Methotrexate (MTX) is an antimetabolite that inhibits folic acid synthesis. It is widely used in dermatology, rheumatology, and oncology.1 Although MTX has a good safety profile, serious adverse effects such as pneumonitis, hepatic fibrosis, and bone marrow aplasia can occur.1–3 Previous studies have suggested that MTX-induced mucositis may be a sign of bone marrow toxicity.2,3 The aim of this study was to analyze clinical, laboratory, and epidemiological characteristics of patients with MTX-induced mucositis.
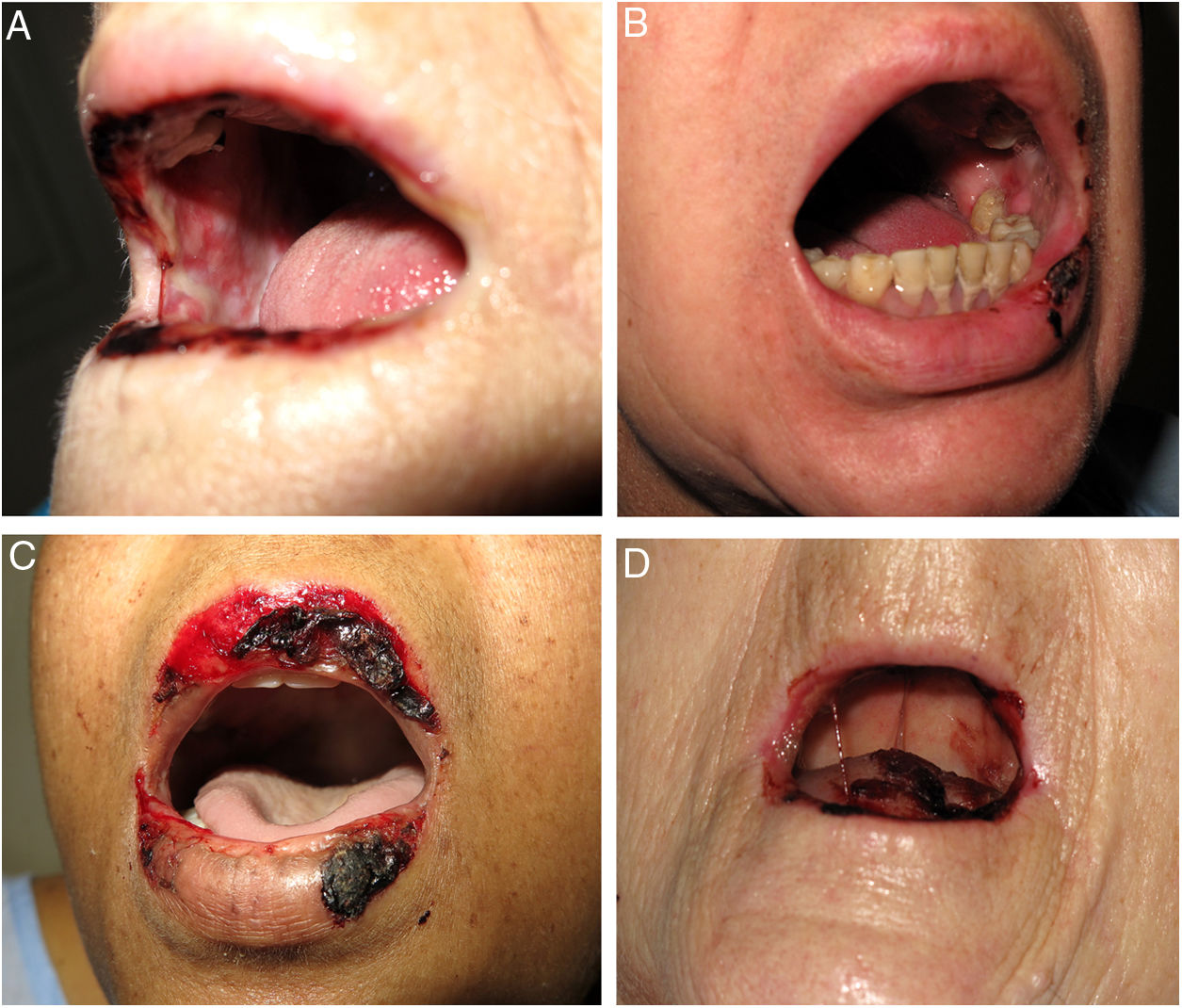
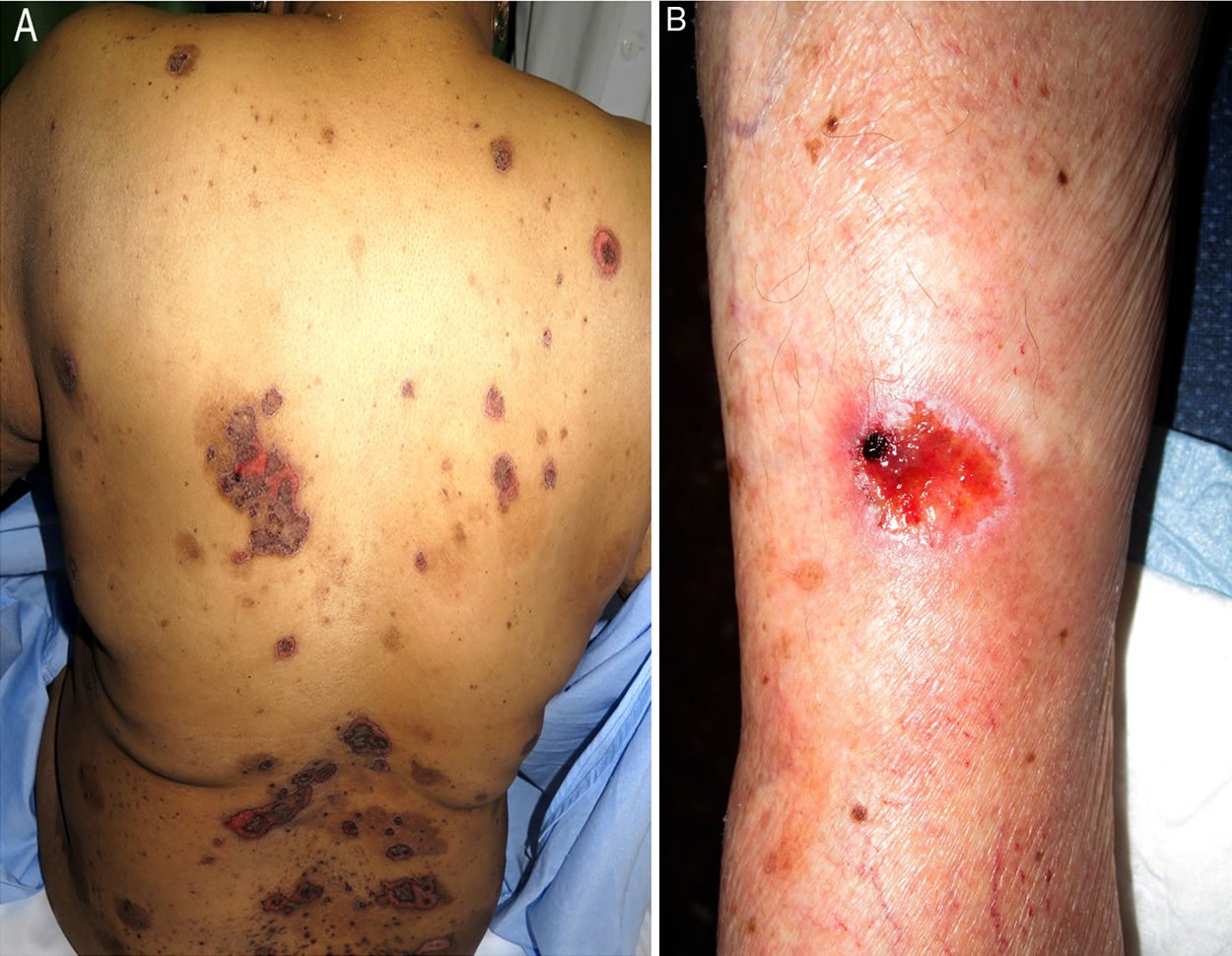
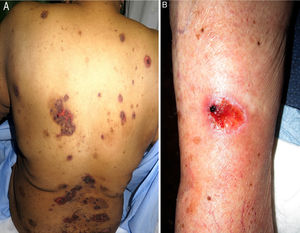
We performed a retrospective study of patients with a diagnosis of MTX-induced mucositis or mouth ulcers evaluated by the dermatology department at our hospital between January 2013 and July 2018. The patients’ medical records were reviewed to collect epidemiological, clinical, and pictorial information. Five patients (1 man and 4 women) with a median age of 72 years (range, 50–90 years) were included. The epidemiological and clinical characteristics are summarized in Table 1. The median MTX dose was 15mg/wk (range, 10–35mg/wk). The route of administration was subcutaneous in 3/5 patients and oral in 2/5 patients. Two of the patients were taking MTX for psoriasis and 3 were taking it for rheumatoid arthritis. All the patients had erosions and necrotic crusts in the oral cavity (Fig. 1A–D). Additional conditions included pancytopenia or bicytopenia in 4/4 patients, fever in 2/5, and skin ulcers in 2/5 (one had ulcerated psoriatic plaques and the other had an ulcerated basal cell carcinoma on the leg (Fig. 2A and B). Median peripheral blood leukocyte count was 2475×106 (range, 330–4200×106). Additional laboratory values (median [range]) were hemoglobin 73.5g/L (range, 53–92g/L), mean corpuscular volume 98.5fL (range, 93–105fL), and platelets 92500×106 (range, 37000–128000×106). Serum creatinine levels were elevated in 2/4 patients (median, 2.42mg/dL [range, 0.44–5mg/dL]). Only 1 patient had impaired liver function reflected by elevated gamma-glutamyl transferase (156mg/dL [reference value<40mg/dL]) and prothrombin time (international normalized ratio, 1.4). Bone marrow aspiration was performed in 3/5 patients and showed marked hypocellularity, which is consistent with MTX toxicity. The most common trigger was use of a nonsteroidal anti-inflammatory drug (NSAID) (2/5), followed by an administration error (1/5), infection (1/5), and a lack of folic acid intake (1/5). Plasma MTX levels were normal (<0.3mol/L) in 3/3 patients. Treatment included MTX withdrawal in 5/5 patients, intravenous folic acid in 4/5, and granulocyte colony stimulating factor in 1/5. All the patients achieved complete recovery and just 1 was restarted on MTX.
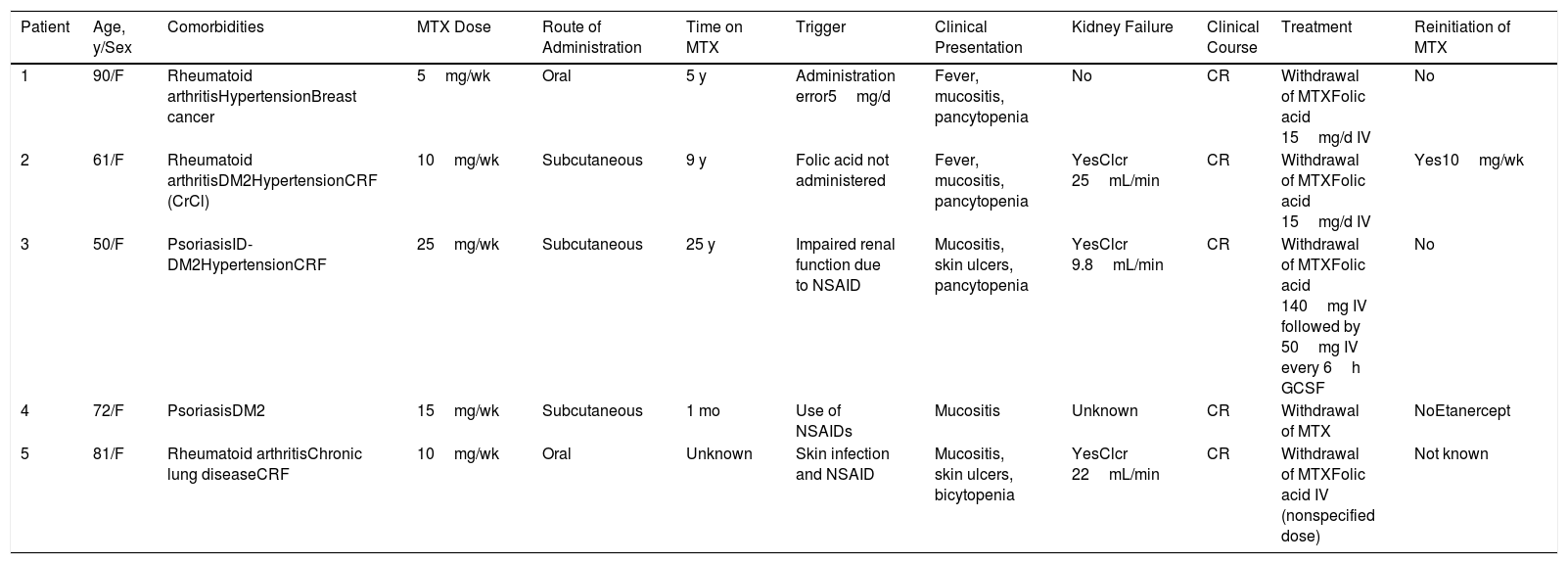
Epidemiological and Clinical Characteristics of Patients With MTX-induced Toxicity and Mucocutaneous Lesions.
| Patient | Age, y/Sex | Comorbidities | MTX Dose | Route of Administration | Time on MTX | Trigger | Clinical Presentation | Kidney Failure | Clinical Course | Treatment | Reinitiation of MTX |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 90/F | Rheumatoid arthritisHypertensionBreast cancer | 5mg/wk | Oral | 5 y | Administration error5mg/d | Fever, mucositis, pancytopenia | No | CR | Withdrawal of MTXFolic acid 15mg/d IV | No |
| 2 | 61/F | Rheumatoid arthritisDM2HypertensionCRF (CrCl) | 10mg/wk | Subcutaneous | 9 y | Folic acid not administered | Fever, mucositis, pancytopenia | YesClcr 25mL/min | CR | Withdrawal of MTXFolic acid 15mg/d IV | Yes10mg/wk |
| 3 | 50/F | PsoriasisID-DM2HypertensionCRF | 25mg/wk | Subcutaneous | 25 y | Impaired renal function due to NSAID | Mucositis, skin ulcers, pancytopenia | YesClcr 9.8mL/min | CR | Withdrawal of MTXFolic acid 140mg IV followed by 50mg IV every 6h GCSF | No |
| 4 | 72/F | PsoriasisDM2 | 15mg/wk | Subcutaneous | 1 mo | Use of NSAIDs | Mucositis | Unknown | CR | Withdrawal of MTX | NoEtanercept |
| 5 | 81/F | Rheumatoid arthritisChronic lung diseaseCRF | 10mg/wk | Oral | Unknown | Skin infection and NSAID | Mucositis, skin ulcers, bicytopenia | YesClcr 22mL/min | CR | Withdrawal of MTXFolic acid IV (nonspecified dose) | Not known |
Abbreviations: CR, complete response; CRF, chronic renal failure; DM2, diabetes mellitus type 2; F, female; ID, insulin-dependent; M, male; MTX, methotrexate; NSAID, nonsteroidal anti-inflammatory drug.
The most common serious adverse effect of MTX is bone marrow toxicity, which has been observed in 2.5% to 10% of patients and is potentially fatal.4 Mucocutaneous ulceration is a characteristic finding in patients with acute MTX toxicity.3,5 MTX inhibits cells with a fast turnover and therefore hematopoietic and skin cells are more likely to be affected by its antiproliferative action.6 MTX is mainly eliminated by glomerular filtration, and just a small fraction is metabolized in the liver. Decreased glomerular filtration rate due to dehydration, infection, and use of drugs such as NSAIDs can therefore lead to MTX accumulation and subsequent toxicity.3,7 In our study, 3/4 patients had impaired kidney function and 3/5 were taking NSAIDs. These associations are consistent with previous reports describing dosing errors, kidney failure, drug-drug interactions (NSAIDs, antibiotics, or salicylates), and MTX reintroduction or dose increments as the main causes of MTX toxicity.2,3,5,7
All the patients in our study with blood test results (4/4) had pancytopenia or bicytopenia, and 2 required empirical antibiotic therapy due to febrile neutropenia. These findings highlight the complex management of MTX-induced toxicity, the potential complications, and the need for a high index of clinical suspicion in patients who develop mucocutaneous ulcers while on MTX, particularly if they have risk factors for decreased glomerular filtration rate.2,3 Elevated mean corpuscular volume (present in 2 of our patients) could be an early sign of bone marrow toxicity due to folic acid deficiency. Serum MTX levels were low in 3 of the 4 patients with mucositis and pancytopenia tested. These low levels can be explained by the cellular accumulation of the drug. Plasma concentrations are typically 0.01M 24 hours after withdrawal of MTX, and serum levels do not correlate well with toxicity.7
Skin ulcers due to MTX have mostly been described in association with psoriasis or previous injury.2,5 In our series, ulcers were observed in psoriatic plaques in 1 patient and in a basal cell carcinoma in the other. Ulcers can, however, also affect healthy skin.
MTX toxicity can cause high morbidity and mortality.3 Detection of mucocutaneous ulcers in a patient on MTX should raise suspicion of pancytopenia and kidney failure, even in patients on low MTX doses and with normal serum concentrations. These patients must be hospitalized and treated by a multidisciplinary team.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Morgado-Carrasco D, Riquelme-Mc Loughlin C, Fustà-Novell X, Giavedoni P. La mucositis por metotrexato como marcador de toxicidad medular. Estudio retrospectivo de las características clínicas y epidemiológicas. Actas Dermosifiliogr. 2020;111:436–439.