Lentigo maligna is a type of in situ melanoma. It develops mainly in middle-aged and elderly individuals on areas of the skin chronically exposed to sunlight. It progresses to its invasive form, lentigo maligna melanoma, in 5% to 50% of cases. Management of lentigo maligna is open to debate, with a notable lack of randomized trials and specific guidelines and protocols. Early diagnosis and treatment is necessary to achieve cure if possible and prevent progression to invasive melanoma with the corresponding risk of metastasis. The treatment of choice for lentigo maligna is surgery. When surgery is not possible, other alternatives are available although outcomes and rates of recurrence are variable. The objective of this study was to review the diagnostic methods and criteria for lentigo maligna, as well as the different surgical options and alternatives to surgery, in order to provide information on the best approach in each case.
El lentigo maligno (LM) es una variante de melanoma in situ que se desarrolla principalmente en áreas de exposición solar crónica en pacientes de edad media-avanzada. Puede evolucionar a su forma invasiva lentigo maligno melanoma (LMM) en el 5-50% de los casos. Su manejo en ocasiones en controvertido, destacando la ausencia de estudios prospectivos aleatorizados y de guías específicas o protocolos. Es necesario realizar un diagnóstico y tratamiento precoces para obtener la curación, si es posible, y evitar la evolución a melanoma invasivo con el consiguiente riesgo metastásico. El tratamiento de elección del LM es la cirugía. Cuando esta no es posible pueden utilizarse otras alternativas con resultados y tasas de recidiva variables. El objetivo del presente trabajo es realizar una revisión de los métodos y criterios diagnósticos de LM, así como de las diferentes modalidades de tratamiento quirúrgico y las alternativas al mismo, que favorezca el mejor enfoque en cada caso.
Lentigo maligna (LM) is a variant of melanoma in situ that mainly affects chronically sun-exposed parts of the body in older patients.1 This type accounts for between 79% and 83% of all cases of melanoma in situ2 and can run a very slow course (up to decades) before progressing to its invasive form, lentigo maligna melanoma (LMM). Four to 15% of all melanomas are LMs or LMMs,3 and most affect the head and neck (86% of all cases).4 The incidence of LM is rising, and while most cases are described in patients aged between 65 and 80 years, there have been reports of LM in patients aged 20 to 30 years; in some large series 10% of the patients were younger than 40 years.1,5
No specific protocols have been published for the treatment of LM, and because of its special features, its management is still debated. LM generally occurs in elderly patients, affects parts of the body that have been damaged by the sun or carry a risk of cosmetic or functional defects, has poorly defined clinical and histologic margins, and recurs frequently following treatment.6 We review the diagnosis of LM, including methods and criteria, and provide an overview of the different treatment modalities available, with a description of the advantages and disadvantages of each in order to guide clinical decision making.
MethodsWe performed a literature search using the Cochrane and MEDLINE databases.
The search terms entered in PubMed were Hutchinson melanotic freckle, lentigo maligna, diagnosis, therapy, surgery, and radiotherapy. Preference was given to reviews and articles published in English or Spanish. We also reviewed the main clinical management guidelines for melanoma.
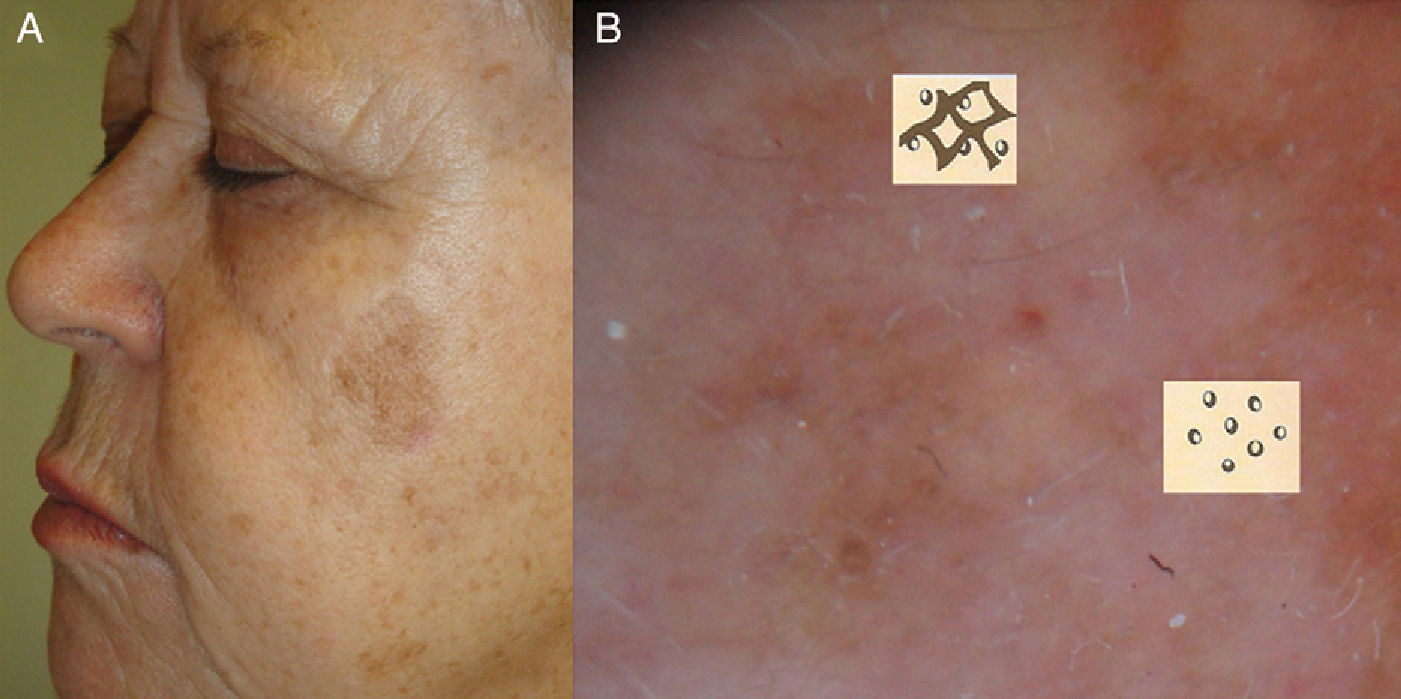
Diagnosis of LMLM presents clinically as a slow-growing, asymmetric macule with irregular, poorly defined borders and varied pigmentation (dark gray, light brown, dark brown, black, and white [areas of regression]).3,7 Albeit rarely, primary or recurrent LM can present clinically and histologically as an amelanotic lesion.8,9
Histology remains the diagnostic method of choice in LM.10 Characteristic histologic findings are the proliferation of atypical melanocytes along the dermal-epidermal junction; cells are often oriented perpendicular to the surface, and involvement of the periadnexal structures of the epithelium is a common finding. In more advanced lesions, nests of cells at the dermal-epidermal junction and multinucleated giant cells may be observed. Occasionally, there may be pagetoid spread, epidermal atrophy, and solar elastosis in the dermis. The papillary dermis may contain melanophages and a chronic inflammatory infiltrate.8
It is sometimes difficult to distinguish atypical melanocytes from the atypical junctional melanocytic hyperplasia seen in actinically damaged skin. A useful approach is to take a control biopsy from the contralateral side of the face.11
In general, biopsy of suspicious or changing pigmented lesions on the face is recommended.12 Excisional biopsy is the most accurate diagnostic method in LM, but because lesions are often large and located in cosmetically sensitive areas, punch biopsy is the most widely used procedure,13 despite the problem of sampling error.14 Based on observational studies by Stevens and Cocherell,15 deep incisional biopsy extending as far as the subcutaneous tissue with a punch of at least 5mm can minimize this source of error. For large lesions, mapping and taking multiple punch biopsies of the most representative sites (darker or palpable areas suspicious for invasion) offers a possible approach. The use of complementary techniques such as dermoscopy and confocal reflectance microscopy (CRM) is also useful.
Some facial lesions that are clinically insignificant, i.e., that do not meet the ABCD criteria (Asymmetry; Borders, uneven; Color, multiple; Diameter, >6mm), are histologically malignant.16,17 Routine dermoscopy is therefore a useful complementary tool that can help to identify such lesions early so that even lesions that appear clinically benign can be excised. Schiffner and Stoltz18 reported that dermoscopy has a sensitivity of 89% and a specificity of 96% for the early diagnosis of LM when several dermoscopic features are detected and that the presence of a single feature is not diagnostically useful.
Main Dermoscopic Criteria for LMThe most characteristic dermoscopic finding in facial LM lesions is a pigmented pseudonetwork, which is an area of homogeneous pigmentation disrupted by follicular or adnexal openings on the face.19
Other dermoscopic criteria for LM/LMM are asymmetric pigmented follicular openings (corresponding to follicular invasion by tumor melanocytes); dark rhomboidal structures (proliferation of melanocytes around the interfollicular spaces); slate-gray dots and globules (melanin-laden macrophages in the superficial dermis that may form granular, ring-like images in several areas); dark streaks (rows of melanoma cells in the epidermis or superficial dermis); homogeneous areas (complete obliteration of follicular openings by invading melanoma cells); and isobar structures (concentric pigmented circles around the follicular openings).
Another recently described pattern is a series of brown or blue-gray dots in lines that form a zigzag.20
Dermoscopy is also useful for selecting biopsy sites, distinguishing LM from other pigmented lesions on the face,18,19 delineating surgical margins, and assessing and monitoring treatment response.
Dermoscopy as a Guide for Punch Biopsy/Mapping/Incisional BiopsyBiopsy sites should contain rhomboidal structures or homogeneous areas, and sites with regression or melanophages should be avoided. If histology is negative and suspicion is still high, a second biopsy should be performed or the entire lesion excised if possible. Because lesions can contain both benign and malignant features, areas with benign features, such as light brown fingerprint-like structures (solar lentigines), comedo-like openings (seborrheic keratosis), or opaque yellowish areas (seborrheic keratosis) should not be sampled.20
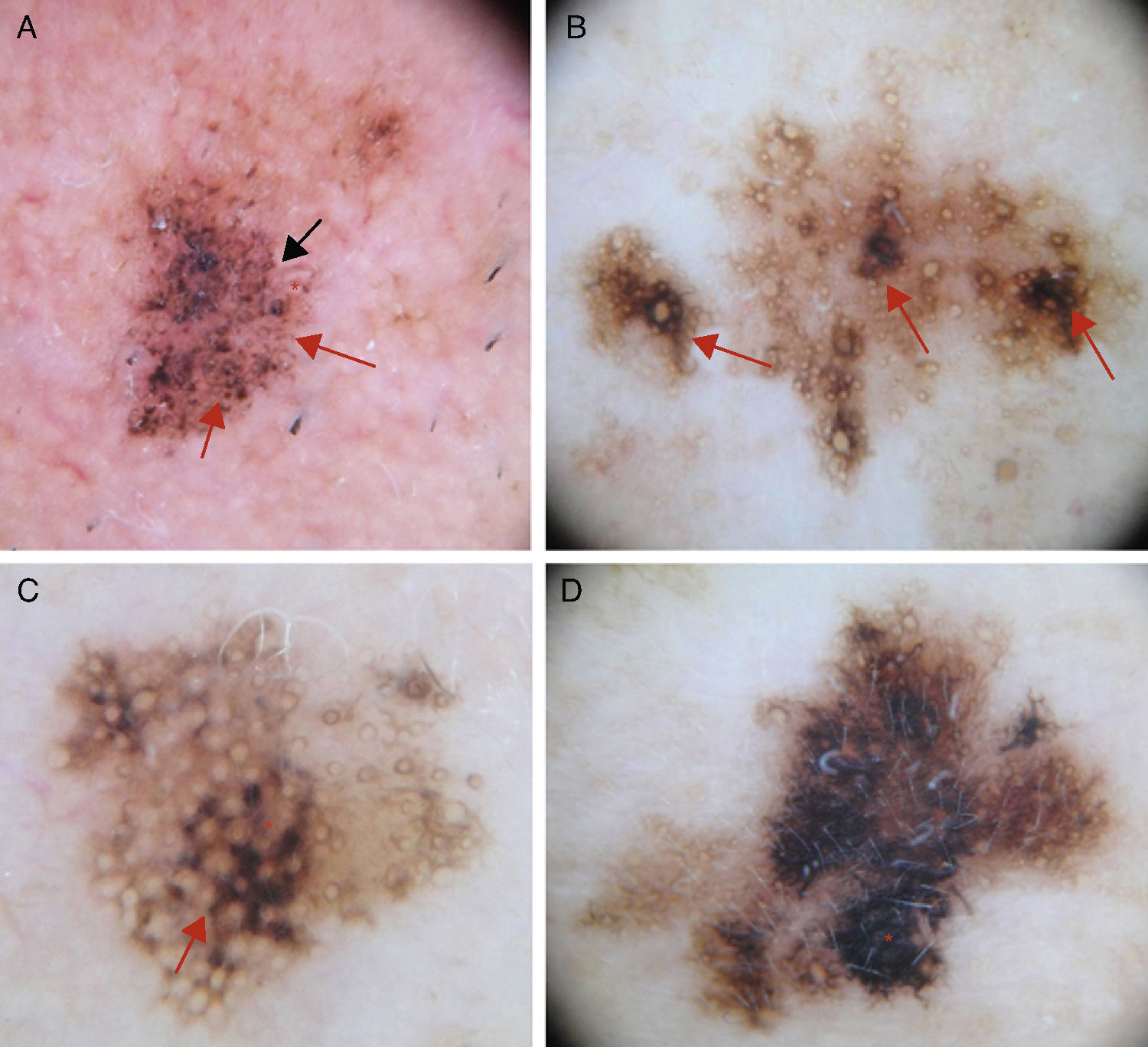
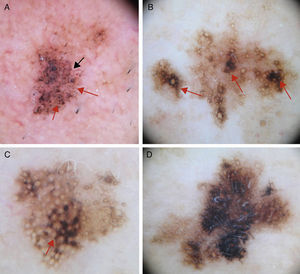
Early Diagnosis: The Schiffner Dermoscopic Progression ModelRapid recognition of the first dermoscopic signs of primary or recurrent LM is important during follow-up. Schiffner and colleagues18,21 designed a progression model for LM that shows the characteristic dermoscopic structures of LM lesions from the earliest to the latest phases in the following order: dots around the hair follicle, short streaks, rhomboidal structures, follicular openings, and homogeneous areas-obliteration of follicular openings (Figs. 1 and 2).
A, Dermoscopic image of early-phase lentigo maligna showing irregularly distributed slate-gray dots and globules (red arrows), short dark streaks, early rhomboidal structures (black arrow), and asymmetric perifollicular pigmentation (asterisks). B, Dermoscopic image of lentigo maligna showing follicular openings with asymmetric pigmentation, some of which have signs of early obliteration (red arrows). C, Image showing isobar structures (asterisk), rhomboidal structures (red arrow), and follicular openings with asymmetric pigmentation. D, Image of histologically confirmed lentigo maligna melanoma following biopsy taken of area marked by the asterisk, showing homogeneous areas with obliterated follicular openings.
It can be difficult to distinguish LM from other pigmented lesions (solar lentigines, pigmented actinic keratosis, seborrheic keratosis, benign lichenoid keratosis) by simple visual examination.
Furthermore, dermoscopy alone may not be sufficient as LM shares several dermoscopic features with other lesions.22,23
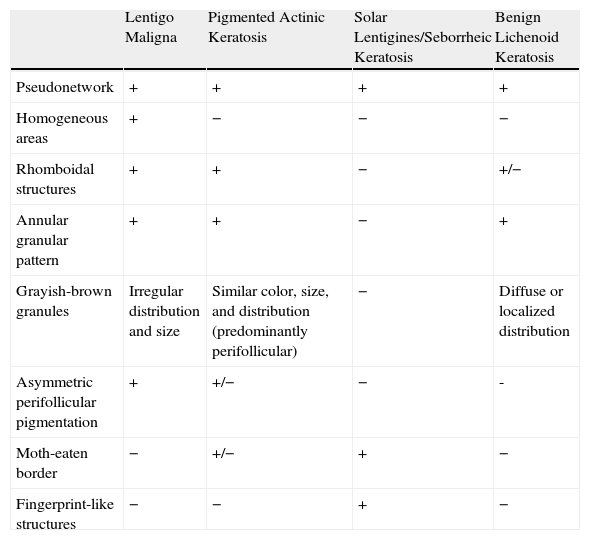
The most useful noninvasive diagnostic approach is the correlation of dermoscopic and clinical findings. Table 1 summarizes several key dermoscopic features for distinguishing between pigmented lesions on the face.
Differential Diagnosis of Pigmented Facial Lesions by Dermoscopy.
| Lentigo Maligna | Pigmented Actinic Keratosis | Solar Lentigines/Seborrheic Keratosis | Benign Lichenoid Keratosis | |
| Pseudonetwork | + | + | + | + |
| Homogeneous areas | + | − | − | − |
| Rhomboidal structures | + | + | − | +/− |
| Annular granular pattern | + | + | − | + |
| Grayish-brown granules | Irregular distribution and size | Similar color, size, and distribution (predominantly perifollicular) | − | Diffuse or localized distribution |
| Asymmetric perifollicular pigmentation | + | +/− | − | - |
| Moth-eaten border | − | +/− | + | − |
| Fingerprint-like structures | − | − | + | − |
LM and pigmented actinic keratosis are known to be clinically similar, but there are cases in which histologic distinction can be difficult. In such cases, immunohistochemical staining with human melanoma black 45 and Melan-A may be useful; these markers can be combined with Giemsa staining, whereby melanin deposits acquire a greenish color, allowing melanocytes to be distinguished from melanophages.
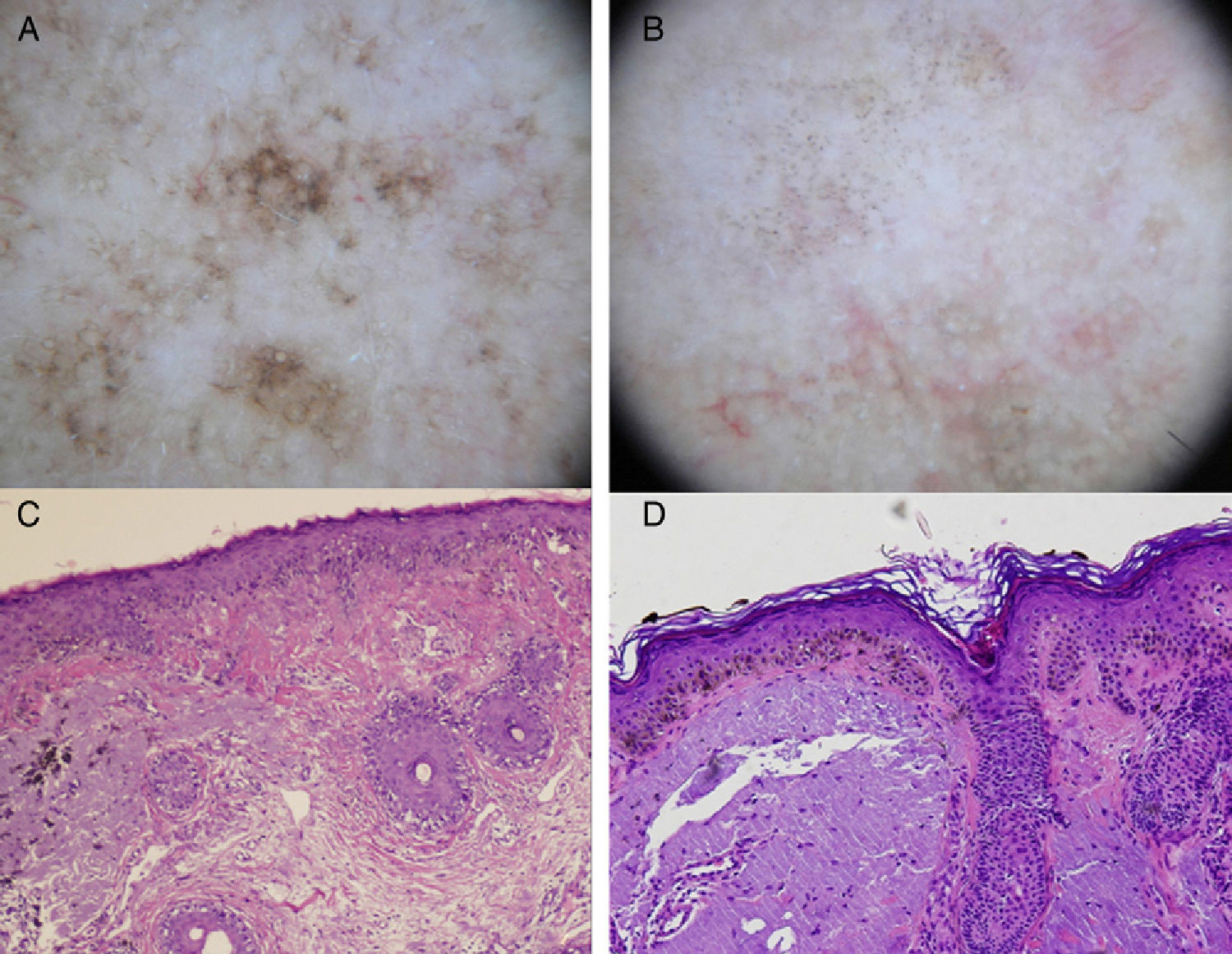
Almost all of the dermoscopic features of LM are also found in pigmented actinic keratosis, with the exception of homogeneous areas, which are highly specific for LM/LMM.10
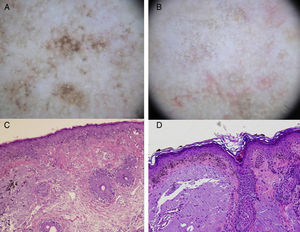
In summary, the most specific dermoscopic structures seen in pigmented actinic keratosis are multiple brown-grayish dots and globules with a similar size and color and a perifollicular arrangement (more regular than in LM), rhomboidal structures, and an annular granular pattern. These first 3 structures are the most common (Fig. 3). Other patterns are a brown to gray pseudonetwork, a superficial fragmented pseudonetwork, and follicular openings with yellowish keratotic material surrounded by a whitish halo giving a target-like appearance.10,24
A, Dermoscopic image of lentigo maligna (rhomboidal structures asymmetric perifollicular pigmentation, and granular pattern with asymmetrically distributed irregular globules). B, Dermoscopic image of pigmented actinic keratosis (multiple similarly shaped and colored brown-gray dots with a predominantly perifollicular distribution, and rhomboidal structures). C, Histologic image (hematoxylin-eosin, original magnification ×100) of the lesion in Figure 3A, showing proliferation of atypical melanocytes along the dermal-epidermal junction with involvement of the perifollicular epithelium. Melanophages and solar elastosis can also be observed in the dermis. D, Histologic image (hematoxylin-eosin original magnification ×100) of the lesion in Figure 3B, showing atypical pigmented keratinocytes at the dermal-epidermal junction and solar elastosis in the dermis.
Margins are clinically difficult to define in LM, as these lesions occur in areas of chronic sun damage. While margins can be seen more clearly under Wood's light, as Robinson25 showed, greater accuracy can be achieved if Wood's light is used in combination with dermoscopy as there is a correlation between dermoscopic and histologic findings. Robinson showed that margins excised with Mohs micrographic surgery were larger (and more accurate) than those determined by dermoscopy, but that the margins determined by dermoscopy were in turn more accurate than those determined using Wood's light.
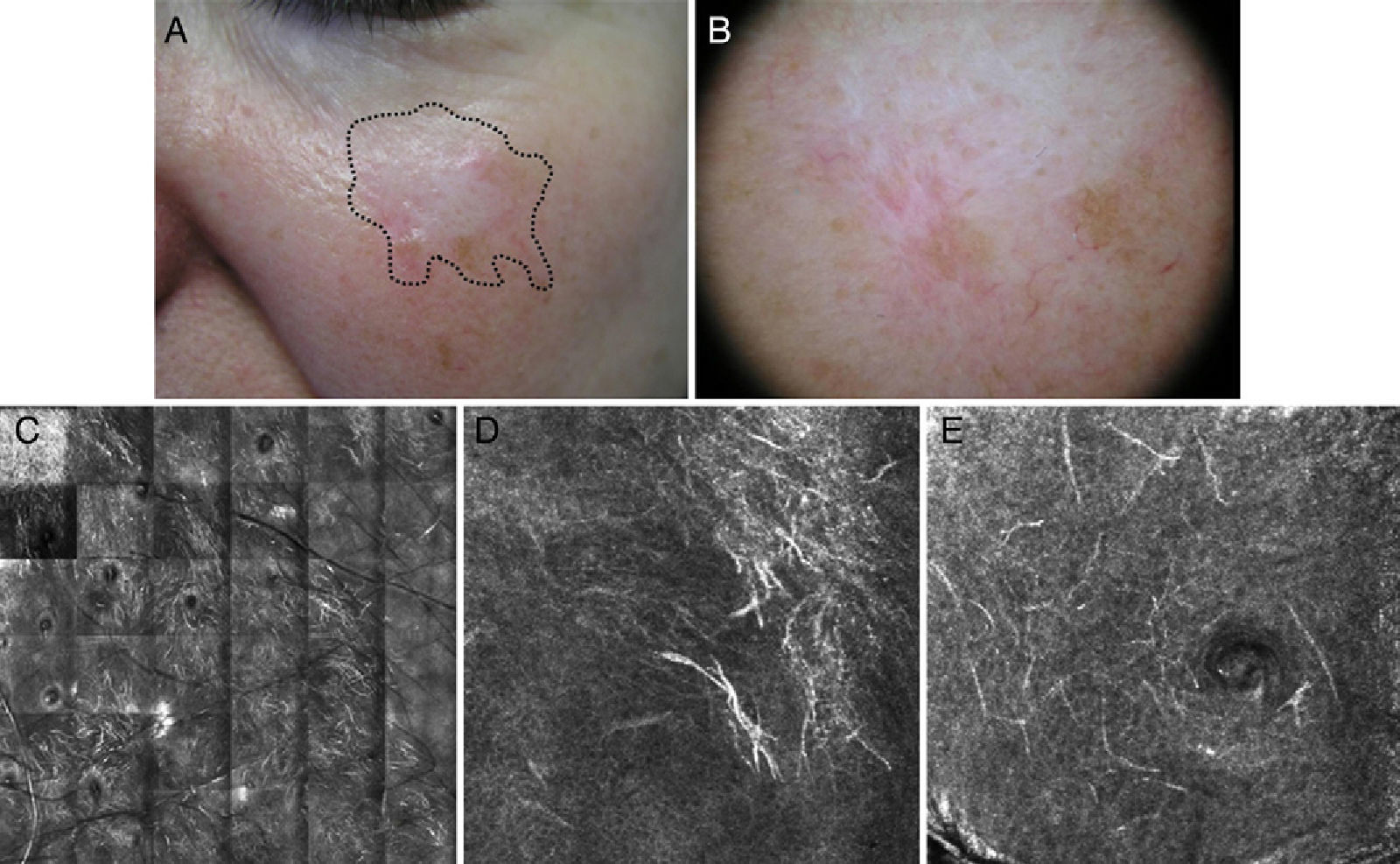
CRM is a noninvasive technique that permits the visualization of skin cells and structures in real time. The images this in vivo diagnostic tool provides have very similar resolution to those assessed in conventional histology. CRM also shows a depth of 250 to 350 μm, which is sufficient to visualize the epidermis, the papillary dermis, and the superficial reticular dermis in horizontal sections. This tool serves as a bridge between dermoscopy and histology.
Uses of CRM in LM- 1.
As a diagnostic tool for equivocal cases following dermoscopy CRM is particularly useful in hypopigmented or amelanotic lesions, as it reflects even small traces of melanin.26,27
- 2.
To guide the selection of biopsy sites so that common sampling errors are avoided.
- 3.
To complement Wood's light examination and dermoscopy for identifying poorly defined margins prior to surgery.28
- 4.
Tool for monitoring treatment response. For detecting subclinical recurrence or recurrence in which dermoscopic features are not visible due to scarring or lack of pigment29–31 (Fig. 4).
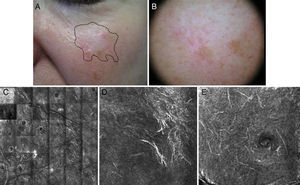
Figure 4.Seventy-year-old man with recurrent lentigo maligna in a cryotherapy scar on the left cheek; no previous biopsy. Confocal reflectance microscopy (CRM) showing the presence of atypical cells, compatible with the diagnosis of lentigo maligna, confirmed by biopsy. A, A hypochromatic scar lesion, possibly due to cryotherapy, with slight, nonspecific light brown pigmentation. Visualization of the positive margins provides a guide for treatment. B, Dermoscopic image of a lesion with a homogeneous pigmented pattern without signs of malignancy. The lesion has a scar that distorts the structure of the skin in the area and erases the typical pseudonetwork pattern; the image is therefore not informative. C, CRM in vivo (VivaScope 1500). Mosaic in the stratum spinosum showing typical honeycomb pattern and abundant hyperrefractile linear structures at low magnification consistent with the characteristic dendritic melanocytic of lentigo maligna melanoma. D, Image of the spinous stratum, showing an area 500μm×500μm with atypical hyperrefractile dendritic cells. E, Image of the stratum granulosum, showing an area 500μm×500μm with atypical dendritic cells consistent with pagetoid melanocytes. (Images were kindly provided by Dr Josep Malvehy of Hospital Clínic de Barcelona.)
The following CRM patterns have been described for LM: a) epidermal disarray (areas with complete or partial loss of honeycomb pattern) and loss of definition of keratinocyte borders; b) pagetoid spread of atypical melanocytes (isolated round or dendritic cells in the superficial layers of the epidermis); c) atypical melanocytes in the superficial dermis; d) melanophages in the superficial dermis; e) focal increase of atypical melanocytes and nests around the adnexal openings (corresponding to the dots around the follicular openings seen in dermoscopy); f) confluence of atypical dendritic melanocytes between the adnexal openings or obliterating them (corresponding to the rhomboidal or rhomboidal and homogeneous structures seen in dermoscopy); g) crests in a cordlike arrangement at the dermal-epidermal junction (reduced number of crests and flattening of dermal-epidermal junction, seen as branched, tube-like structures with a heterogeneous reflectance) and poorly visible dermal papillae with an undefined pattern; and h) infiltration of adnexal structures (round to dendritic nucleated cells along the adnexal structures).25,26,32
CRM has certain limitations as the depth of analysis is limited to the superficial reticular dermis; when it is suspected that a palpable lesion is a recurrence, it should be biopsied, regardless of the CRM results. This technique is also not valid for palmoplantar or crusted lesions. Additional disadvantages are that trained staff must be available to interpret the findings and that the procedure is laborious and very time consuming. It is also not widely used.
Prognosis of LMThe exact percentage of cases of LM that progress to LMM is unknown, but based on studies published to date, progression rates would appear to be highly variable, ranging from 5% to 50%.3,33,34
No clinical, histologic, or biologic predictors of malignant transformation have been identified. An invasive component has been histologically demonstrated in 22% of fully excised LMs following diagnosis by incisional biopsy.2,7 The risk of LMM may be proportional to lesion size, as foci of invasive melanoma are seen more frequently in large lesions. The period of latency between the development of LM and progression to LMM can be long (10-50 years), but rapid progression (6 months) has also been reported. LMM should be managed like other invasive melanomas since prognosis for all these tumors is similar.35–38 Recurrence rates vary from 3% to 50%, depending on treatment. Rates of 3% have been associated with microscopically controlled excision, while rates of 50% (and even 100%) have been seen in LMs managed nonsurgically. Most recurrences present as in situ lesions,3 but invasive forms, which have a poorer prognosis due to the risk of metastasis, have been described.1,39,40
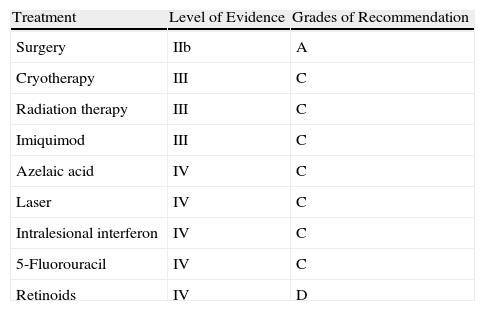
TreatmentThere is a notorious lack of prospective, randomized studies and specific guidelines and protocols on the management of LM. Nevertheless, the main guidelines on the management of melanoma11,12,41,42 address LM and should be consulted. The aim of treatment is to achieve cure and thereby prevent progression to invasive melanoma (LMM), which carries a risk of regional and distant metastasis.1 Treatment should be effective and initiated as early as possible; the treatment of choice is surgical excision whenever possible, especially in younger patients.5 The following factors should be taken into account when deciding on the best treatment modality: the patient's age and general state of health, comorbidity, life expectancy, size of lesion, location in cosmetically or functionally sensitive areas, accessibility and convenience of treatment, and patient preferences.43 Both patients and their relatives should be informed about the advantages and disadvantages of each treatment option. The levels of evidence and grades of recommendation associated with the different treatment options are shown in Table 2. (See Appendices A and B for the meaning of these classifications.)
Levels of Evidence and Grades of Recommendation for the Treatments of Lentigo Maligna.
| Treatment | Level of Evidence | Grades of Recommendation |
| Surgery | IIb | A |
| Cryotherapy | III | C |
| Radiation therapy | III | C |
| Imiquimod | III | C |
| Azelaic acid | IV | C |
| Laser | IV | C |
| Intralesional interferon | IV | C |
| 5-Fluorouracil | IV | C |
| Retinoids | IV | D |
Surgery is the mainstay of treatment, with a safety margin depending on Breslow thickness. A margin of 0.5cm is recommended for melanoma in situ and LM, but in certain cases this may be insufficient to remove all affected tissue and might lead to recurrence. Zalla et al.44 reported on a series of 46 LMs in which margins of 6mm achieved full excision in only 50% of cases. In another study of 92 LMs, 58% of lesions required safety margins of over 5mm to achieve complete clearance.45 These data confirm that the margins recommended by the National Institutes of Health for the treatment of LM46 may be insufficient.
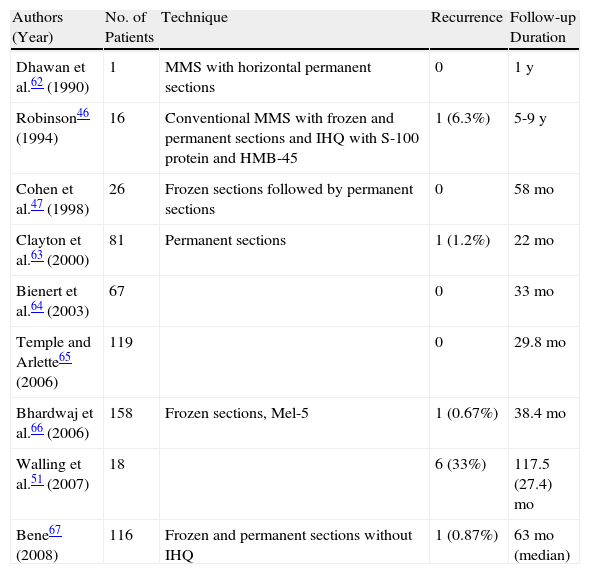
The best surgical procedure for the treatment of LM and LMM in terms of ensuring complete excision involves the 3-dimensional study of the excised tumor before any type of reconstruction using Mohs micrographic surgery or microscopically controlled surgery with frozen or paraffin-embedded sections. One group of authors reported a 97% cure rate with the frozen-section technique in patients with LM and LMM.47 Mohs micrographic surgery was introduced in 1941 by Frederic Edward Mohs,48,49 although the technique and name have changed over the years. In all the variations, the entire excised tumor is histologically examined using horizontal and vertical sections or a section that includes the peripheral and deep margins after the tissue is flattened with a cryostat. Variations of histographic surgery that do not involve the flattening of peripheral margins but rather study peripheral and deep margins separately using vertical and horizontal sections are perfectly applicable in LM and are technically less complex than the conventional Mohs procedure.
Following a review of the literature on the treatment of LM and LMM, McLeod et al.50 showed that recurrence rates were lower after Mohs micrographic surgery and its variations than after conventional surgical excision with safety margins. This finding is consistent with what we have discussed here because the radial growth of atypical melanocytes may not be uniform, meaning that subclinical disease may exist beyond one of the resection margins, even in the case of wide excision. LM and LMM are mainly located on the face, and therefore better cosmetic and functional outcomes are achieved when the operator uses healthy tissue–sparing approaches, which are not incompatible with oncologic control. This principle is particularly relevant in recurrent LM, where lesions are less well defined and the chances of treatment success are lower.
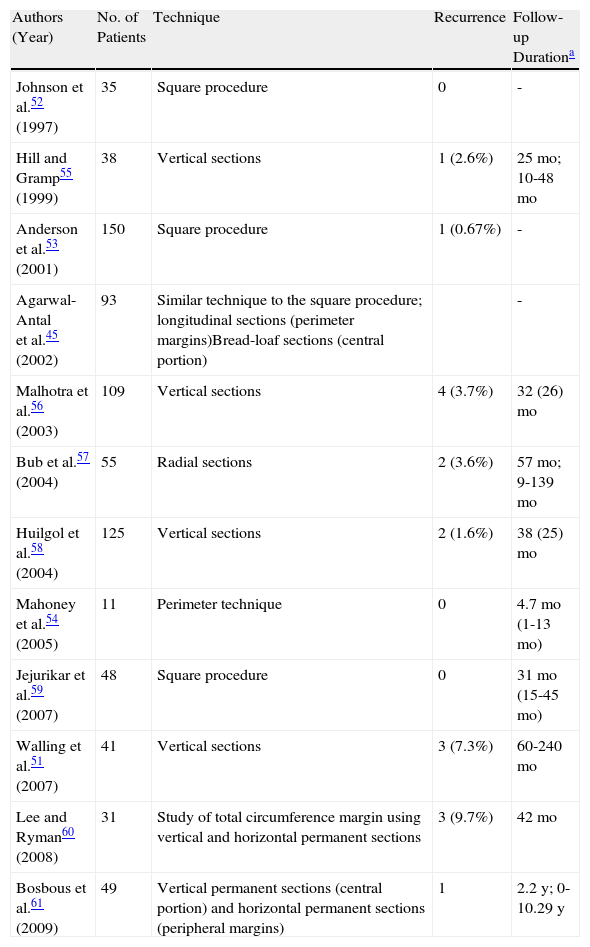
Staged excision is a simple, acceptable alternative for treating LM and LMM and offers comparable results to those seen in Mohs micrographic surgery (Tables 3 and 4, Fig. 5). One recent study found that staged excision was associated with a significantly lower recurrence rate than Mohs, and there were no differences in the final size of the surgical defect.51 This technique, which was first referred to as the square procedure, consists of enclosing the target lesion and safety margins within a geometric figure (in this case a square) and histologically examining the peripheral margins using paraffin-embedded sections.52,53
Studies on the Treatment of Lentigo Maligna Using Staged Surgical Excision.
| Authors (Year) | No. of Patients | Technique | Recurrence | Follow-up Durationa |
| Johnson et al.52 (1997) | 35 | Square procedure | 0 | - |
| Hill and Gramp55 (1999) | 38 | Vertical sections | 1 (2.6%) | 25mo; 10-48mo |
| Anderson et al.53 (2001) | 150 | Square procedure | 1 (0.67%) | - |
| Agarwal-Antal et al.45 (2002) | 93 | Similar technique to the square procedure; longitudinal sections (perimeter margins)Bread-loaf sections (central portion) | - | |
| Malhotra et al.56 (2003) | 109 | Vertical sections | 4 (3.7%) | 32 (26)mo |
| Bub et al.57 (2004) | 55 | Radial sections | 2 (3.6%) | 57mo; 9-139mo |
| Huilgol et al.58 (2004) | 125 | Vertical sections | 2 (1.6%) | 38 (25)mo |
| Mahoney et al.54 (2005) | 11 | Perimeter technique | 0 | 4.7mo (1-13mo) |
| Jejurikar et al.59 (2007) | 48 | Square procedure | 0 | 31mo (15-45mo) |
| Walling et al.51 (2007) | 41 | Vertical sections | 3 (7.3%) | 60-240mo |
| Lee and Ryman60 (2008) | 31 | Study of total circumference margin using vertical and horizontal permanent sections | 3 (9.7%) | 42mo |
| Bosbous et al.61 (2009) | 49 | Vertical permanent sections (central portion) and horizontal permanent sections (peripheral margins) | 1 | 2.2y; 0-10.29y |
Adapted from McLeod et al.50
Studies on Treatment of Lentigo Maligna Using Mohs Micrographic Surgery (MMS).
| Authors (Year) | No. of Patients | Technique | Recurrence | Follow-up Duration |
| Dhawan et al.62 (1990) | 1 | MMS with horizontal permanent sections | 0 | 1y |
| Robinson46 (1994) | 16 | Conventional MMS with frozen and permanent sections and IHQ with S-100 protein and HMB-45 | 1 (6.3%) | 5-9y |
| Cohen et al.47 (1998) | 26 | Frozen sections followed by permanent sections | 0 | 58mo |
| Clayton et al.63 (2000) | 81 | Permanent sections | 1 (1.2%) | 22mo |
| Bienert et al.64 (2003) | 67 | 0 | 33mo | |
| Temple and Arlette65 (2006) | 119 | 0 | 29.8mo | |
| Bhardwaj et al.66 (2006) | 158 | Frozen sections, Mel-5 | 1 (0.67%) | 38.4mo |
| Walling et al.51 (2007) | 18 | 6 (33%) | 117.5 (27.4)mo | |
| Bene67 (2008) | 116 | Frozen and permanent sections without IHQ | 1 (0.87%) | 63mo (median) |
Adapted from McLeod et al.50
Abbreviations: HMB-45, human melanoma black; IHQ, immunohistochemistry.
aExpressed as mean (SD) unless otherwise specified.
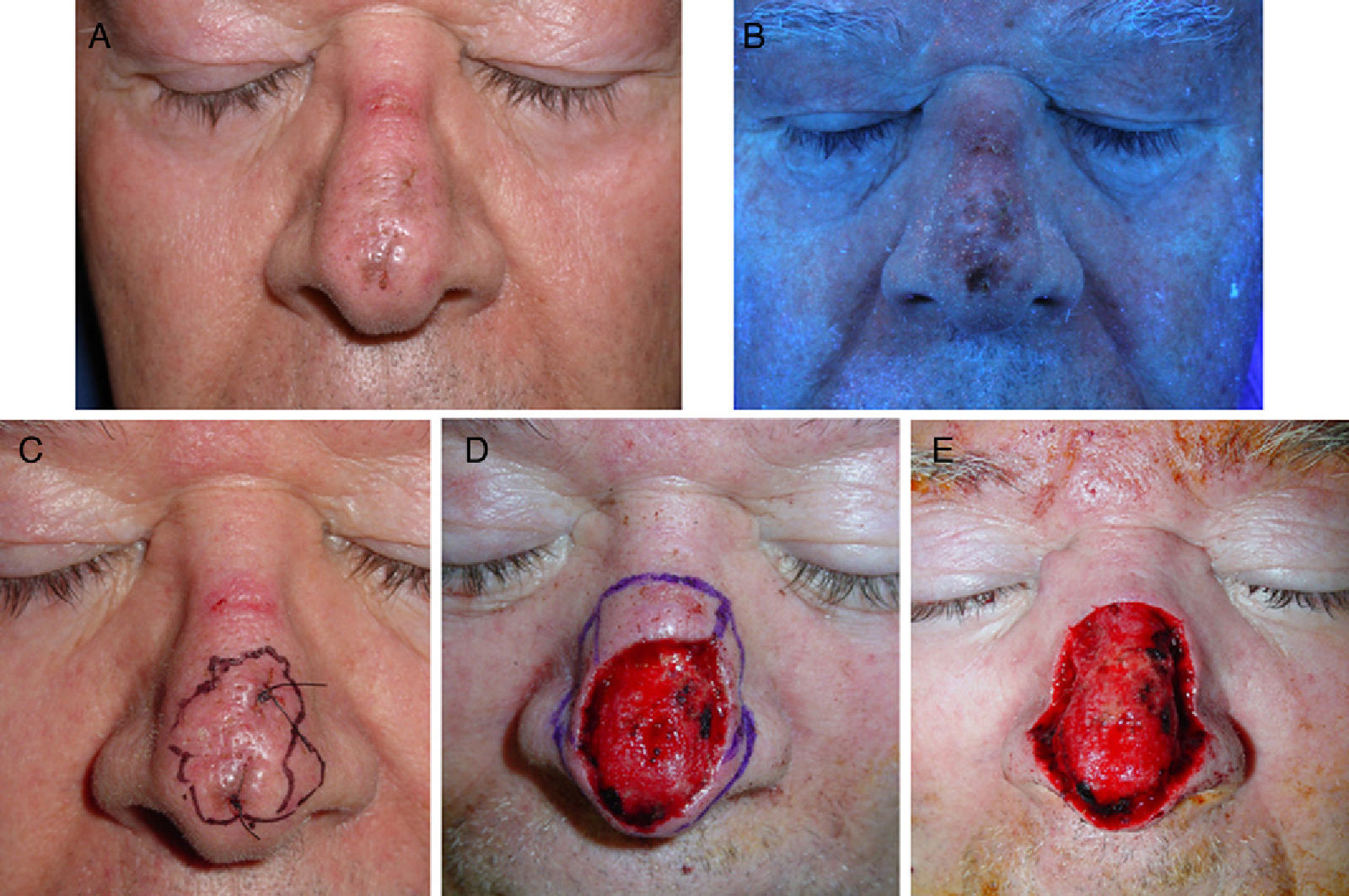
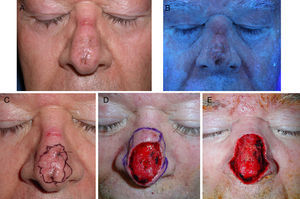
Sixty-five-year-old man with lentigo maligna. A, The lesion on the nasal dorsum. B, Under Wood's light the margins are more clearly defined. Note the ulcer on the bridge of the nose, caused by the use of continuous positive airway pressure. C, Inking of margins guided by Wood's light examination after biopsy confirmation of the diagnosis. D, Surgical defect after 2 steps of Mohs micrographic surgery using paraffin-embedded sections; all margins were positive. E, Final defect with clear margins after 3 steps.
More recent alternatives are the perimeter technique54 and the spaghetti technique,68 which involve the same basic principle—evaluation of all peripheral margins before reconstruction—but differ from the previously described excision methodologies.
The main obstacle to the successful treatment of LM is the difficulty of distinguishing between atypical or dysplastic melanocytes and normal melanocytes in chronically sun exposed parts of the body. Failure to make this distinction can complicate diagnosis and lead to slow tumor recurrence.69 Paraffin-embedded sections are preferable to frozen sections, in which it is difficult to identify dysplastic melanocytes. In some cases, immunohistochemical staining may be needed to reach a definitive diagnosis. Nonetheless, several recent articles describing the use of immunohistochemical stains on Mohs frozen sections have reported rapid staining protocols (19 and 20 minutes for MART-1 and 35 minutes for MITF) that give results similar to those seen in paraffin-embedded sections.60,70–72 These results, however, need to be confirmed in larger series or controlled studies.
Nonsurgical TreatmentBecause LM frequently affects older patients with comorbidities and involves large lesions requiring wide excision margins in cosmetically or functionally sensitive areas, nonsurgical treatments are sometimes required. These treatments are also necessary for patients who decline to undergo surgery. While the main advantages of nonsurgical treatments are good cosmetic results and low morbidity, these modalities are unfortunately associated with higher recurrence rates. In a study of melanoma in situ, Zalaudek et al.73 reported a mean (SD) 5-year recurrence of 6.8% (1.3%) for surgical excision and of 11.3%±8.5% for nonsurgical alternatives as a whole. The authors concluded that radiation therapy, which has similar recurrence rates to surgery, was the best alternative.73 Based on a survey conducted among British dermatologists in 2001, the most common nonsurgical treatments were cryotherapy and radiation therapy.74 The general limitations of nonsurgical treatments are summarized below.
- 1.
Recurrence rates are higher because the whole tumor is not evaluated histologically and so invasive areas may be missed (sampling error).2 It is also difficult to treat deep periadnexal melanocytes, which explains why any treatment must reach a depth of at least 3 to 5mm, regardless of treatment modality.
- 2.
Residual hypopigmentation following certain treatments may lead to delayed detection of recurrence during follow-up.
- 3.
The efficacy of nonsurgical treatments has not yet been well established due to the lack of randomized controlled studies.
Regardless of the treatment modality employed, long-term follow-up is essential. In the following sections, we review the main nonsurgical treatments used in LM.
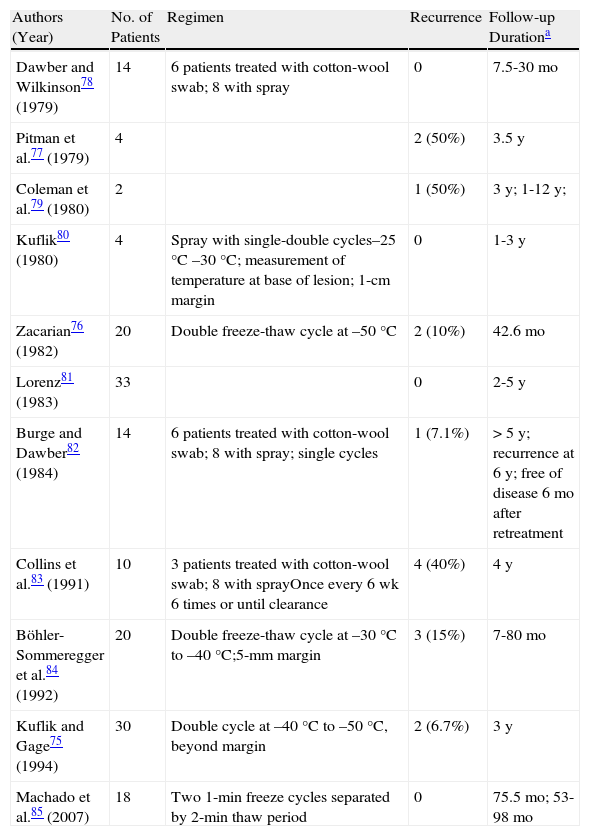
CryotherapyCryotherapy is one of the most common treatment alternatives for LM in clinical practice. As melanocytes are very sensitive to damage induced by freezing, cryotherapy selectively destroys melanocytes but not keratinocytes at temperatures of –4¿C to –7¿C, although there is no evidence that it also destroys neoplastic melanocytes. The fall in temperature must penetrate to at least 3mm so that atypical melanocytes extending into normal skin and down to the hair follicles are also destroyed.75 Some authors have proposed monitoring the temperature at the base of the tumor to ensure adequate penetration depth, but there does not appear to be any correlation between this temperature and cure rates.72 No cryotherapy protocols have been established for LM and the technique varies from one center to the next. The treatment should generally be aggressive, with double freeze-thaw cycles and a spray distance of 2 to 3cm to achieve a temperature of –40¿C to –50¿C at the base of the tumor. Freezing duration depends on the size of the lesion, with periods of 45 to 60 seconds required to freeze a lateral margin of apparently healthy skin of 1cm (minimum 5mm). This cycle is then repeated after a thaw period of 2 to 3 minutes.3,76 Treating large lesions in several sessions ensures adequate treatment of lateral margins with fewer complications. Treatment duration depends on the size of the lesion. Response is variable, with reported rates varying according to technique, histologic evaluation, duration of follow-up, etc. Over 200 cases of LM treated with cryotherapy have been described since 1979; the overall recurrence rates are under 9%, but specific studies have reported rates of 0%, 8% to 10%, and even 50%.1,75–79 There have also been reports of lesions recurring 7 to 9 years after treatment (Table 5).
Main Studies on Lentigo Maligna Treated With Cryotherapy.
| Authors (Year) | No. of Patients | Regimen | Recurrence | Follow-up Durationa |
| Dawber and Wilkinson78 (1979) | 14 | 6 patients treated with cotton-wool swab; 8 with spray | 0 | 7.5-30mo |
| Pitman et al.77 (1979) | 4 | 2 (50%) | 3.5y | |
| Coleman et al.79 (1980) | 2 | 1 (50%) | 3y; 1-12y; | |
| Kuflik80 (1980) | 4 | Spray with single-double cycles–25°C –30°C; measurement of temperature at base of lesion; 1-cm margin | 0 | 1-3y |
| Zacarian76 (1982) | 20 | Double freeze-thaw cycle at –50°C | 2 (10%) | 42.6mo |
| Lorenz81 (1983) | 33 | 0 | 2-5y | |
| Burge and Dawber82 (1984) | 14 | 6 patients treated with cotton-wool swab; 8 with spray; single cycles | 1 (7.1%) | >5y; recurrence at 6y; free of disease 6mo after retreatment |
| Collins et al.83 (1991) | 10 | 3 patients treated with cotton-wool swab; 8 with sprayOnce every 6 wk 6 times or until clearance | 4 (40%) | 4y |
| Böhler- Sommeregger et al.84 (1992) | 20 | Double freeze-thaw cycle at –30°C to –40°C;5-mm margin | 3 (15%) | 7-80mo |
| Kuflik and Gage75 (1994) | 30 | Double cycle at –40°C to –50°C, beyond margin | 2 (6.7%) | 3y |
| Machado et al.85 (2007) | 18 | Two 1-min freeze cycles separated by 2-min thaw period | 0 | 75.5mo; 53-98mo |
The advantages of cryotherapy are that it is fast and easy to apply and the good cosmetic outcome. Retreatment can be effective. Disadvantages are the general limitations of nonsurgical treatment as well as long healing times (up to 3 months, which is even longer than with surgery) and a risk of hypertrophic scarring.
Radiation TherapyRadiation therapy is the most effective nonsurgical treatment for LM.2,86,87 It has been used mainly in older patients with large lesions located in anatomically sensitive areas, as it is generally associated with minimal morbidity and good cosmetic results.88 Reports of good results after conventional orthovoltage irradiation in LM and LMM have been published in the United States,89,90 with all authors stressing that conventional fractionated radiation therapy is highly effective because the doses administered in the subcutaneous tissue (50% of the dose between 6mm and 4cm) prevent subepidermal recurrences. The disadvantage of using high-energy x-rays is that they can reach underlying tissues, especially bone tissue, in the treated area and cause radiation necrosis.91 This adverse effect can be significantly minimized with the Miescher technique, which has been used in Europe as a primary treatment or adjuvant to surgery since it was first described in the 1950s92 and involves the application of high doses of Grenz rays or superficial x-rays with a very low voltage (12-50 kV) and a maximum penetration depth of 2 to 3mm. In the Miescher technique and its variations, the technician uses an X-ray tube with a beryllium window of 12kV, 15 MAamp at a distance of 20cm to administer 50% of the dose at 1 to 1.3mm. Because only the epidermis and the superficial dermis are affected, the treatment induces acute radiodermatitis, but less atrophy.93 A very common and well-tolerated regimen is a total fractionated dose of 100Gy administered over 10 sessions (5 sessions a week for 2 weeks).
The treatment must be carefully planned to ensure inclusion of the entire tumor with a sufficiently wide margin: 1cm for lesions smaller than 2cm and 2cm for larger lesions.
In practice, both superficial x-rays (100-200kV) and high-energy electrons (6-9MeV) are adequate. Doses of 45 to 50Gy in 15 to 25 fractions are sufficient to achieve disease control in the majority of patients. The recommended dose per fraction is 2 to 3.5Gy depending on the size and thickness of the tumor and the treatment regimen used.
Postoperative radiation therapy is useful for recurrent lesions, large lesions with insufficient surgical margins, and lesions with a lentiginous part that cannot be fully excised due to the risk of considerable morbidity.
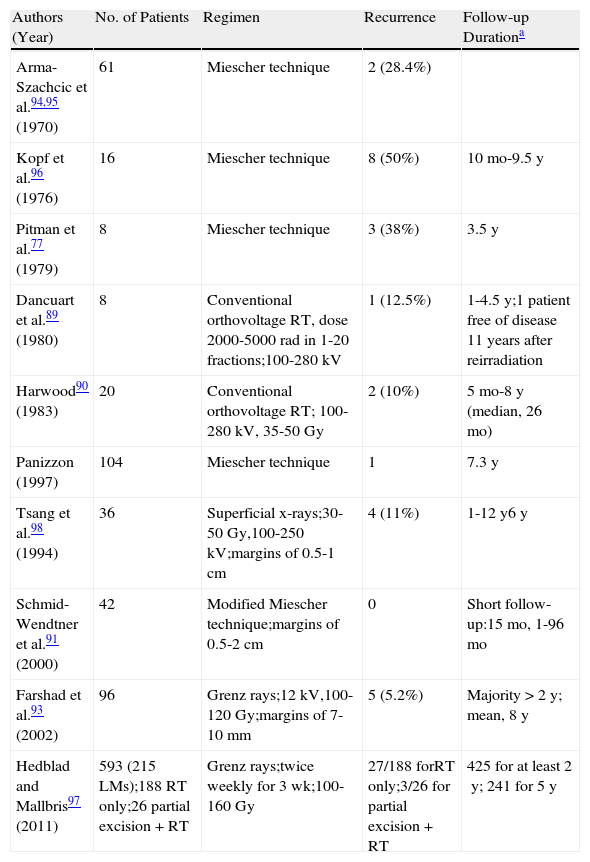
Cure rates of 86% to 95% have been reported for radiation therapy with Grenz rays or superficial x-rays using the Miescher technique, but they are from small series with short follow-up times (2-5 years)2 (Table 6). Clinical regression has been observed 2 to 8 months after treatment.91 In 2002, Farshad et al.93 published a retrospective study of 150 patients (93 patients with LM, 54 with LMM, and 3 with LM and LMM) in which the overall recurrence rate was 7% (5.2% in LMs).
Main Studies on the Treatment of Lentigo Maligna Using Radiation Therapy (RT).
| Authors (Year) | No. of Patients | Regimen | Recurrence | Follow-up Durationa |
| Arma-Szachcic et al.94,95 (1970) | 61 | Miescher technique | 2 (28.4%) | |
| Kopf et al.96 (1976) | 16 | Miescher technique | 8 (50%) | 10mo-9.5y |
| Pitman et al.77 (1979) | 8 | Miescher technique | 3 (38%) | 3.5y |
| Dancuart et al.89 (1980) | 8 | Conventional orthovoltage RT, dose 2000-5000 rad in 1-20 fractions;100-280kV | 1 (12.5%) | 1-4.5y;1 patient free of disease 11 years after reirradiation |
| Harwood90 (1983) | 20 | Conventional orthovoltage RT; 100-280kV, 35-50Gy | 2 (10%) | 5mo-8y (median, 26mo) |
| Panizzon (1997) | 104 | Miescher technique | 1 | 7.3y |
| Tsang et al.98 (1994) | 36 | Superficial x-rays;30-50Gy,100-250kV;margins of 0.5-1cm | 4 (11%) | 1-12y6y |
| Schmid-Wendtner et al.91 (2000) | 42 | Modified Miescher technique;margins of 0.5-2cm | 0 | Short follow-up:15mo, 1-96mo |
| Farshad et al.93 (2002) | 96 | Grenz rays;12kV,100-120Gy;margins of 7-10mm | 5 (5.2%) | Majority >2 y; mean, 8y |
| Hedblad and Mallbris97 (2011) | 593 (215 LMs);188 RT only;26 partial excision + RT | Grenz rays;twice weekly for 3 wk;100-160Gy | 27/188 forRT only;3/26 for partial excision + RT | 425 for at least 2y; 241 for 5y |
Adapted from Stevenson et al.1
Radiation therapy appears to be less effective in LMM than in LM. In many cases, it is used as adjuvant therapy after the surgical excision of the nodular part of the lesion.88,91
Adverse EffectsIn most cases, radiation therapy causes erythema, scaling, and local hair loss from the second week of treatment onwards. Late complications include atrophy, flattening of the epidermis, fibrosis, telangiectasis, pigmentary changes (hyperpigmentation or hypopigmentation), and an increased risk of squamous cell carcinoma.
The advantages of radiation therapy are good tolerance and cosmetic results and shorter treatment periods than with topical treatments. Cure rates are similar to those observed with conservative surgery. Because the skin in exposed areas of the head and neck is very thin in older patients, a penetration depth of 1.1mm is sufficient to reach a considerable portion of the dermis.91
The disadvantages of radiation therapy are the limited penetration of x-rays (which explains why the Miescher technique can fail in cases where melanocytes extend below the adnexal structures91), the need for multiple sessions (generally done only in urban areas with adequate infrastructure93), and the difficulty of detecting recurrence due to treatment-induced pigmentary changes.
ImiquimodImiquimod, a synthetic imidazoquinoline amine, is a topical immune response modifier that exerts its effect through the stimulation of both innate and acquired immunity. It has also been shown to induce apoptosis99 and exert antiangiogenic effects.100
After the first reported case of imiquimod-treated LM was published in 2000,101 by 2006 there were descriptions of 68 cases treated in this way according to a review of 15 reports in the English-language literaure.102 The authors of the review concluded that, because of the risk of leaving invasive or residual disease, “without controlled evidence and prolonged follow up, the use of imiquimod for LM must still be considered experimental.”102 The literature now contains reports of the use of imiquimod in 234 patients with LM, all from uncontrolled studies. A recent review evaluated 161 of these cases.2 However, the authors included highly variable types of studies (Table 7): case series, individual case reports, and open-label studies. There were also differences in lesion size and type (primary or recurrent), previous treatment, treatment regimens (from twice-weekly to daily applications) and duration (8 days-9 months), margin width, confirmation of treatment response (not always by biopsy), and follow-up times (shorter than the standard 5-year period used in oncology). Many of the studies had a follow-up time of less than a year.
Main Studies on Lentigo Maligna (LM) Treated Using Imiquimod (IMQ).
| Authors (Year) | No. of Patients | Regimen | Margin | Response | Histologic Confirmation | Follow-up Durationb | Other |
| Ahmed and Berth-Jones101(2000) | 1 | 3 times a wk for 4 wk; daily for 2 wk Alternating days for 7 mo | NR | CR | Yes | 9mo | |
| Ormond et al.124 (2002) | 5 | Daily for 6-12mo | NR | CR and PR | Only 1 case | Up to 6mo | |
| Chapman et al.114 (2003) | 1 | Once/twice daily | NR | CR | Yes | 3mo | |
| Epstein125 (2003) | 1 | Alternating days | NR | CR | NR | 18mo | |
| Fisher and Lang109 (2003) | 1 | Test: Alternating days for 3 mo; rest: 3 times a week for 14 wk | NR | PR. Developed amelanotic LM with satellite lesions | Yes | Free of disease 17mo after MMS and IMQ | |
| Naylor et al.5 (2003) | 30 | Daily for 12wk | 2cm | 26/28 | Yes | 12mo | 1 case resolved in 8d |
| Powell et al.126 (2004) | 11 | 3 times a wk for 4-20 wk | 2cm | 6 CR, 4 PR | Yes | 9mo | |
| Powell and Jones9 (2004) | 2 | 3 times a wk for 12 and 20 wk | NR | CR | Yes, 3mo posttreatment | 9y 18mo | Treated 2 amelanotic LMs |
| Kupfer et al.127 (2004) | 2 | 3 times a wk for 3mo3 times a wk for 4mo | NR | CR | Yes, 3mo posttreatment | 14 and 13mo | |
| Flemming et al.106 (2004) | 6 | Daily for 6wk | 1cm | 4/6 | Yes (standard excision with 5-mm margin after treatment) | First study with complete excision | |
| Michapoulos et al.128 (2004) | 1 | 5 times a wk for 2 wk3 times a wk for 16wk | NR | CR | Yes | 9mo | Study of inflammatory infiltrate |
| Muñoz et al.129 (2004) | 1 | Daily for 1 wk2-3 times a wk for 16wk | NR | CR | Yes | 4mo | |
| Kamin et al.130 (2005) | 1 | 5 times a wk for 6 wk Second 3-mo period after 1mo | NR | CR | Yes | 12mo | |
| Wolf et al.131 (2005) | 6 | Daily for 5-13 wk | 0.5-1cm | 5/6 | Yes | 3-18mo | Response in 1 amelanotic LM |
| Noel and Kunzle132 (2005) | 1 | Twice a week for 12 wk | NR | CR | Yes | 12mo | |
| Ray et al.133 (2005) | 3 | 3 times a wk for6-12 wk | 0.5-1cm | CR | Yes | 7-12mo | |
| Van Meurs et al.111 (2007) | 10 | 3 times a wk/d depending on inflammation6-13 wk (minimum of 3mo proposed) | 2cm | 9/10 | One biopsy after 4 wk and second one after 1y | 31mo | 4 recurrences including 1 case of histologic confirmation without clinical evidence |
| Spenny et al.121 (2007) | 12 | 2-7 times a wk for 7-44 wk | NR | CR | Four unconfirmed | 18.3mo | |
| Mahoney et al.118 (2008) | 7 | Variable depending on inflammationMean, 12.4 wk | 0.5-1cm | 6/7 | Yes | 19.1mo | 1 case of herpes simplex infection and elevated liver enzymes |
| Troya-Martin et al.119 (2008) | 2 | 5 times a wk for 16 wk and 12 wk | NR | CR | Yes | 3y | |
| Buetticker et al.110 (2008) | 34 | 1-2 times daily with/without occlusion2-20 wk | No margin | CR | Yes (not all) | 17.2mo | Vitiligo recurrence after 30mo in a patient with B-cell lymphoma |
| Cotter et al.105 (2008) | 40 | 5 times daily for 3mo | 2cm | Clinical, 83%; histologic, 75% | 2 mo after staged excision with 2-mm margin | 18mo; 12-34mo | 6 cases with different clinical and histologic findings |
| Powell et al.113 (2009) | 48a | 3 times a wk/d for 6-12 wk | 2cm | 37/48 | Yes, 4 mo after treatment | 25-72mo | Follow-up of >5y without recurrence |
| Ventura et al.134 (2009) | 1 | Daily for 4mo | NR | CR | Yes, 3 mo after treatment | 1y | LMMNodules excised and rest treated with IMQ |
| Woodmansee and McCall123 (2009) | 1 | Daily for 6-8 wk; alternating days for 5wk | 1cm | CR but recurrence | Cure at 4mo, but recurrence as LMM | 24mo before recurrence | Free of disease for 11 mo after MMS |
| Ramsdell and Zeitouni135 (2009) | 1 | 3 times a week for up to 1 y depending on area | NR | CR | after treatment and at 3 y | 3y | 10-cm lesion (largest treated) |
| Ly et al.107 (2011) | 43 | 5 times a wk for 12wk | 1cm | 20/38 | Complete excision after treatment |
Abbreviations: CR, complete response; LMM, lentigo maligna melanoma; MMS, Mohs micrographic surgery; NR, not reported, PR, partial response.
In this article, 37 patients were added to the cases reported in another article126 by the same author.
Imiquimod is generally not recommended in invasive tumors or in young patients due to the risk of progression to invasive disease, or in patients who cannot be closely monitored.103,104
Response and Response-Related FactorsImiquimod applied 5 times a week for at least 12 weeks has been proposed as the most effective treatment regimen. Response rates vary from series to series.104 While an 85% clinical response rate with histologic confirmation has been reported,91 in most cases evaluation was done by punch biopsy of a previously positive area.2 In 3 studies, after initial imiquimod treatment full tumor excision was achieved, with histologic clearance rates of 75%, 63%, and 53%.105–108 Progression to LMM was reported during treatment in 3 patients.5,109,110
Because of variations in study design, it is not possible to identify optimal parameters or predictors of treatment failure or absence of response.110,111
Imiquimod has been associated with a hypothetical risk of favoring tumor progression.103,112 One study showed that treatment efficacy was significantly related only to a moderate to severe clinical inflammatory response.113 Different strategies have been used to improve inflammatory response, including increasing frequency of application (twice a day) or using occlusion, cryotherapy, or tazarotene 0.1% in gel.110,114,115 Clinical response should not be used on its own to evaluate treatment response, as clinical and histologic findings may not match (Fig. 6). All lesions should be biopsied after treatment.116 In some cases, at least 3 months should be left before biopsy unless an inflammatory response is absent or abnormal pigmentation persists. Biopsies performed too soon are difficult to interpret because of the frequent presence of florid interface dermatitis. Several authors have proposed performing a second biopsy 1 or 3 years after treatment, as late recurrences have been described.110 As mentioned earlier, dermoscopy and CRM may be useful for monitoring response.
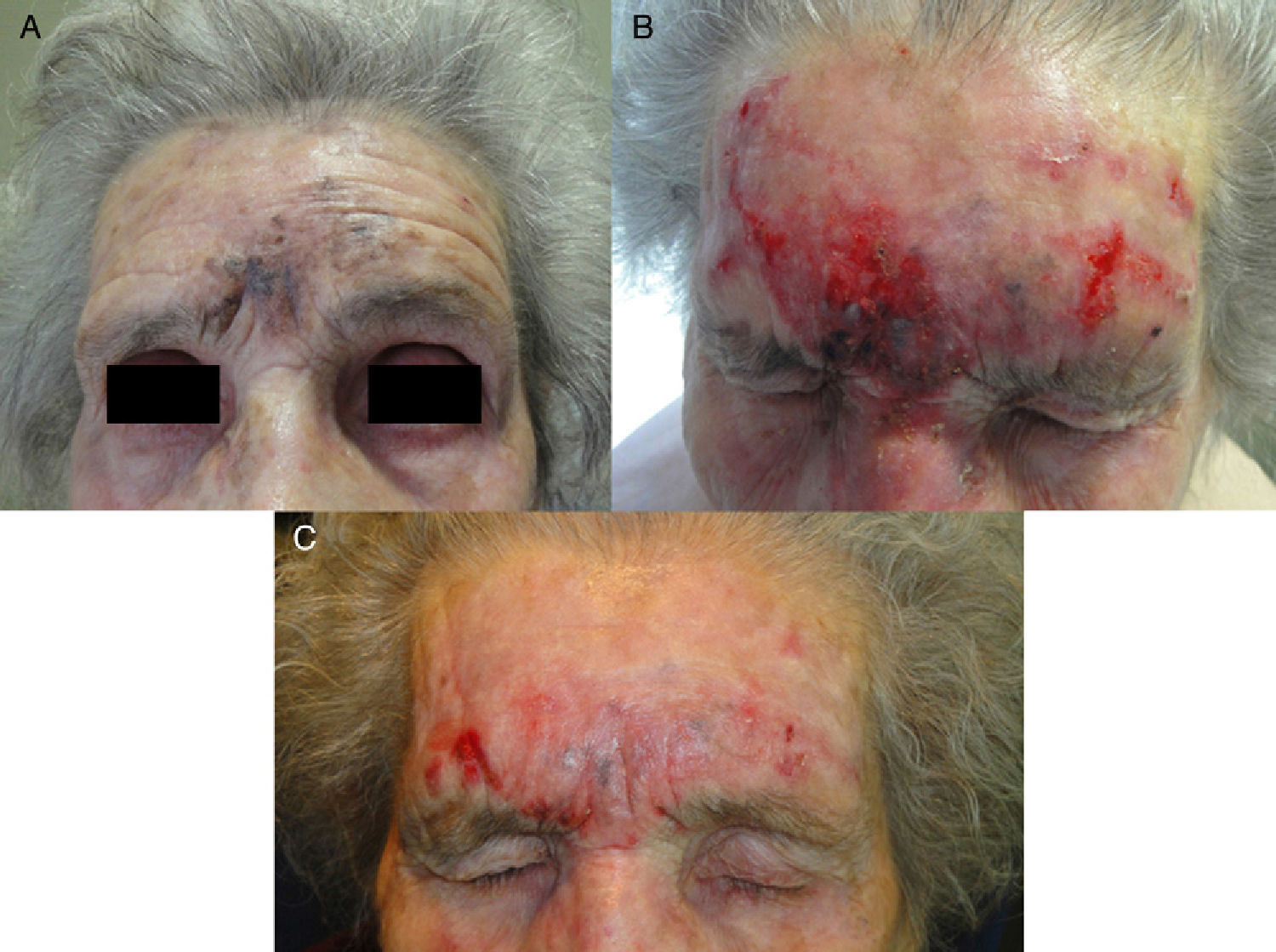
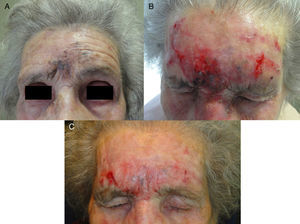
A, Lentigo maligna melanoma (0.125-mm Breslow depth after diagnostic biopsy) on the forehead of a 94-year-old woman. B, Marked inflammatory response after 2 months of treatment. C, Residual grayish hyperpigmentation between the eyebrows after 3 months of treatment. Tumor persistence ruled out by histology.
The adverse effects associated with imiquimod treatment are postinflammatory dyschromia, frequent erythema and edema, erosions, telangiectasia,110 superinfection, and reversible punctate keratopathy with reduced visual acuity in periocular lesions.117 Systemic effects include cytokine release syndrome (headache, gastrointestinal discomfort, fever, and general malaise). There have also been reports of vitiligo,110 herpes simplex infection, and elevated liver enzymes.118
The advantages of imiquimod are the same as those associated with other nonsurgical treatment modalities.
Disadvantages include the lack of standardized protocols and regimens and of clinical and histologic response criteria.111 Furthermore, residual hyperpigmentation following treatment may be confused with recurrence.119
In conclusion, the use of imiquimod should be reserved for cases in which surgery and other nonsurgical treatments such as radiation therapy are contraindicated, at least until further evidence is available on its efficacy and safety.7
Imiquimod can be used as adjuvant therapy before surgery to reduce tumor size108,113 or after surgery to treat extensive melanoma in situ around the lesion excised.120 Some authors have recommended its use in recurrent LM following conventional excision or Mohs micrographic surgery, in cases with positive margins (as adjuvant therapy), or to prevent recurrence.37,100,121,122
Other Nonsurgical TreatmentsAzelaic AcidAzelaic acid (C9 dicarboxylic acid) 20% in cream or 15% to 35% in ointment has been used to treat LM since the 1970s. This agent is selectively cytotoxic for abnormal melanocytes due to the reversible inhibition of tyrosinase and the inhibition of mitochondrial enzymes.136 It is applied twice a day for 2 to 12 weeks, depending on response.1 Treatment results have been highly variable,137–139 and there is insufficient evidence to recommend its use over other modalities in clinical practice.
LaserSeveral types of lasers are capable of destroying melanocytes, and argon, carbon dioxide, ruby, and Q-switched Nd:YAG lasers have all been used to treat LM140,141 (Table 8). While promising results have been reported, there is still insufficient evidence to recommend laser therapy over other alternatives.1 Finally, atypical melanocytes that have lost their pigment-forming capacity may be resistant to laser therapy (Table 9).
Main Studies on Lentigo Maligna Treated With Laser.
| Authors (Year) | No. of Patients | Regimen | Recurrence | Follow-up Durationa |
| Arndt et al.145(1984) | 1 | Argon laser, 5-cmdistance; 1-mm spot, 3.8W, 50 ms | No | 8mo |
| Arndt140 (1986) | 3 | Argon laser (as above) | 33% (1/3) | 75wk |
| Kopera141 (1995) | 4 | 10 600-nm carbon dioxide laser, 12W, 1-mm spot, 2 passes | 0% (0/4) | 15mo |
| Thissen and Westerhof142 (1997) | 1 | 694-nm Q-switched ruby laser | 0 | 12mo |
| Orten et al.143 (1999) | 1 | Q-switched Nd:YAG laser4 -11J/cm2, 532 and/or 1064nm, 10-20ns, 2- to 3-mm spot, 10Hz | 37.8% (3/8) | 8mo-3.5y |
| Iyer and Goldman144 (2003) | 1 | Q-switched alexandrite laser; 5 different treatments | 1/1 | 3.5y |
| Niiyama et al.146 (2007) | 1 | Q-switched ruby laser 5J/cm2, 4-mm spot | 1/1 | 2y |
| Madan and August147 (2009) | 22 | 532-nm-switched Nd:YAG laser, 6J/cm2, 2-mm spot, 5ns; 755-nm alexandrite laser, 12J/cm2, 2-mm spot, 50ns | 23% (5/22) | 2-5y |
Adapted from McLeod et al.50
Summary of Key Diagnostic and Therapeutic Issues in Lentigo Maligna (LM).
| - Histology is the method of choice for the diagnosis of LM.10 Excisional biopsy has the highest diagnostic accuracy. Imaging techniques such as dermoscopy or confocal reflectance microscopy (CRM) can help to select biopsy areas. Dermoscopy is a useful complementary tool in clinical practice for the early diagnosis of LM. CRM is useful as a follow-up to dermoscopy for the diagnosis of equivocal cases, particularly in hypopigmented and amelanotic lesions.- The treatment of choice is surgery. Mohs micrographic surgery (MMS) and its variants are associated with a lower recurrence rate than conventional surgery with safety margins. MMS is particularly indicated in large or recurrent lesions, lesions with poorly defined clinical borders, and lesions in anatomically sensitive areas. Wood's light examination, dermoscopy, and CRM can help to define margins prior to surgery.- In cases where surgical treatment is not possible:- Radiation therapy is considered to be the most effective nonsurgical treatment for patients whose general health and life expectancy are good.- Alternatives such as imiquimod or cryotherapy can be used in patients with a poor state of health, a short life expectancy, or who do not wish to undergo other treatments. These alternatives are not currently supported by scientific evidence; reliable, noninvasive methods are needed to completely rule out an underlying invasive component.- Long-term monitoring is essential. Dermoscopy and CRM are useful monitoring tools. Posttreatment biopsy is advisable when recurrence is suspected and is necessary after topical treatment. |
Very few studies have analyzed the use of curettage and electrocoagulation in the treatment of LM since the first cases were published over 20 years ago79 (mean recurrence of 27% for 11 patients mentioned in a review1 discussing that and another study). This treatment modality cannot currently be recommended over others due to a lack of evidence. Furthermore, the histologic sample obtained is generally inadequate for confirming complete excision/destruction, and melanocytes extending beyond the adnexal structures may remain untreated.1,77,79
Intralesional InterferonAlthough a group of authors reported the clearance of 11 lesions in 10 patients treated with intralesional interferon, only 4 cases were confirmed histologically.148 A report of 6 LMs successfully treated in a patient with xeroderma pigmentosum has also been published.149 The follow-up periods reported have been very short and there is little evidence to support the use of intralesional interferon in LM.,1
5-FluorouracilThe mechanism of action of 5-fluorouracil is related to the inhibition of DNA synthesis. Litwin et al.150 used it for the first time in 1975 to treat 3 patients with twice-daily application regimens of 6, 9, and 13 months. The treatment was successful in all 3 cases, although 1 patient required retreatment for recurrence. There have also been reports of clearance followed by recurrence in an additional 3 patients.79 Little evidence supports the use of 5-fluorouracil to treat LM in clinical practice.1,79,150
RetinoidsRetinoids have been used to treat LM due to their ability to inhibit the proliferation and induce the differentiation of murine melanocytes in cultures. A recent article reported the resolution of LM in 2 elderly patients following treatment with tazarotene 0.1% gel, with no signs of recurrence at 18 and 30 months.151 There are currently no data to support the use of retinoic acid or topical tretinoin in the treatment of LM, although these retinoids have been used in combination with other treatments, such as imiquimod, to enhance inflammatory response.110 More studies are needed.1
Table 9 summarizes key points related to the diagnosis and treatment of LM.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Dr Estefanía Palacios of the Radiotherapy Oncology Department at the Complejo Asistencial Universitario de León for her assistance with radiation therapy section. Dr Malvehy of the Dermatology Department of Hospital Clínic de Barcelona for providing the CRM images.
| Ia | Evidence obtained from meta-analysis of randomized clinical trials |
| Ib | Evidence obtained from at least 1 randomized clinical trial |
| IIa | Evidence obtained from at least one well designed controlled study without randomization |
| IIb | Evidence obtained from at least 1 type of well designed quasi-experimental study |
| III | Evidence obtained from nonexperimental descriptive studies |
| IV | Evidence obtained from expert opinion or clinical experience |
Adapted from Stevenson et al.1
| A | There is good evidence to support use of the procedure |
| B | There is fair evidence to support use of the procedure |
| C | There is poor evidence to support use of the procedure |
| D | There is fair evidence to support rejection of use of the procedure |
| E | There is good evidence to support rejection of use of the procedure |
From Stevenson et al.1
Please cite this article as: Samaniego E, Redondo P. Lentigo maligno. Actas Dermosifiliogr. 2013;104:757–775.