/β-catenin pathway regulates activation of the nest of stem cells localized in the follicular bulge, a process necessary for the initiation and maintenance of the follicular anagen phase and whose inhibition has been related with the loss of hair density in AGA.36–38
- c)
It has recently been reported that pharmacological inhibition of 2 of the signaling pathways implicated in downregulation of the proliferation and differentiation of the follicular bulge stem cells and in initiation of the follicular resting phase, JAK/STAT and mTOR/PI3K, effectively activates hair growth in mouse models and in human follicles in vitro.39,40
- d)
It has also been shown that the transient production of reactive oxygen species by photodynamic therapy in vivo in mouse skin induces several signaling pathways that ultimately activate of the nest of hair follicle stem cells, accelerating hair growth.41
- e)
Finally, we would like to comment on stem cell treatments. Basically, there are 2 types of stem cell treatment: E1, which involves hair cloning by the injection of follicular stem cells previously expanded in vitro, producing the growth of new follicles; and E2, adipose-derived stem cells. In the first case, few advances have been reported since the initial studies in mouse models published by McElwee et al. in 2003,42 and the preliminary results of the only clinical trial performed in humans were very limited, with an increase in hair growth of only 6% (McElwee, communication at the World Trichology Conference in Barcelona, 2012). Although this treatment will be a landmark in the treatment of AGA, further research is still required. The second technique consists of performing liposuction to obtain mesenchymal stem cells, which are then conditioned and injected into the scalp to stimulate hair growth. Preliminary studies have shown the possible usefulness of adipose-derived stem cells in AGA,43–45 but further studies are needed to confirm the safety and efficacy of this therapy.
The therapeutic novelties in the treatment of AA fall into 2 groups: a) new data and forms of application of traditional treatments, and b) new therapies.
- a)
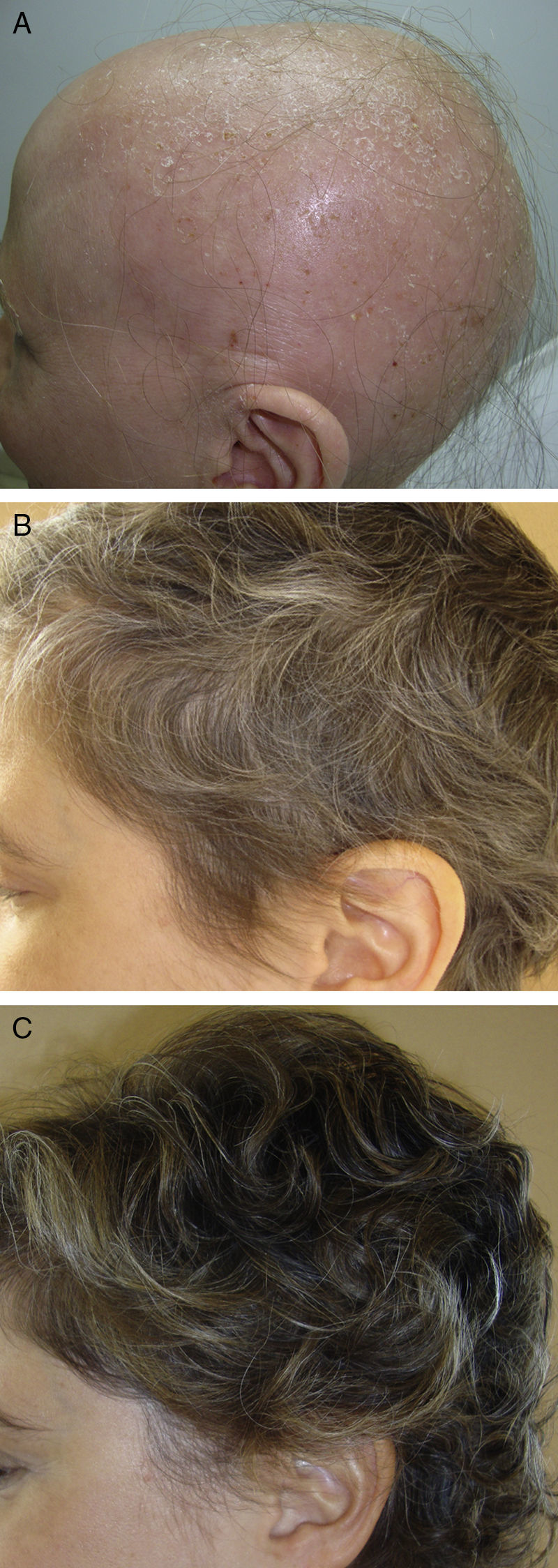
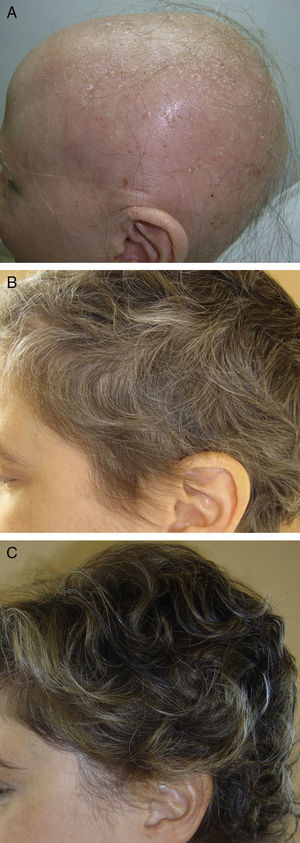
In the first group, we draw attention to a review article by Lamb et al.46 on the use of immunotherapy with diphencyprone in 133 patients with AA. A response was observed in 72% of patients, with regrowth of over 90% in 16% of cases. Another important novelty in this first group is the use of pulsed systemic corticosteroids in AA. There is evidence of greater safety and at least the same efficacy when corticosteroids are used in pulses.47,48 In a study performed by the research group of Hospital Ramón y Cajal in Madrid,48 the use of oral dexamethasone at a dose of 0.1mg/kg/d on 2 consecutive days every week produced regrowth in 25/31 patients with alopecia totalis or universalis (Fig. 2), with mild adverse effects in 32%.
- b)
In the second group of novel therapies, the possible usefulness of the combination of simvastatin and ezetimibe in AA stands out for its immunomodulator effect, demonstrated in a study in which 14 of 19 patients with extensive AA responded.49 However, other authors have reported significantly lower response rates with this treatment (1/20 patients).50 The combination of simvastatin and ezetimibe may be a useful concomitant therapy to improve the response to other treatments, but its action in monotherapy would appear to be limited.
The greatest novelty in the field of AA, and one of the most important in trichology in recent years, is the therapeutic usefulness of the anti-Janus kinase (anti-JAK) agents,51–58 not only for their apparent efficacy, but also because these are the first agents that act against a specific pathogenic target in AA. The JAK pathway is implicated in the activation of CD8 cytotoxic T cells and in the production of interferon-γ, which are fundamental to the onset of AA. Their inhibition appears to induce regrowth in these patients. The drugs reported to be potentially useful in AA are tofacitinib55–58 (whose approved indication is rheumatoid arthritis), ruxolitinib51,52,54 (approved for use in myelofibrosis and polycythemia vera) and baricinitib53 (pending approval of indication in rheumatoid arthritis). These drugs are administered orally and have an acceptable safety profile; because of their indications, they are usually prescribed for long-term administration and are well tolerated. In addition, the first study has been published on the usefulness of topical ruxolitinib in AA of the eyebrows,52 and clinical trials are already underway with new topical anti-JAK agents, such as LEO-124249 (NCT02561585). The main limitation of these treatments is their cost. However, they open new lines of therapeutic research intoAA.
The etiology and pathogenesis of frontal fibrosing alopecia (FFA) are currently considered to involve a double autoimmune and hormonal mechanism.59–61 This justifies the use both of anti-inflammatory drugs (corticosteroids) to reduce the autoimmune inflammation and of antiandrogens (finasteride and dutasteride). In recent years, several studies have been published that show the potential usefulness of these drugs in FFA,59–64 including a multicenter Spanish study of 355 patients.59 The mechanism by which the antiandrogens act in the FFA is not fully understood, but it would appear that inhibition of the action of male hormones on the root of the follicle helps to stabilize the disease.59,61 Some authors have even reported regrowth after the administration of antiandrogens.64 However, others remain skeptical regarding the use of these drugs in FFA.65 Although their mechanism of action remains unclear, there is evidence that a hormonal factor is responsible forFFA.61
Another important therapeutic novelty in FFA and lichen planopilaris (LPP) is the use of the oral antidiabetic agent pioglitazone, which blocks the peroxisome proliferator-activated receptor-γ (PPARγ). Recent studies have suggested that altered function of PPAR-γ may play a role in the initiation of inflammation in LPP and FFA.66 Four studies have been published in which pioglitazone has been used to treat LPP and FFA.67–70 Results have been variable, with an efficacy of between 20% and 70% and adverse effects in up to 50%. Marquez and Camacho71 performed a study in 68 women with FFA, with favorable results in 64% after the use of pioglitazone. The authors believe that pioglitazone could be effective in some cases, but intolerance is not uncommon, mainly in the form of lower limb edema and weight gain, leading to drug withdrawal in a significant number of cases.
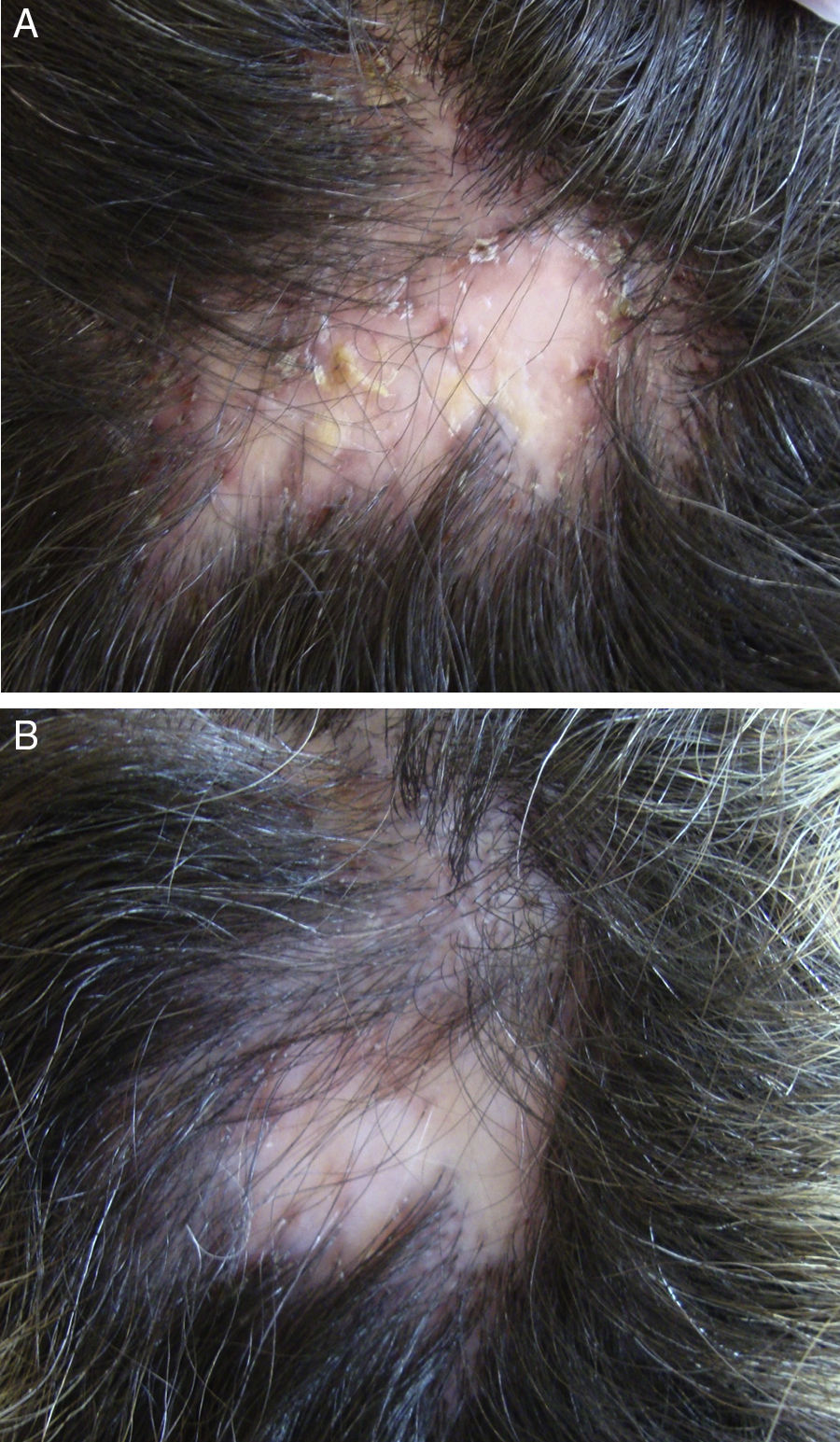
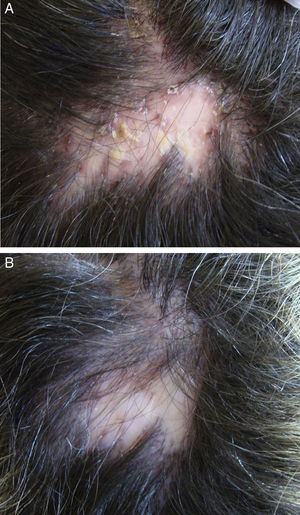
In the case of folliculitis decalvans (FD), a Spanish multicenter study including 82 patients72 concluded that the most effective treatment (improvement in 15/15 patients treated) with the longest period of remission after treatment (7.2 months) was the combination of rifampicin plus clindamycin administered for 10 weeks (Fig. 3). Another therapeutic novelty reported in the literature is the possible usefulness of photodynamic therapy (PDT) with benefit in 9 out of 10 patients with FD.73 However, other authors have reported a negative experience with PDT in 3 out of 3 patients.74
Hair transplantTo complete this review, 2 novel methods stand out in the field of hair transplant. First, the arrival of the new automated robotic systems that reduce operating times and, in some cases, improve the rates of follicular transection in the follicular unit extraction technique.75 However, we consider that results depend more on surgical dexterity than on the device used.
The other novelty is a new technique of follicular extraction, known as partial longitudinal extraction.76 This consists of only partially extracting the follicular units, so that the part of the follicle that remains in the donor region can survive and regenerate an intact follicular unit, thus avoiding the progressive depopulation that occurs in the donor region. The concept is interesting, but still needs to be developed.
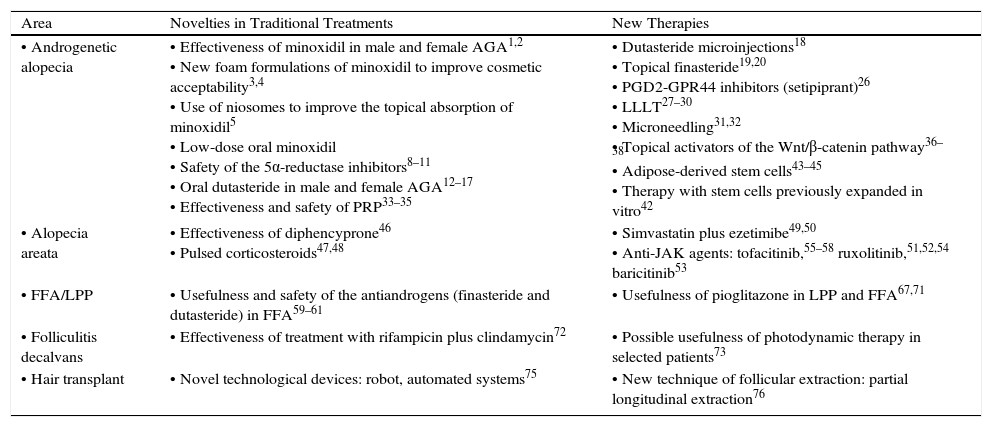
A summary of the most relevant therapeutic novelties in trichology is presented in Table 1.
Summary of the Most Important Therapeutic Novelties in Trichology.
| Area | Novelties in Traditional Treatments | New Therapies |
| • Androgenetic alopecia | • Effectiveness of minoxidil in male and female AGA1,2 • New foam formulations of minoxidil to improve cosmetic acceptability3,4 • Use of niosomes to improve the topical absorption of minoxidil5 • Low-dose oral minoxidil • Safety of the 5α-reductase inhibitors8–11 • Oral dutasteride in male and female AGA12–17 • Effectiveness and safety of PRP33–35 | • Dutasteride microinjections18 • Topical finasteride19,20 • PGD2-GPR44 inhibitors (setipiprant)26 • LLLT27–30 • Microneedling31,32 • Topical activators of the Wnt/β-catenin pathway36–38 • Adipose-derived stem cells43–45 • Therapy with stem cells previously expanded in vitro42 |
| • Alopecia areata | • Effectiveness of diphencyprone46 • Pulsed corticosteroids47,48 | • Simvastatin plus ezetimibe49,50 • Anti-JAK agents: tofacitinib,55–58 ruxolitinib,51,52,54 baricitinib53 |
| • FFA/LPP | • Usefulness and safety of the antiandrogens (finasteride and dutasteride) in FFA59–61 | • Usefulness of pioglitazone in LPP and FFA67,71 |
| • Folliculitis decalvans | • Effectiveness of treatment with rifampicin plus clindamycin72 | • Possible usefulness of photodynamic therapy in selected patients73 |
| • Hair transplant | • Novel technological devices: robot, automated systems75 | • New technique of follicular extraction: partial longitudinal extraction76 |
Abbreviations: AGA, androgenetica alopecia; FFA, frontal fibrosing alopecia; JAK, Janus kinase; LLLT, low-level laser therapy; LPP, lichen planopilaris; PGD2-GPR44, prostaglandin D2 receptor; PRP, platelet-rich plasma.
In recent years we have seen great advances in the therapeutics of trichology, but more importantly, new lines of research have been opened that will allow us to continue advancing. The developments discussed in this review, which has looked at the past 3 years, are more than hope, they are now a reality.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Vañó-Galván S, Camacho F. Novedades terapéuticas en tricología. Actas Dermosifiliogr. 2017;108:221–228.