Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma in adults and children. The prevalence has increased in some countries, but no descriptive studies of MF in the pediatric population have been done in Colombia to date.
MethodsA combined prospective–retrospective study of 128 patients with a diagnosis of MF confirmed by the dermatology department and dermatopathology laboratory of Universidad de Antioquia between 2008 and 2017. We describe the clinical and histopathologic variants, response to treatment, and progression of the disease in 23 patients under 18 years of age.
ResultsThe pediatric cases of MF accounted for 18% of all the cases on record. The median age of onset of lesions was 9 years, the median age at diagnosis was 11 years, and the median time between onset of lesions and diagnosis was 2 years. All patients were in early stages of the disease. Hypopigmented MF was the most common clinical presentation (in 52.2%), followed by classical MF (in 30.4%). Folliculotropic MF was identified in 17.4%. All patients were treated with topical corticosteroids and phototherapy. One patient received chemotherapy while still in the early stage of disease. Complete remission was achieved in 59.1% and a partial response in 40.9%. Only 2 patients remained asymptomatic for 5 years.
ConclusionWe found hypopigmented MF to be the most common clinical presentation in patients under 18 years of age. The disease did not progress to advanced stages in any of the patients, although recurrence after treatment interruption was common.
La micosis fungoide (MF) es el tipo más común de linfoma cutáneo de células T tanto en adultos como en niños. En algunos países se ha observado un aumento de la prevalencia de MF en niños. Hasta la fecha, no existen estudios descriptivos de MF en la población pediátrica colombiana.
MétodosEn una revisión ambispectiva de 128 pacientes con el diagnóstico confirmado de MF en la Sección de Dermatología y del Laboratorio de Dermatopatología de la Universidad de Antioquia entre 2008–2017, se describen las variantes clínicas e histopatológicas, la respuesta al tratamiento y progresión de la enfermedad de 23 pacientes menores de 18 años de edad.
ResultadosLos casos de MF en niños constituyeron el 18% del total. La mediana de edad de inicio de las lesiones fue 9 años; la mediana de edad al momento del diagnóstico fue 11 años y la mediana del tiempo promedio entre el inicio de las lesiones y el diagnóstico fue 2 años. Todos los pacientes se encontraban en estadios tempranos de la enfermedad. La MF hipopigmentada fue la presentación clínica más frecuente (52.2%), seguida por la MF clásica (30.4%). La MF foliculotropa se presentó en el 17.4%. Todos los pacientes recibieron terapias dirigidas a la piel con esteroides tópicos y fototerapia, uno recibió quimioterapia, aún en estadio temprano. El 59.1% obtuvieron remisión completa y el 40.9% respuesta parcial. Solo dos casos permanecieron asintomáticos durante 5 años.
ConclusiónEn nuestra experiencia, la MF hipopigmentada fue la presentación clínica más común en pacientes menores de 18 años. No se presentaron progresiones a estadios avanzados. Sin embargo, las recurrencias después de la interrupción del tratamiento fueron comunes.
Primary cutaneous lymphomas constitute a heterogeneous group of malignant lymphoproliferative disorders classified within the non-Hodgkin lymphomas.1–3 Depending on the strain, they are classified as cutaneous T-cell lymphoma (CTCL) and cutaneous B-cell lymphoma. The former is more common in all age groups (73.8%), whereas the latter account for 23.2% of cases.4 In childhood, primary cutaneous lymphomas are more common than secondary lymphomas, although skin manifestations may be the first sign of systemic disease in a secondary lymphoma.2,3
Mycosis fungoides (MF) is the most common form of CTCL.5 Most cases occur in adults aged > 50 years, and, although uncommon in children, MF is the most frequently diagnosed cutaneous lymphoma in this age group.2,6,7 The percentage of children with CTCL in the first reported series of pediatric MF ranged from 0.5% to 5% of all cases8,9; however, in recent years, frequencies of pediatric MF of 9% and 16.6% have been reported in Brazil and Kuwait, respectively.10,11 Diagnosis is delayed because of the clinical similarity to other inflammatory skin diseases such as pityriasis alba, vitiligo, pityriasis versicolor, and atopic dermatitis.12 The time to diagnosis may be as long as 7.5 years.13,14 MF is diagnosed based on the correlation between clinical and histopathology findings, although flow cytometry, immunohistochemistry, and analysis of T-cell receptor rearrangement can also be used. There are no specific guidelines for the management of MF in children, who generally receive the same therapy as adults. This is selected based on the severity of the disease. The first-line treatments comprise topical corticosteroids and phototherapy, especially narrowband UV-B, although psoralen-UV-A (PUVA) and UV-A 340–400 nm (UVA-1) are also used.15,16 The prognosis of MF is good in children, with survival rates of 95% and 93% at 5 and 10 years, respectively. Disease progression is 5% at 5 years and 29% at 10 years; progress to later stages and fatal cases are exceptional, and only isolated cases have been reported.17 No descriptive studies on MF in Colombian children have been reported to date in the indexed literature we reviewed. Below, we report our experience with MF in children and adolescents at the Dermatology Department of the University of Antioquia, Medellín, Colombia. Our objectives were to characterize the disease in our population, compare our findings with data from the literature, and to describe the frequency of the clinical variants of MF, response to treatment, and disease progression, all of which have been widely discussed in the various case series.
Material and MethodsStudy data were obtained by means of an ambispective review of the clinical and histopathology records of all patients diagnosed with MF between August 2008 and January 2017 in the lymphoma database of the Dermatopathology Laboratory of Universidad of Antioquia. For purposes of the study, we included patients aged ≤ 18 years with a confirmed diagnosis of MF.
This was a minimal risk study. The identity of the patients, who attended the Dermatology Department accompanied by their parents or guardians, was preserved. The parents gave their informed consent. The clinical database was approved by the Bioethics Committee of the School of Medicine of the University of Antioquia.
MF was diagnosed based on the relationship between clinical and histopathological features. The histopathology studies were reviewed by 2 lecturers in dermatopathology, who reported their findings in descriptive terms. Diagnosis sometimes required several biopsy specimens. T-cell receptor rearrangement analysis was not available. Severity was assigned by means of a physical examination, review of the clinical history, and extension studies in all cases (chest x-ray, abdominal ultrasound, and complete blood count, with an extension for peripheral blood [automatic and manual], and/or flow cytometry). Immunohistochemistry studies were also carried out.
Patient follow-up was based on clinical evaluations and telephone calls every 1–3 months after diagnosis depending on the treatment modality. The body surface area involved was calculated using the palmar method (including the fingers), which corresponded to 1% of the total surface.18 Response to treatment was classified based on the United States Cutaneous Lymphoma Consortium and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer, namely, complete (100% response, resolution of all lesions), partial (50%–99% response), and none (< 50% response).19
We recorded epidemiological variables (sex and age), clinical variables (age at onset, time since onset, age at diagnosis, type of lesion, stage of severity, phototype), and treatment (skin-directed therapies, systemic therapy, type of phototherapy, phototherapy regimen, response to the first cycle of phototherapy, follow-up).
A univariate analysis of absolute and relative frequencies was performed. In addition, tables were constructed to describe the qualitative characteristics. Age and time since diagnosis were expressed using the median and range.
ResultsA total of 128 patients with MF were seen at the Dermatology Department of the University of Antioquia between August 2008 and January 2017. Of these, 23 (18%) were children and adolescents aged ≤18 years (Table 1). The median age at onset of the lesions was 9 (2–17) years. The median age at diagnosis was 11 (4–18) years, and the median time since the onset of lesions and diagnosis was 2 (0–8) years. There were 13 boys and 10 girls, with a ratio of males to females of 1.3:1. The predominant phototype was III (43.8%), followed by II (30.4%).
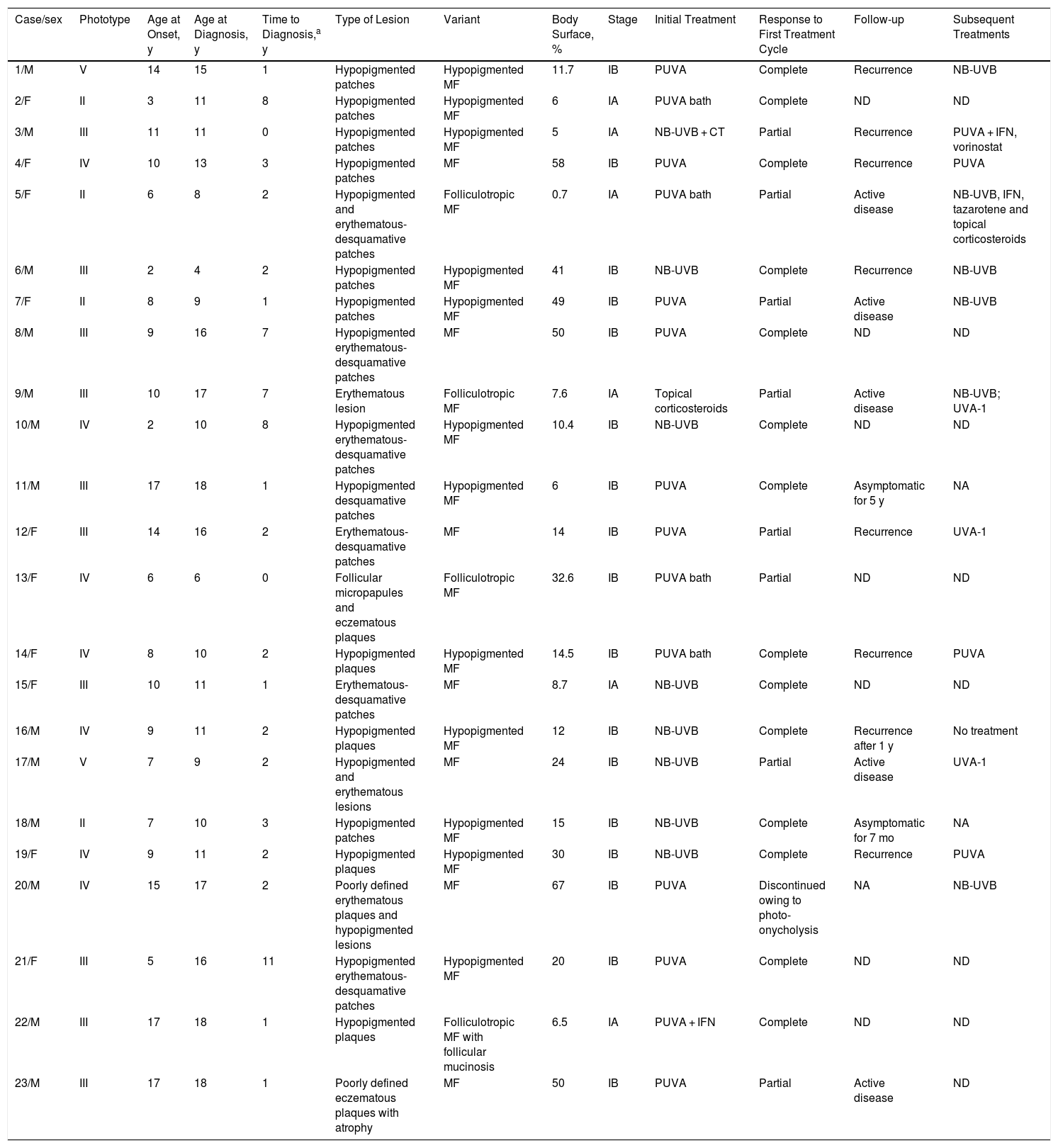
Demographic and Clinical Data of Children and Adolescents With Mycosis Fungoides.
| Case/sex | Phototype | Age at Onset, y | Age at Diagnosis, y | Time to Diagnosis,a y | Type of Lesion | Variant | Body Surface, % | Stage | Initial Treatment | Response to First Treatment Cycle | Follow-up | Subsequent Treatments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/M | V | 14 | 15 | 1 | Hypopigmented patches | Hypopigmented MF | 11.7 | IB | PUVA | Complete | Recurrence | NB-UVB |
| 2/F | II | 3 | 11 | 8 | Hypopigmented patches | Hypopigmented MF | 6 | IA | PUVA bath | Complete | ND | ND |
| 3/M | III | 11 | 11 | 0 | Hypopigmented patches | Hypopigmented MF | 5 | IA | NB-UVB + CT | Partial | Recurrence | PUVA + IFN, vorinostat |
| 4/F | IV | 10 | 13 | 3 | Hypopigmented patches | MF | 58 | IB | PUVA | Complete | Recurrence | PUVA |
| 5/F | II | 6 | 8 | 2 | Hypopigmented and erythematous-desquamative patches | Folliculotropic MF | 0.7 | IA | PUVA bath | Partial | Active disease | NB-UVB, IFN, tazarotene and topical corticosteroids |
| 6/M | III | 2 | 4 | 2 | Hypopigmented patches | Hypopigmented MF | 41 | IB | NB-UVB | Complete | Recurrence | NB-UVB |
| 7/F | II | 8 | 9 | 1 | Hypopigmented patches | Hypopigmented MF | 49 | IB | PUVA | Partial | Active disease | NB-UVB |
| 8/M | III | 9 | 16 | 7 | Hypopigmented erythematous-desquamative patches | MF | 50 | IB | PUVA | Complete | ND | ND |
| 9/M | III | 10 | 17 | 7 | Erythematous lesion | Folliculotropic MF | 7.6 | IA | Topical corticosteroids | Partial | Active disease | NB-UVB; UVA-1 |
| 10/M | IV | 2 | 10 | 8 | Hypopigmented erythematous-desquamative patches | Hypopigmented MF | 10.4 | IB | NB-UVB | Complete | ND | ND |
| 11/M | III | 17 | 18 | 1 | Hypopigmented desquamative patches | Hypopigmented MF | 6 | IB | PUVA | Complete | Asymptomatic for 5 y | NA |
| 12/F | III | 14 | 16 | 2 | Erythematous-desquamative patches | MF | 14 | IB | PUVA | Partial | Recurrence | UVA-1 |
| 13/F | IV | 6 | 6 | 0 | Follicular micropapules and eczematous plaques | Folliculotropic MF | 32.6 | IB | PUVA bath | Partial | ND | ND |
| 14/F | IV | 8 | 10 | 2 | Hypopigmented plaques | Hypopigmented MF | 14.5 | IB | PUVA bath | Complete | Recurrence | PUVA |
| 15/F | III | 10 | 11 | 1 | Erythematous-desquamative patches | MF | 8.7 | IA | NB-UVB | Complete | ND | ND |
| 16/M | IV | 9 | 11 | 2 | Hypopigmented plaques | Hypopigmented MF | 12 | IB | NB-UVB | Complete | Recurrence after 1 y | No treatment |
| 17/M | V | 7 | 9 | 2 | Hypopigmented and erythematous lesions | MF | 24 | IB | NB-UVB | Partial | Active disease | UVA-1 |
| 18/M | II | 7 | 10 | 3 | Hypopigmented patches | Hypopigmented MF | 15 | IB | NB-UVB | Complete | Asymptomatic for 7 mo | NA |
| 19/F | IV | 9 | 11 | 2 | Hypopigmented plaques | Hypopigmented MF | 30 | IB | NB-UVB | Complete | Recurrence | PUVA |
| 20/M | IV | 15 | 17 | 2 | Poorly defined erythematous plaques and hypopigmented lesions | MF | 67 | IB | PUVA | Discontinued owing to photo-onycholysis | NA | NB-UVB |
| 21/F | III | 5 | 16 | 11 | Hypopigmented erythematous-desquamative patches | Hypopigmented MF | 20 | IB | PUVA | Complete | ND | ND |
| 22/M | III | 17 | 18 | 1 | Hypopigmented plaques | Folliculotropic MF with follicular mucinosis | 6.5 | IA | PUVA + IFN | Complete | ND | ND |
| 23/M | III | 17 | 18 | 1 | Poorly defined eczematous plaques with atrophy | MF | 50 | IB | PUVA | Partial | Active disease | ND |
Abbreviations: F, female; IFN, interferon; M, male; MF, mycosis fungoides; NA, not applicable; ND, no data; NB-UVB, narrowband UV-B; ND, no data; PUVA, psoralen UV-A; CT, chemotherapy.
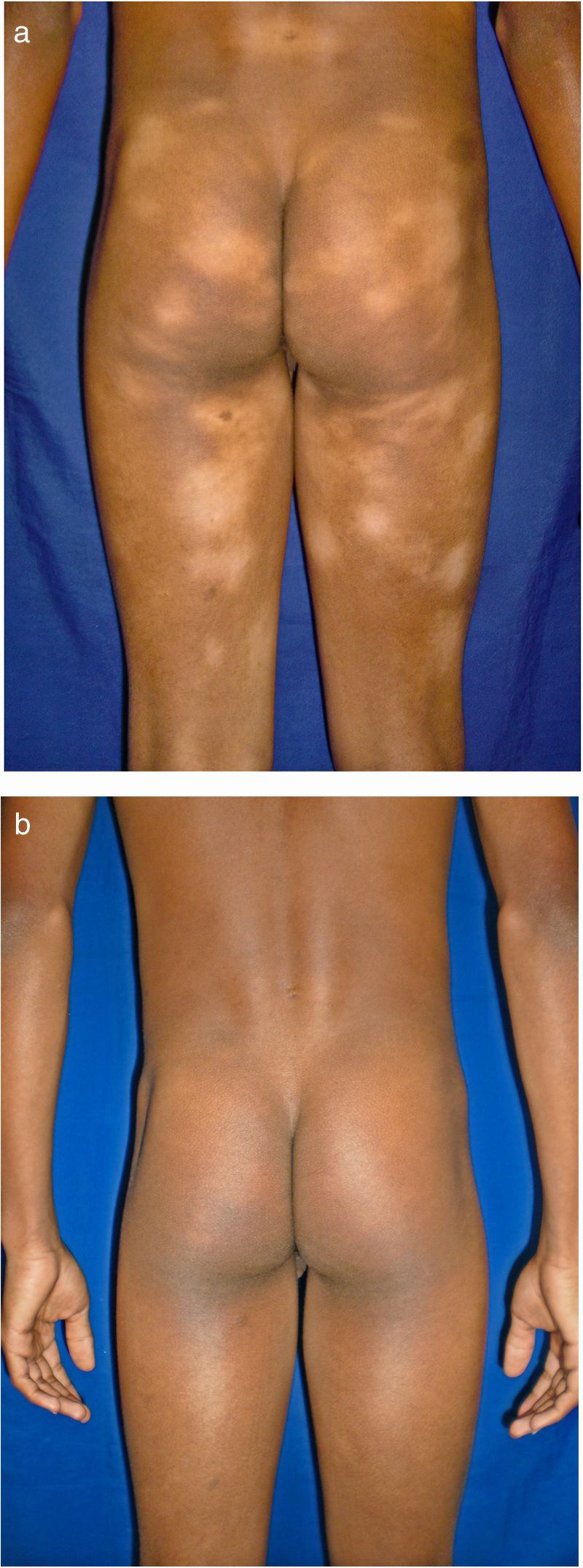
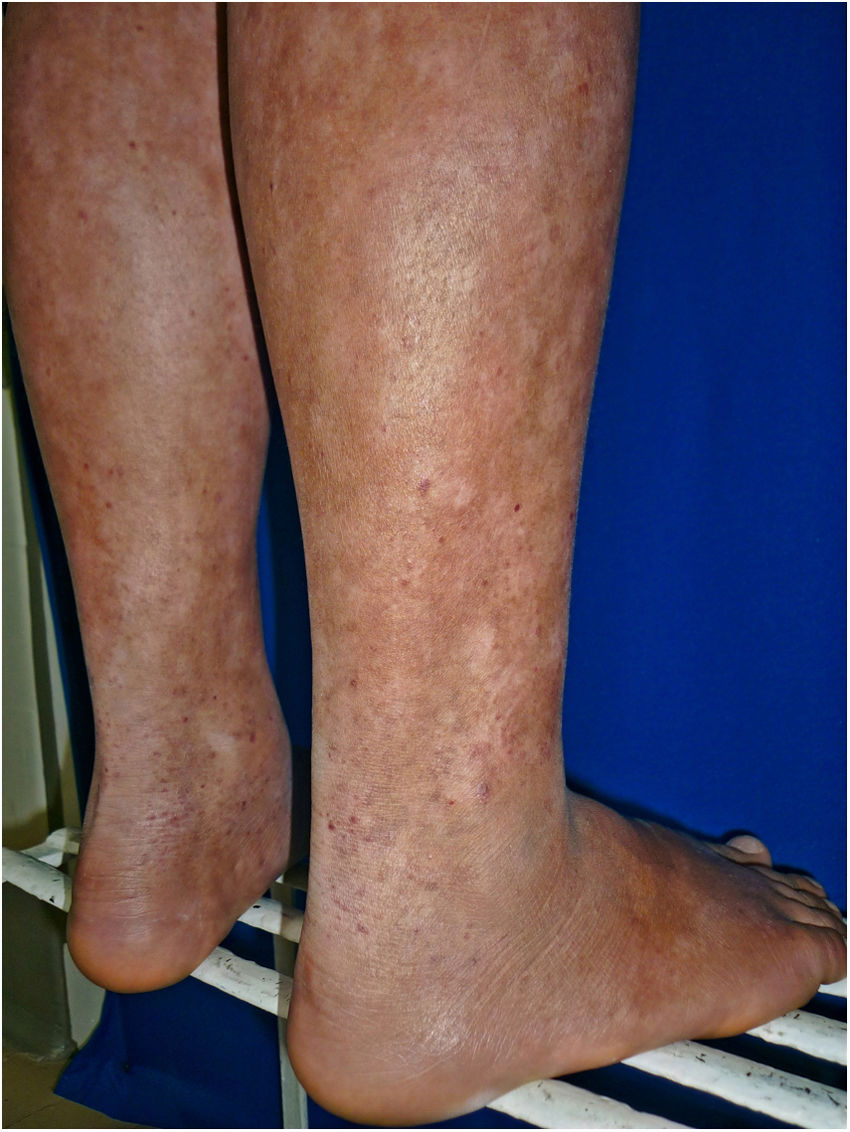
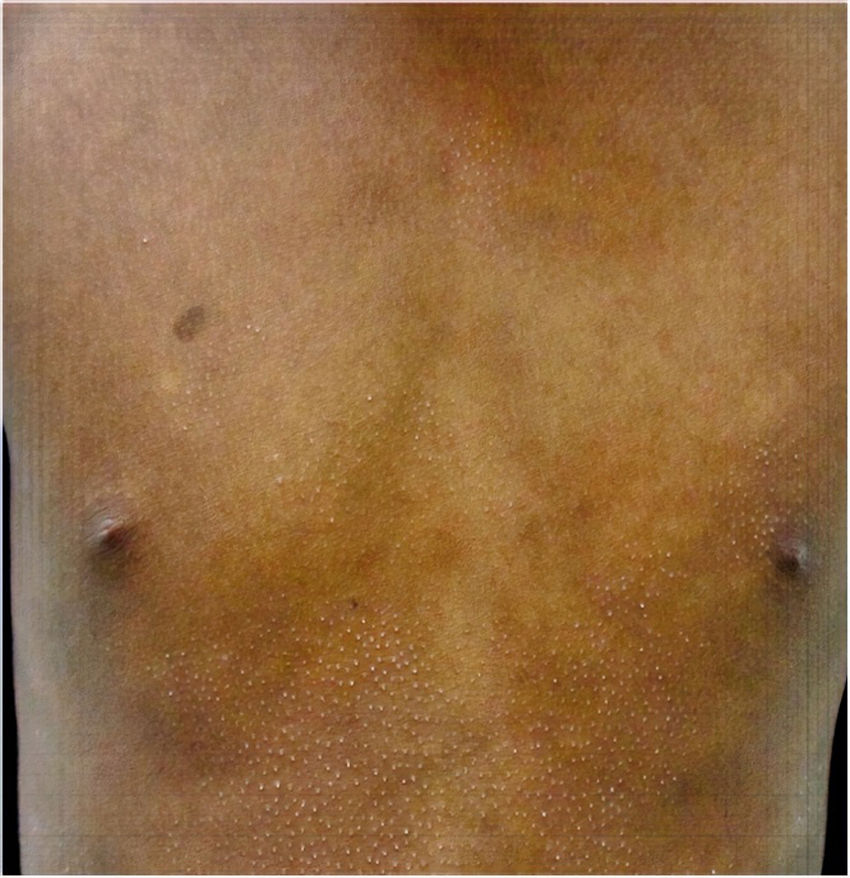
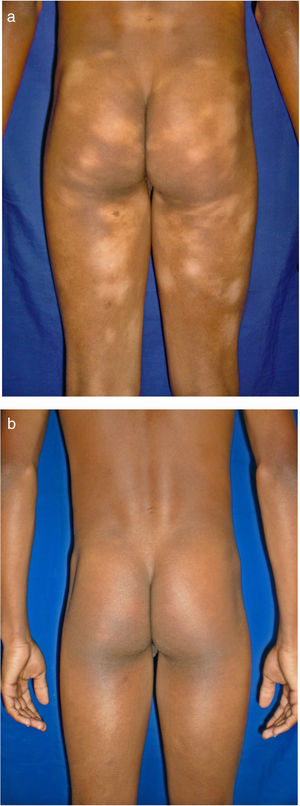
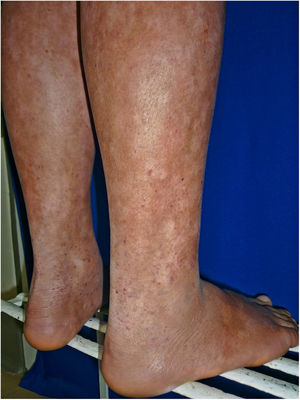
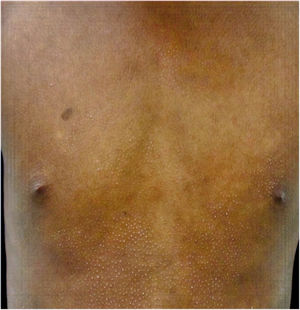
All of the patients were in early stages of the disease: 6 in IA (26.1%) and 17 in IB (73.9%). Hypopigmented MF was the most common clinical presentation (12 patients, 52.2%) (Fig. 1). Interestingly, 1 case of hypopigmented MF was characterized by numerous hypopigmented macules not preceded by a lesion and accompanied by others that mimicked pityriasis lichenoides chronica (PLC), that is, round erythematous plaques measuring 0.5 cm–3 cm in diameter, covered by compact whitish scaling. Analysis of the clinical, histopathology, and immunohistochemistry findings for the 2 types of lesion revealed that they were MF. The second most common type was classic MF in 7 cases (30.43%), 1 of which was associated with capillaritis (Fig. 2). Folliculotropic MF was found in 4 cases (17.4%), 1 of which was similar to lichen spinulosus (Fig. 3 and Table 2).
Demographic Characteristics of 23 Children and Adolescents With Mycosis Fungoides.
| Characteristics | Value |
|---|---|
| Distribution by sex | |
| Male | 13 (56.5) |
| Female | 10 (43.48) |
| Male-to-female ratio | 1.3:1 |
| Age at onset of symptoms, y | |
| Median | 9 (2–17) |
| Age at diagnosis, y | |
| Median | 12 (4–18) |
| Time since the onset of symptoms until diagnosis, y | |
| Median | 2 (0–8) |
| Clinical presentation, No. (%) | |
| Classic MF | 6 (26.1) |
| Hypopigmented MF | 12 (52.2) |
| Folliculotropic MF | 4 (17.4) |
| Classic MF with capillaritis | 1 (4.3) |
| Phototype | |
| I | 0 (0) |
| II | 4 (17.4) |
| III | 10 (43.8) |
| IV | 7 (30.4) |
| V | 2 (8.7) |
| Stage at diagnosis, No. (%) | |
| IA | 6 (26.1) |
| IB | 17 (73.9) |
Abbreviation: MF, mycosis fungoides.
All of the children and adolescents received skin-directed therapy with topical corticosteroids (mainly mometasone and clobetasol), both previous to and alongside phototherapy (in those cases where it was administered). The initial treatment was phototherapy in 22 cases (14 with PUVA), including 4 treated with a PUVA bath and 8 with narrowband UV-B. One patient initially received chemotherapy in the Oncology Department (methotrexate, cytarabine, vincristine). A patient with folliculotropic MF initiated treatment with PUVA and interferon (3 million units 3 times per week). Of the 22 patients who received phototherapy, 14 (64%) achieved complete remission and 8 (36%) achieved partial remission. Phototherapy was well tolerated, except for 1 patient, who experienced photo-onycholysis associated with PUVA after 48 sessions. This patient’s therapy was suspended, and treatment with narrowband UV-B was started.
The narrowband UV-B phototherapy regimen was calculated based on the phototype, with a frequency of 3 times per week and an average of 52–62 sessions per cycle. In the case of UVA-1, low or medium doses were used (maximum, 50 J/cm2, 3 sessions per week, with an average of 40 sessions).
During follow-up, 6 of the patients who had achieved a complete response relapsed and required a second cycle of phototherapy. Time to relapse was 3 months to 7 years. Only 2 cases have remained asymptomatic. In one case, the patient had a family history of MF (grandfather) and has remained symptom-free for 5 years; in the other, the patient has been symptom-free for 7 months. Six of the patients who achieved a complete response have not returned to our institution for follow-up (Table 3).
Response to the First Cycle of Phototherapy.
| Phototherapy, No. % | Complete Response, % | Partial Response, % |
|---|---|---|
| PUVA (14) | 8 (57) | 6 (43) |
| PUVA bath (4) | 2 (50) | 2 (50) |
| PUVA + interferon (1) | 1 (100) | |
| Narrowband UV-B (8) | 6 (75) | 2 (25) |
| With chemotherapy (1) | 1 (100) | |
| Total: 22a | 14 (64) | 8 (36) |
Abbreviation: PUVA, psoralen-UV-A.
The Photodermatology Unit at the University of Antioquia administers UVA-1 phototherapy. Three of the patients who achieved a partial response with narrowband UV-B or PUVA were treated with UVA-1: 2 patients achieved a partial response > 90%, and 1 remains in treatment with satisfactory results to date.
MF has not progressed to advanced stages in any of the patients who remain in follow-up. The longest follow-up to date is 10 years.
DiscussionMF is the most common form of CTCL. It mainly affects older patients, with 75% of cases diagnosed after 50 years of age and 0.5%–5% of cases diagnosed before age 20 years.10 Reports of MF affecting children have recently increased in frequency. However, the real incidence in children has not been determined with any degree of accuracy. The same is true of the clinical, immunohistochemical, immunophenotypic characteristics of the disease.20 Of the 128 patients diagnosed with MF, 23 were children and adolescents (18%). This percentage is similar to that reported in Asian children (16.6%), although lower values have been reported in Latinos (9%) and North Americans (2%),10,11 probably because of the increase in the incidence of this condition, earlier diagnosis, or a greater prevalence of MF in this age group in specific geographic areas. The male-to-female ratio for MF in the present study was 1.3:1, which is similar to that reported in most pediatric series (1.25:1 in Arab series and 3.6:1 in Korean series17,21). Consistent with the literature, hypopigmented MF was the most common clinical presentation,15,22 in contrast with adults, who account for only 3.5%.23 The differential diagnosis is with postinflammatory hypopigmentation, pityriasis alba, pityriasis versicolor, vitiligo, leprosy, and, in some cases, PLC.7,20 In addition, while not recognized as a variant in the classification of the World Health Organization/European Organisation for Research and Treatment of Cancer (WHO/EORTC),24 the higher frequency of cases makes hypopigmented MF especially interesting in Latinos and Asians, and it has been associated with a potentially more favorable prognosis.25 According to the literature, hypopigmented MF is considered the type in which only hypopigmented lesions are observed.26 In our opinion, it should be included in the WHO/EORTC classification as a variant of MF. Dermatologists should bear MF in mind as a differential diagnosis in patients with recurrent eczematous and inflammatory lesions that respond poorly to treatment. In the present series, we observed uncommon forms of MF, such as folliculotropic MF and MF with capillaritis. Folliculotropic MF is the most common nonclassic variant in adults and has a potentially more aggressive course that may require combined management with systemic therapy.27 In contrast, folliculotropic MF is rare in pediatric patients. It responds better to therapy, and its prognosis is similar to that of adults.22 Cases of MF with capillaritis are unusual in children. There is a report of one patient (2.2%) in a series from Singapore28 and of another in a series from New York, USA,20 with no differences in the stage, response to therapy, or prognosis compared with classic MF in children. In 1 case of MF, the lesions mimicked PLC. This condition is a benign lymphoproliferative disorder that mainly affects young patients and in which monoclonal T-lymphocyte populations with loss of CD7 expression have been identified. The benign nature of the lesion has been attributed to an antitumor immune response in CD8+ lymphocytes. In the case of a patient with PLC-type lesions, it is important to bear in mind that these may occasionally precede MF. Alternatively, MF can mimic PLC; hence, the recommendation for clinical follow-up and new biopsies when the plaques increase in size or progress slowly.7 The progress of MF may be brought to light through increased nuclear atypia in lymphocytes and reduced numbers of apoptotic keratinocytes and CD7+ and CD8+ lymphocytes.29
The histopathology findings for the patients in the present study were consistent with MF. We do not use T-cell receptor rearrangement analysis in our department because it is not available. We found 1 case of familial MF, where the patient’s grandfather had psoriasiform MF, possibly similar to cases of MF associated with human leukocyte antigen (HLA), specifically, HLA-DQB1*03.5,6 The literature contains a published report of 15 cases of familial MF in first-degree relatives. Consistent with available data, it seems that familial MF is no different from sporadic MF in terms of clinical outcome.30 In the present series, all patients were in early stages of the disease (IA/IB), with frequent recurrences, and none has since progressed to later stages, as reported elsewhere.10,15
Prognosis of MF is generally favorable in young people. The factors associated with progress to MF include poikilodermatous presentation, African American race, and advanced-stage disease at diagnosis. Prognosis of hypopigmented presentations is favorable.31
In children, MF mostly has a chronic and indolent course.21 Patients should be accompanied and followed up. We should avoid aggressive treatments that affect quality of life and could potentially diminish the immune response and, in the long term, affect the course of the disease.25
Conflicts of interestThe authors declare that they have no conflicts of interest.
FundingThis study was performed with local resources and received no specific grants from the public, private, or nonprofit sector.
We are grateful to the Skin Lymphoma Support Group and to the Dermatology Department of the School of Medicine, University of Antioquia at IPS Universitaria and Hospital Universitario San Vicente Fundación.
Please cite this article as: Valencia Ocampo OJ, et al. Micosis fungoide en niños y adolescentes: descripción de una serie de 23 casos. Actas Dermosifiliogr. 2020;111:149–156.