Noonan syndrome with multiple lentigines (NSML), also known as LEOPARD syndrome, is a rare genetic skin disease whose acronym comes from its main clinical manifestations: lentigines (multiple), electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormalities of the genitalia, retarded growth, and deafness (sensorineural). NSML is usually associated with a mutation in the PTPN11 gene leading to alterations in the RAS-mitogen activated protein kinase (MAPK) transduction pathway. This signal transduction pathway plays a key role in the tumorigenesis of various cancers. There have also been reports of mutations in other genes, such as RAF1 and BRAF,1 thus enabling us to classify the syndrome as belonging to the so-called RASopathy group.2 Abnormal function of the abovementioned signal transduction pathways can increase the risk of cancer, with reports of an increased risk of diseases such as leukemia and neuroblastoma.3 However, the association with melanoma has received little attention in the literature, with only 4 cases published to date.4–7
A 44-year-old man with NSML confirmed by a genetic study of the mutation p.Tyr279Cys (c.8364 > G) in heterozygosity in the PTPN11 gene consulted in the dermatology department for follow-up of his skin lesions. The typical clinical manifestations of the syndrome included multiple lentigines, electrocardiographic abnormalities (incomplete right bundle branch block and anterior fascicular block), surgically treated infundibular pulmonary stenosis, and auditory abnormalities.
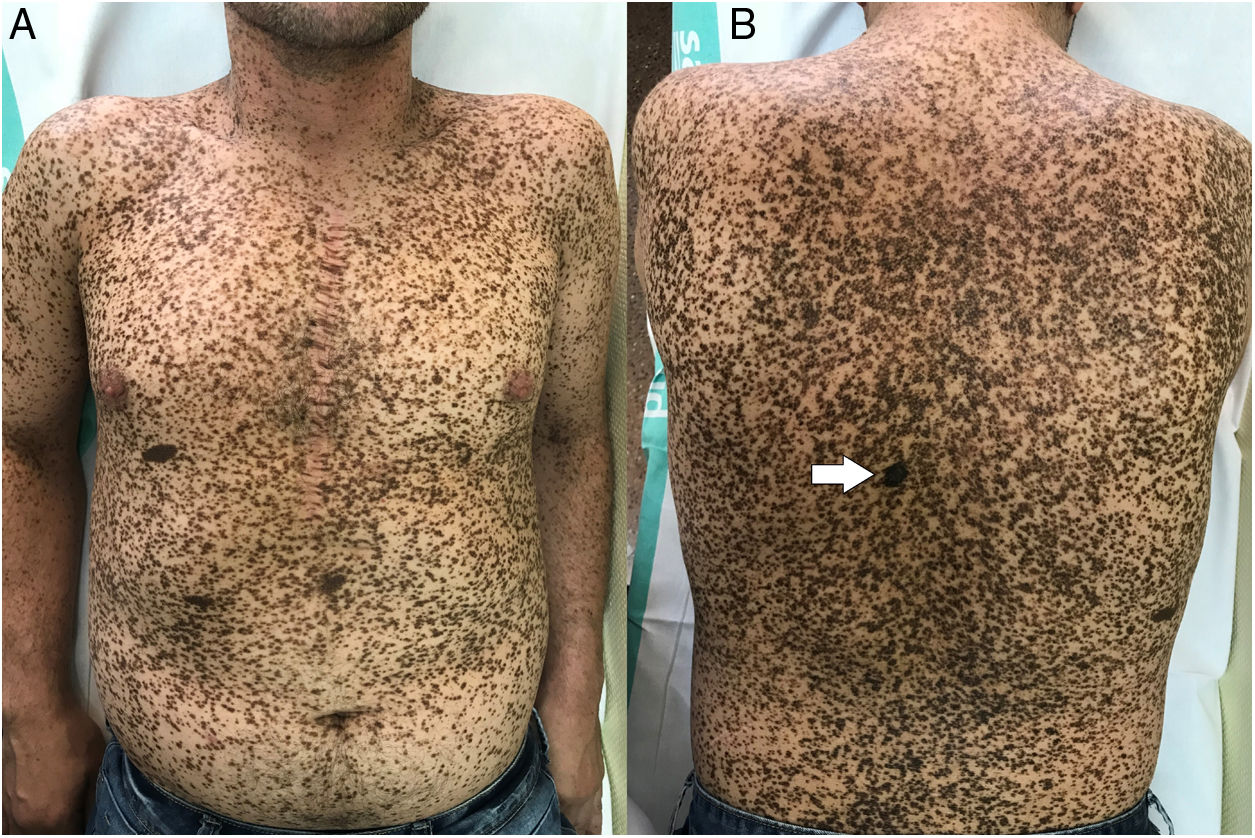
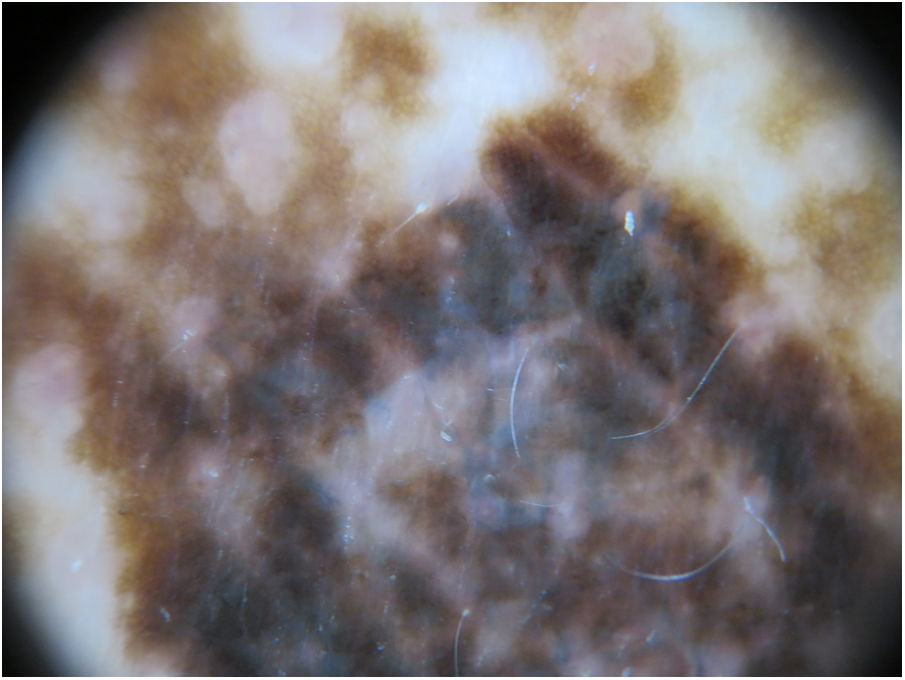
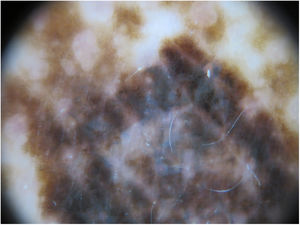
The physical examination revealed marked generalized multiple lentigines on the trunk and extremities, with most lesions measuring <1cm and other, larger lesions that were smaller in number. One of the most noticeable lesions was at the dorsal level. It had appeared recently and differed from the other lesions, with an irregular morphology. It measured approximately 2×1.2cm and had a more intense pigmentation. Polarized light dermoscopy revealed an atypical pigment network and gray-blue areas (Figs. 1 and 2).
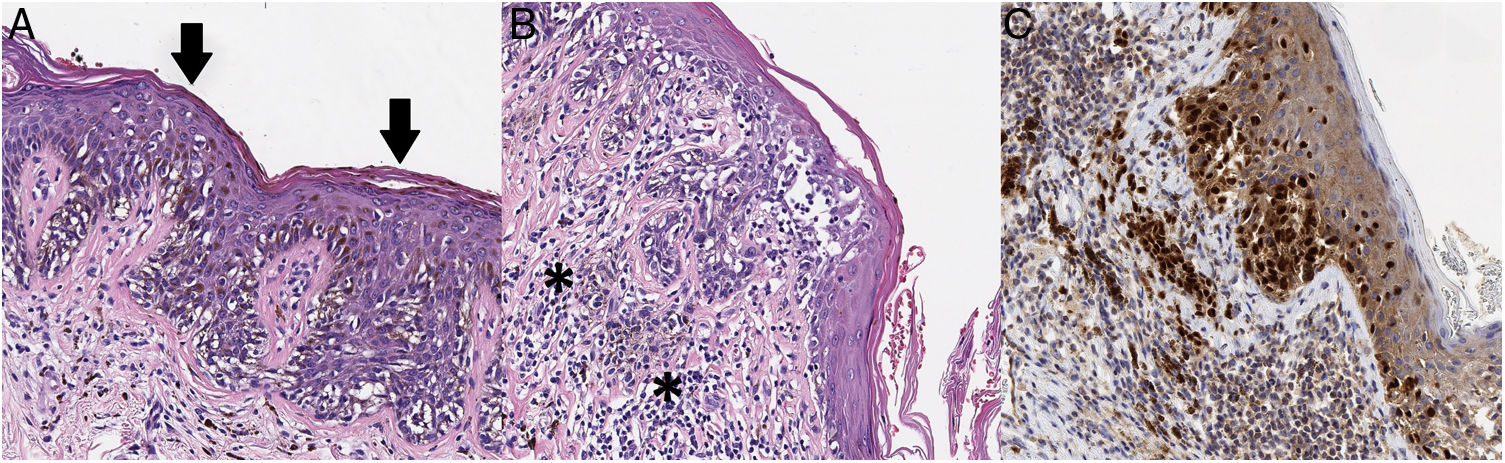
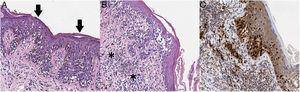
Given its clinical and dermoscopic characteristics and progress, the lesion was completely removed. Histopathology revealed superficial spreading melanoma with a Breslow depth of 0.15mm, and no ulceration, mitosis, vascular invasion, or evident neurotropism (Fig. 3).
A, The histopathology analysis reveals an atypical melanocytic neoplasm with a lentiginous growth pattern, pagetoid spread (arrows), and a moth-eaten pattern in the epidermis (hematoxylin-eosin, ×200). B, In other areas, we can see atypical melanocytes infiltrating the most superficial part of the dermis (asterisk), together with fibroplasia of the papillary dermis and inflammatory infiltrate (hematoxylin-eosin, ×200). C, Immunohistochemistry reveals nuclear positivity in intradermal and intraepidermal melanocytes (Sox10, ×200).
The patient was staged as IA according to the American Joint Committee on Cancer (2017).
A recent description of the clinical, histopathologic, and dermoscopic characteristics of NSML8 included both simple lentigines (lesions <1cm in diameter) and café noir spots (lesions >1cm in diameter), which, depending on their pigmentation and histologic and dermoscopic characteristics, have been subclassified as dark and medium-colored or pale.
Despite the low number of cases published, it is thought that patients with NSLM could have an increased risk of developing melanoma. This increased risk is explained because 90% of patients with NSML have a mutation in the PTPN11 gene.1 This gene codes for a protein tyrosine phosphatase (SHP-2), which regulates the activity of the RAS signaling pathway. The protein acts as a cytoplasmic transducer of various growth factors, cytokines, hormones, and integrins that produce the NSML phenotype.
The PTNPN11 gene was the first protooncogene identified that coded for a protein tyrosine phosphatase that is capable of promoting activation of the RAS-ERK signaling pathway involved in various types of cancer.9 The hypothesis has also been put forward that this gene could behave in some types of cells as a tumor suppressor gene by means of inhibition of the RAS-ERK pathway. Thus, suppression of protein SHP-2 favors tumorigenesis owing to abnormality of the STAT3 pathway, which is also involved in the pathogenesis of the melanoma.10
Despite the fact that few cases have been reported in the medical literature, we must take into account the probable increased risk of melanoma in patients with NSML. Therefore, patients should undergo periodic and exhaustive dermatologic follow-up to identify atypical and/or recent lesions. The phenotypic characteristics in this syndrome make this task an authentic challenge for dermatologists, although digital follow-up based on body mapping could help in its management.
Declaration of Competing InterestThe authors declare that there is no conflict of interest.
Please cite this article as: García-Gil MF, Álvarez-Salafranca M, Valero-Torres A, Ara-Martín M. Melanoma en el síndrome de Noonan con lentigos múltiples (síndrome de LEOPARD): presentación de un nuevo caso. Actas Dermosifiliogr. 2020;111:619–621.