In this review, we analyze the 3 clinical scenarios related to the development of melanoma in solid organ transplant recipients: melanoma in patients with a history of the tumor prior to a transplant, de novo melanoma following a transplant, and melanoma of donor origin. The main factors to consider in organ-transplant candidates with a history of melanoma are tumor stage, presence or absence of residual disease, and time from diagnosis to transplantation. Solid organ transplant recipients have a greater risk of melanoma than immunocompetent individuals. Mortality is also higher in this population, especially in patients with advanced melanoma, as treatment is especially challenging. Clinical history and physical examination provide the most useful information for preventing donor-to-recipient transmission of melanoma. Donor-derived melanoma has a very poor prognosis.
El melanoma en receptores de un trasplante de órgano sólido (RTOS) puede aparecer en tres situaciones clínicas, objeto de esta revisión: pacientes con historia de melanoma previa al trasplante, pacientes que desarrollan el melanoma posteriormente al trasplante y pacientes con melanoma procedente del donante. Los factores más relevantes a considerar en pacientes con antecedentes de melanoma candidatos a un trasplante son el estadio del tumor, la presencia o no de enfermedad residual y el periodo entre el diagnóstico y el trasplante. Los RTOS tienen mayor riesgo de padecer un melanoma que la población inmunocompetente. La mortalidad por melanoma es también mayor, especialmente en aquellos con estadios avanzados, que suponen un verdadero reto terapéutico. Finalmente, la historia clínica y la exploración física del donante son las herramientas más útiles para evitar la transmisión de un melanoma al receptor, situación con pronóstico infausto.
Approximately 120 000 solid organ transplants (SOTs) are performed worldwide every year.1 Survival in this setting has improved with time thanks to advances in surgical techniques and better immunosuppressive regimens.2 Chronic immunosuppression increases the risk of malignancy, and in SOT recipients 40% to 50% of all malignancies are nonmelanoma skin cancers.3 In the general population, melanoma accounts for up to 80% of skin cancer deaths.4,5 Early diagnosis significantly reduces melanoma-specific mortality.6 Because melanoma is a highly immunogenic tumor, it would be expected to be more common and more aggressive in SOT recipients due to their immunosuppressed state.7,8 Melanoma accounts for 6.2% of all malignancies in adult SOT recipients and for 15% of those in pediatric recipients.9 In this review, we analyze the different clinical scenarios in which SOT recipients can develop melanoma and describe the corresponding clinical and epidemiologic characteristics, treatments, and prognosis.
Search Strategy and Selection of ArticlesThe PubMed database was used to search for articles whose title or abstract contained combinations of the following MESH terms: “melanoma”, “organ transplantation”, “transplantation”, “nivolumab”, “pembrolizumab”, “ipilimumab”, “dabrafenib”, “vemurafenib”, “photocarcinogenesis”, and “voriconazole”.
A hand search was also made of the articles selected to identify additional studies.
Clinical ScenariosThree clinical scenarios related to the development of melanoma in SOT recipients are generally recognized: melanoma in patients with a pretransplant history of melanoma, de novo melanoma after a transplant, and melanoma of donor origin.6 In our experience, there is a fourth scenario in which an SOT recipient was not diagnosed or treated for melanoma until seen by a dermatologist after the transplantation.
History of Pretransplant MelanomaUnfortunately for anyone whose survival depends on an SOT, a history of melanoma is a classic contraindication for SOT.10 There is little evidence on whether SOT recipients with a history of melanoma have an increased risk of recurrence or progression after transplantation.11 The authors of a series of 31 SOT recipients with a past history of melanoma reported an alarming recurrence rate of 19% and recommended leaving a period of at least 5 years between melanoma treatment and SOT.12 The authors, however, did not provide details of Breslow depth, the most important prognostic factor in melanoma.13 These high recurrence rates were not confirmed by more recent work.10 Dapprich et al.,14 in a series of 12 SOT recipients previously treated for melanoma (mean Breslow depth, 0.35 mm), found no cases of posttransplant recurrence or melanoma-specific mortality. Similarly, the European Skin Care in Organ Transplant Patients, Europe (SCOPE) group reported no melanoma recurrences or deaths after SOT in a series of 9 recipients with a history of melanoma.4 Brewer et al.15 found no significant differences in recurrences (after a period of 10.5 years) or metastasis rates in 59 SOT recipients with pretransplant melanoma. It should be noted, however, that Breslow depth measurements were available for just 17 cases.15 It should also be noted that the results of these studies were probably affected by selection bias, as patients with a history of melanoma selected for SOT are likely to have a better prognosis (lower Breslow depth) and to have been free of disease for longer. Another study showed that 336 SOT recipients with a history of melanoma had a higher risk of posttransplant melanoma-specific mortality (hazard ratio [HR], 27; 95% CI, 11–64; P < .0001), overall mortality, and incident melanoma than recipients without a history of melanoma.16 Despite these alarming rates, the authors explained that because melanoma-specific deaths are, in absolute terms, rare among SOT recipients, the difference in 5-year mortality due to melanoma between recipients with and without pretransplant melanoma was just 1.2%. They were of the opinion that these data would probably not justify a change in patient selection strategies, although they would indicate the need for close dermatologic follow-up.16 Based on these studies and the melanoma survival curves in the American Joint Commission on Cancer (AJCC) Cancer Staging Manual (7th Edition), the International Immunosuppression and Transplant Skin Cancer Collaborative (ITSCC) drew up a series of recommendations on the wait time between melanoma treatment and transplant (Table 1).17 Based on a 5-year posttransplant survival rate of 60% and the AJCC 7th Edition survival curves, the authors proposed that patients with melanoma stages Ia, Ib, IIa, IIb, or IIIa would be candidates for SOT. They also considered that the point at which the survival curve for each stage flattened was the minimum wait time between a diagnosis of melanoma and SOT. Sentinel lymph node biopsy results are particularly useful when evaluating the candidacy of patients with a history of melanoma for SOT.6
Minimum Time From Melanoma Treatment to SOT According to the ITSCC.
| Stage (AJCC CSM, 7th Edition) | Recommended Time to SOT |
|---|---|
| Stage 0 | No need to defer SOT |
| Stage Ia | 2 y |
| Stage Ib | 5 y |
| Stage IIa, IIb, IIIa | 5-10 y |
| Stages IIc, IIIb, IV | Not candidates for SOTa |
Abbreviations: AJCC CSM, American Joint Commission on Cancer Cancer Staging Manual; ITSCC, International Transplant Skin Cancer Collaborative; SOT, solid organ transplant.
Source: Adapted from Zwald et al.17
SOT recipients have a 2- to 8-fold increased risk of developing melanoma compared with members of the general population18–24 (Table 2). The higher number of dermatology examinations in SOT recipients has probably led to increased awareness of the risk among other health care professionals and consequently more diagnoses. The risk of melanoma may be 17.2 times higher in African American SOT recipients.25
Studies of the Incidence of Melanoma in SOT Recipients and Immunocompetent Individuals in Spain.
| Reference | No. of SOT Recipients | Study Location | Incidence Rate (Annual Cases per 100 000 Population) |
|---|---|---|---|
| Garret et al.20 | 10649 | United States | 75 |
| Brown et al.21 | 861 | London, United Kingdom | 82 |
| Vajdic et al.22 | 8152 | Australia | 134 |
| Reference | Study | Study Location | Incidence Rate (Annual Cases per 100 000 Population) |
|---|---|---|---|
| Tejera-Vaquerizo et al.23 | Meta-analysis | Spain | 8.2 |
Abbreviation: SOT, solid organ transplant.
While SOT recipients have a higher risk of melanoma than immunocompetent people, they have an even higher risk of squamous cell carcinoma (50- to- 250-fold increased risk) and basal cell carcinoma (10-fold increased risk).11
Risk FactorsMelanoma is a highly immunogenic tumor and responds very well to new immunotherapies.17 Like other tumors, however, it has developed mechanisms to evade immune surveillance, enabling it to spread.11 In immunosuppressed patients, one would expect melanoma to have an even higher incidence and greater metastatic potential.15 Apart from immunosuppression itself, other factors specific to each immunosuppressive agent may contribute to the higher incidence and faster progression of melanoma in immunosuppressed individuals,8 as occurs with nonmelanoma skin cancer (NMSC). There have even been reports of good response and outcomes in patients with melanoma following withdrawal of immunosuppressants.22 Calcineurin inhibitors and azathioprine increase the risk of skin cancer by reducing immune surveillance, increasing vascularization and tumor invasive capacity, and enhancing DNA damage (e.g., after exposure to UV-B radiation) or inhibiting its repair.26 Replacing classic immunosuppressive agents with a mammalian target of rapamycin (mTOR) inhibitor, which has antiproliferative properties, is known to be effective for the secondary prevention of NMSC.2,27,28 Very little data, however, are available on the benefits of switching from an immunosuppressive regimen to an mTOR inhibitor in melanoma. The current evidence is based on animal studies and the CONVERT trial, which showed a lower incidence of melanoma in kidney transplant recipients who received sirolimus than in those who did not, although the incidence was very low in both groups.29,30 It is noteworthy that a meta-analysis of data from 5876 patients from 21 randomized controlled trials showed that the use of sirolimus in SOT recipients was associated with a 43% increased risk of death (HR, 1.43; 95% CI, 1.21–1.71; P < .001) in patients treated with high doses. The main causes of death were cardiovascular or infectious disease. These data, together with a high incidence of adverse effects (many of which increase cardiovascular risk), are limiting factors for the use of sirolimus as a first-line immunosuppressive treatment.28
Vajdic et al.22 showed that the risk of melanoma in SOT recipients peaked in the second year posttransplant, and then decreased linearly. Additional risk factors were age and induction immunosuppression with monoclonal antibodies. Female sex, non-Caucasian race, and a longer time since SOT, by contrast, were protective factors.
Several studies have reported a possible association between a higher number of nevi in SOT recipients and duration of immunosuppressive therapy. A high number of nevi is a risk factor for melanoma.6,31 In a Swedish series, 63% of melanomas in SOT recipients were histologically associated with the presence of a dysplastic nevus.32 In another 2 series, just 33% to 36% of melanomas in SOT recipients arose in a previous nevus.18,24
A number of studies on melanoma in SOT recipients have mentioned that a past history of NMSC is common.4,24
Voriconazole is used to treat invasive fungal infections in SOT recipients, particularly following a lung transplant. Its use is considered an independent risk factor for skin cancer, in particular squamous cell carcinoma. Apart from its photosensitizing properties, voriconazole appears to enhance DNA damage when exposed to UV radiation and to inhibit its repair. Several articles thus have pointed to a possible role for voriconazole in the development of melanoma. Discontinuation of this drug is recommended in SOT recipients who develop melanoma or squamous cell carcinoma.3,33
Disease Course and PrognosisStage at diagnosis is one of the main prognostic factors in patients with melanoma, whether they are immunosuppressed or immunocompetent.17,34 Breslow depth is the most significant histologic prognostic factor.17 The 2008 multicenter SCOPE study comparing outcomes between 95 SOT recipients who developed melanoma posttransplant and a cohort of immunocompetent patients with melanoma found no significant differences in mortality rates among patients with stage T1 or T2 disease. Mortality in patients with stage T3 or T4 disease, however, was significantly higher in SOT recipients (HR, 11.49; 95% CI, 3.59–36.82). Another study found that SOT recipients had more advanced melanoma at diagnosis than members of the general population (OR for stages III-IV, 4.2; 95% CI, 1.6–10.8; P = .003).32 The risk of melanoma-specific mortality was also higher in SOT recipients (adjusted HR, 3; 95% CI, 1.7–5.3; P < .001). Finally, a Canadian study of 51 patients with posttransplant melanoma also showed a higher risk of melanoma-specific mortality compared with immunocompetent individuals (adjusted HR, 1.93; 95% CI, 1.03–3.63; P = .04).35 All-cause mortality was also higher (2 to 8 times depending on melanoma stage) While it is true that changes to the immunosuppressive regimen could explain some of the increase in mortality, the authors did not believe that these changes made a significant contribution.35
Clinical and Histologic CharacteristicsBased on findings from the main series of posttransplant melanoma to date, the clinical and histologic characteristics of the tumors appear to be indistinguishable from those observed in immunocompetent individuals.4 The mean age at diagnosis was 54 years (range, 26–77 years) and there a predominance of male patients (66%), supporting previous findings.35 Mean time from the first transplant to onset of melanoma was 8.7 years (range, 0.1–24.9 years), which is somewhat shorter than the mean of 12 years reported by Brocard et al.36 Overall, 95% of patients had a Fitzpatrick skin type I-III and, contrasting with reports for the general population, there were no sex-related differences in tumor location. Park et al.35 found that SOT recipients were more likely to have melanomas on the head and neck than immunocompetent patients. In another study of melanoma in SOT recipients, the predominant locations were the trunk (51%) and the head and neck (26%) for men and the trunk (50%) and the extremities (42%) for women. The most common site for melanoma in women from the general population is the lower extremities (36%).32
In the series by Brocard et al.,36 2 (10%) of 20 SOT recipients with melanoma had mucosal melanoma.36 In our (unpublished) series of 8 patients, 2 (25%) had melanoma involving the oral mucosa. These rates of mucosal melanoma are higher than those reported for the general population (1%–2%).37 Further studies, however, are needed to confirm these data, but they might indicate differential etiologic or pathogenic mechanisms for melanoma in the context of SOT.
Although all histologic subtypes of melanoma were represented in the SCOPE series, superficial spreading melanoma was the most common form of invasive melanoma, supporting findings for the general population and other series.4,24,35 The mean Breslow depth observed in invasive melanomas was 1.5 to 2 mm.4,32 In the series described by Krynitz et al.,32 82% of melanomas diagnosed in SOT recipients had a Clark level of III to V compared with 66% of those in immunocompetent patients (OR, 2.2; 95% CI, 1.01–4.7). The authors also observed a less prominent lymphocytic infiltrate in SOT recipients who died of melanoma and suggested that this feature might be of prognostic value. It is probably a reflection of the underlying iatrogenic immunosuppression.32
Management and TreatmentThe initial treatment of melanoma in SOT recipients should be no different to that in immunocompetent patients: simple excision followed by widening of margins depending on Breslow depth and, where necessary, SLN biopsy. Proper staging is essential for correct management. The worse the prognosis, the more aggressive the approach, and consequently, the greater the risk to the survival of the transplanted organ.6,38
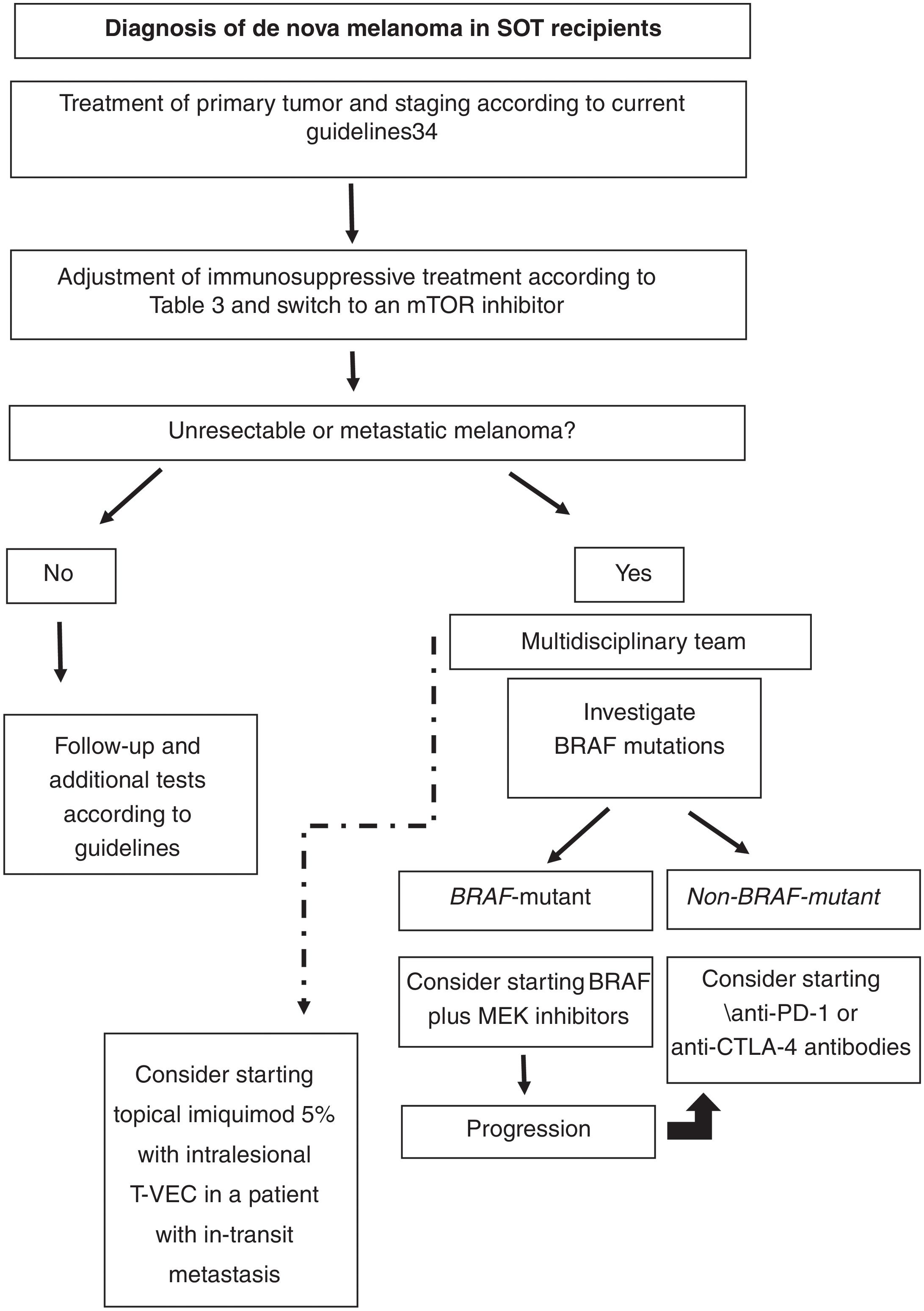
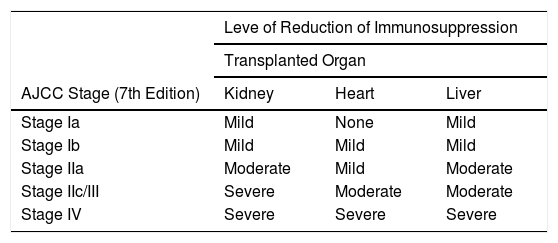
Immunosuppressive therapy should be revised in SOT recipients following a diagnosis of melanoma. Options include drug withdrawal, reduction of drug blood levels, and switching to another drug with antiproliferative and antiangiogenic activity to reduce the risk of tumor spread. It is important to strike a balance between a level of immunosuppression that will not favor the spread of the tumor and one that will not result in rejection of the transplanted organ. Important factors to bear in mind are tumor stage and prognosis, the type of organ transplanted and the possibility of artificially reproducing its function (e.g., dialysis in the case of kidneys), and the patient’s general health. The ITSCC recommendations on the level of reduction of immunosuppression following a diagnosis of melanoma or NMSC are shown in Table 3.6,39 Despite the limited evidence available for melanoma, it would seem reasonable to consider an immunosuppressive regimen involving an mTOR inhibitor where possible.28
ITSCC and SCOPE Recommendations on the Level of Reduction of Immunosuppression for Solid Organ Transplant Recipients With Nonmelanoma Skin Cancer or Melanoma.
| Leve of Reduction of Immunosuppression | |||
|---|---|---|---|
| Transplanted Organ | |||
| AJCC Stage (7th Edition) | Kidney | Heart | Liver |
| Stage Ia | Mild | None | Mild |
| Stage Ib | Mild | Mild | Mild |
| Stage IIa | Moderate | Mild | Moderate |
| Stage IIc/III | Severe | Moderate | Moderate |
| Stage IV | Severe | Severe | Severe |
Abbreviations: AJCC, American Joint Commission on Cancer (Cancer Staging Manual); ITSCC, International Transplant Skin Cancer Collaborative.
Source: Adapted from Zwald et al.6
Survival in patients with advanced melanoma, and metastatic melanoma in particular, is very low.40 Targeted therapies and immunotherapies have revolutionized the treatment of melanoma, achieving long survival times in certain patients with disseminated disease.41 Evidence, however, is lacking on the safety and efficacy of these treatments in SOT recipients, as these patients have been systematically excluded from the clinical trials.42 Some studies have shown acceptable responses to combined BRAF and MEK inhibition in SOT recipients with advanced BRAF-mutant melanoma.40,43 Nonetheless, several studies have indicated that BRAF mutations are less common in melanomas in SOT recipients.36,44 In our unpublished series of SOT recipients with melanoma, just 29% had a BRAF mutation, compared with 54% of patients with melanoma from the general population.45 It is possible that both immunosuppression and immunosuppressive therapy might have a differential effect on the pathogenesis of melanoma in SOT recipients.44 Brocard et al.36 characterized the mutational profile of melanoma (BRAF, c-KIT, and NRAS genes) in 20 SOT recipients.36BRAF and NRAS mutations were detected in 40% and 23% of cases, respectively. No c-KIT mutations were found. One limitation of the study, however, was that a significant number of samples were inadequate for molecular analysis.36 More studies are therefore needed to investigate differences in melanoma mutations between SOT recipients and immunocompetent patients, as this information would help identify potential therapeutic targets.
Immune checkpoint inhibitors (ICIs) should be considered in SOT recipients with advanced non-BRAF-mutant melanoma. The potential benefits and increased risk of organ rejection must be carefully weighed up, and it is very difficult to find the exact point at which this balance is maintained.46 The decision should be taken by a multidisciplinary committee including members of the transplant team, oncologists, and dermatologists.17 It is also essential to discuss the matter with the patient and involve him or her in the decision. Evidence on the use of ICIs in SOT recipients is based on isolated clinical reports and small series.47–60 Abdel-Wahab et al.42 recently published the findings of the largest series of SOT recipients treated with ICIs to date at the University of Texas MD Anderson Cancer Center. They also performed the first systematic review of the literature on this subject.42 They analyzed organ rejection, survival, and tumor response in 39 SOT recipients with melanoma (metastatic in 62% of cases) treated with ICIs. Allograft rejection occurred in 41% of patients and the median time to rejection was 21 days after ICI initiation; 81% of the patients (10 kidney transplant recipients and 3 liver transplant recipients) experienced graft loss despite treatment (increased immunosuppression and ICI discontinuation). Rejection rates were found to be similar with anti-cytoxic T-lymphocyte antigen 4 and anti-programmed death 1 antibodies, contradicting previous findings suggesting that the former might be safer.56 Of the 22 SOT recipients with metastatic melanoma, 64% experienced tumor progression, while 32% showed a partial or complete response. In a recent study, Hurkmans et al.61 proposed that donor-derived cell-free DNA might be a sensitive biomarker for the early detection of transplant rejection in SOT recipients treated with ICIs.
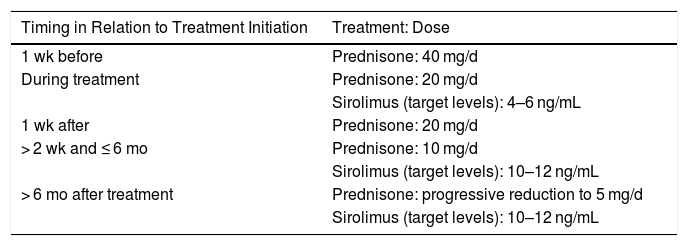
There has been a report of an immunosuppressive regimen being successfully used to preserve renal-allograft function in a recipient with metastatic adenocarcinoma of the duodenum due to receive treatment with nivolumab54 (Table 4).
Immunosuppressive Regimen for Kidney Transplant Recipients Who Are Candidates for Anti-Programmed Death-1 Antibodies.
| Timing in Relation to Treatment Initiation | Treatment: Dose |
|---|---|
| 1 wk before | Prednisone: 40 mg/d |
| During treatment | Prednisone: 20 mg/d |
| Sirolimus (target levels): 4–6 ng/mL | |
| 1 wk after | Prednisone: 20 mg/d |
| > 2 wk and ≤ 6 mo | Prednisone: 10 mg/d |
| Sirolimus (target levels): 10–12 ng/mL | |
| > 6 mo after treatment | Prednisone: progressive reduction to 5 mg/d |
| Sirolimus (target levels): 10–12 ng/mL |
Source: Adapted from Barnett et al.17
Sunshine et al.62 successfully treated a kidney transplant recipient with melanoma and in-transit metastasis with a combination of topical imiquimod 5% and talimogene laherparepvec (T-VEC) injections. No signs of rejection were observed.
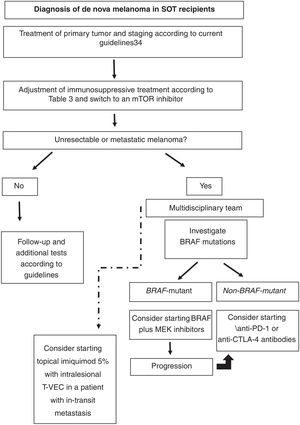
Based on the literature reviewed, our proposed approach to treating de nova melanoma in an SOT recipient is shown in Fig. 1.
Dermatologic Follow-up of SOT RecipientsSOT recipients with advanced melanoma have a significantly worse prognosis than members of the general population with advanced melanoma, and they also face a very high risk of organ rejection after ICI therapy. Close dermatologic surveillance is thus necessary to ensure that any melanoma that may occur is diagnosed early.4 Frequency of follow-up should be determined by individual risk, with particular attention paid to patients with a dysplastic nevus or a personal or family history of melanoma.6,24 Follow-up with dermoscopy, in addition to body mapping and reflectance confocal microscopy, is useful for improving the ratio of melanomas to benign skin lesions excised.24 Patients should be taught how to recognize suspicious lesions or recurrent tumors at previous excision sites and cautioned to avoid recreational sun exposure and to continue to use sun-protection and other measures that minimize sun exposure.17 Strict sun protection measures must be accompanied by monitoring of vitamin D levels and any deficiencies corrected through supplementation.36 Finally, we believe that SOT candidates should be examined by a dermatologist before undergoing a transplant to rule out melanoma. Immunosuppression—and hence risk of tumor progression—is highest during the peritransplant period (Fig. 2).
Donor-Derived MelanomaMelanoma can be transmitted to an SOT recipient via an organ donated by a person with melanoma. This could happen in the case of occult melanomas, which would then spread in an immunosuppressed transplant recipient. It could also happen in the case of donors (especially young donors) who died of metastatic melanoma of the brain misdiagnosed as a brain hemorrhage or a primary brain tumor. The estimated risk of occult donor malignancy is 1.3%, and the risk of transmission to a recipient, 0.2%.6 Melanoma is the donor-derived tumor with the highest risk of metastasis. One review analyzed donor-to-recipient transmission of melanoma in 7 donors whose organs were provided to 44 recipients.6 Thirty-five recipients (80%) developed melanoma within 3 to 24 months of the transplant, and 33 (75%) died as a result.6,11 These data indicate that anyone with a history of melanoma should not be ruled out as an organ donor. In addition, potential donors should be given a full-body examination to search for lesions or scars suspicious for melanoma.3,4
The little evidence available on the treatment of donor-derived melanoma suggests that the best approach may be to excise the transplanted organ together with any resectable metastases and to discontinue immunosuppressive therapy. This approach, however, is only possible in the case of kidney recipients. Recipients of other vital organs face a grim prognosis unless there is another organ available.4 Molecular biology techniques such as fluorescent in situ hybridization, polymerase chain reaction assays, and tandem repeat sequencing can be used to clarify doubts regarding whether metastatic melanoma detected in an SOT recipient is de novo or donor derived. This information has important prognostic and therapeutic implications. In addition, if the tumor is donor derived, any other organs donated by the donor, regardless of the recipient, should also be removed.4
ConclusionsAlthough evidence on the management of melanoma in SOT recipients is scarce and based on case series, it is known that in early-stage melanoma, prognosis in SOT recipients is similar to that of the general population. Dermatologists thus have an essential role in posttransplant follow-up as they are in a position to diagnose thin melanomas. Much work remains regarding the management of advanced melanoma in SOT recipients, who currently face a worse prognosis than immunocompetent patients with metastatic disease. Because SOT recipients have been excluded from clinical trials of ICIs, treatment decisions should be reached by a multidisciplinary committee, with the informed consent of the patient and knowing that there is a high risk of acute rejection. Further research is also necessary on the role of adjuvant therapy in SOT recipients as it has been demonstrated to improve survival in immunocompetent patients.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: González-Cruz C, Ferrándiz-Pulido C, García-Patos Briones V. Melanoma en pacientes receptores de un trasplante de órgano sólido. Actas Dermosifiliogr. 2021;112:216–224.