The aim of this study was to assess the impact of psoriasis on health-related quality of life (HRQOL) using different questionnaires.
Patients and methodsProspective observational study of patients with plaque psoriasis of at least 6 months’ duration stratified by active and stable disease. The patients were evaluated at baseline, 7 days, and 12 weeks. At the 3 visits, the investigators recorded sociodemographic and clinical data and the patients completed the following HRQOL questionnaires: the Dermatology Life Quality Index (DLQI), the Psoriasis Disability Index (PDI), and psoriasis quality of life questionnaire (PSO-LIFE).
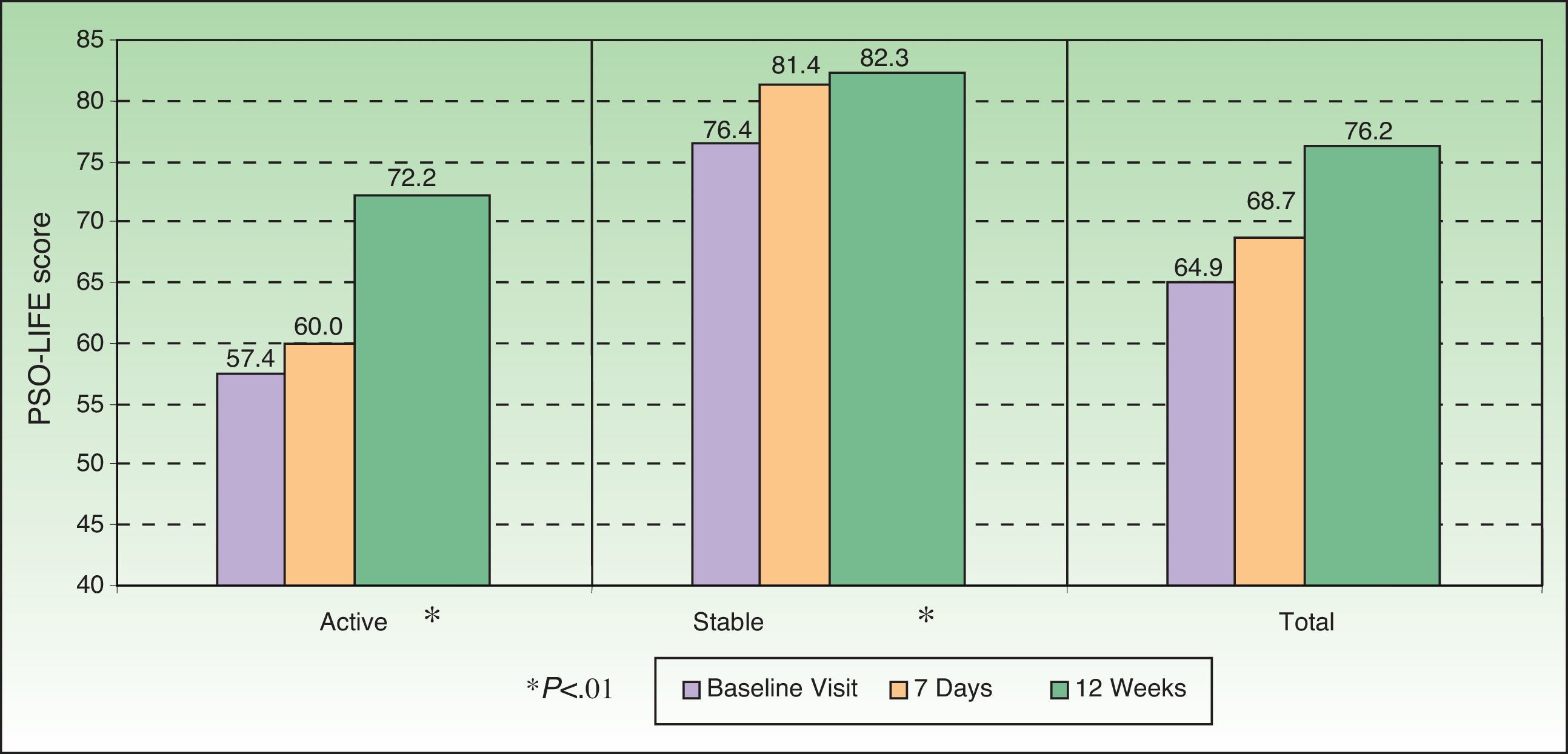
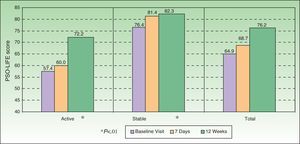
ResultsIn total, 304 patients (182 with active psoriasis and 122 with stable psoriasis) were evaluated. The mean (SD) age was 45.3 (14.5) years, and 56.3% of the group were men. At baseline, the mean (SD) psoriasis and area severity index (PASI) score was 17.0 (7.4) in patients with active disease and 5.6 (5.3) in those with stable disease; a reduction was seen in PASI scores during the evaluation period (P<.01). The mean (SD) score on the PSO-LIFE questionnaire increased significantly from 57.4 (20.4) to 72.2 (19.6) in patients with active psoriasis and from 76.4 (20.6) to 82.3 (18.3) in those with stable disease (P<0.01 in both groups).
The difference in standardized mean scores between the 2 groups was 0.79 for the DLQI, 0.62 for the PDI, and 0.85 for the PSO-LIFE questionnaire.
The impact of psoriasis on HRQOL as assessed by the PSO-LIFE questionnaire was greater in patients with lesions in visible areas than in those with less visible lesions (P<.01).
Changes in PSO-LIFE and PASI scores were moderately and significantly correlated (r=–0.4).
ConclusionsThe impact of psoriasis on HRQOL is higher in patients with active disease. The PSO-LIFE questionnaire showed a greater tendency to discriminate between active and stable psoriasis than either the DLQI or the PDI. PSO-LIFE scores correlated significantly with lesion site and disease severity as measured by PASI.
El objetivo del estudio fue valorar el impacto de la psoriasis en la cali-dad de vida relacionada con la salud (CVRS) mediante diferentes cuestionarios de evaluación.
Pacientes y métodosEstudio observacional prospectivo en pacientes con psoriasis en placas de más de 6 meses de evolución, estratificándose en psoriasis activa (PsoA) y psoriasis estable (PsoE). Se realizó visita basal a los 7 días y al final (12 semanas). En cada una se recogieron características sociodemográficas y clínicas y cuestionarios de CVRS: Dermatology Life Quality Index (DLQI), Psoriasis Disability Index (PDI) y cuestionario de calidad de vida en psoriasis (PSO-LIFE).
ResultadosSe evaluaron 304 pacientes: 182 con PsoA y 122 con PsoE. La edad media (DE) fue 45,3 (14,5) años y el 56,3% eran hombres. La puntuación media (DE) basal del PASI en PsoA fue 17,0 (7,4) y en PsoE fue 5,6 (5,3), disminuyendo durante el estudio (p<0,01). La puntuación media (DE) del cuestionario PSO-LIFE aumentó de 57,4 (20,4) a 72,2 (19,6) en PsoA y de 76,4 (20,6) a 82,3 (18,3) en PsoE (p<0,01 ambos).
Las diferencias de las puntuaciones medias estandarizadas entre los 2 grupos fueron: 0,79 para el DLQI, 0,62 para el PDI y 0,85 para el PSO-LIFE.
Los pacientes con lesiones en las zonas visibles tuvieron una mayor afectación según el cuestionario PSO-LIFE que los pacientes con afectación en zonas no tan visibles (p<0,01).
Los cambios en la puntuación del PSO-LIFE y los cambios en el PASI mostraron una correlación moderada (r=-0,4) y estadísticamente significativa.
ConclusionesLos pacientes con PsoA presentan una mayor afectación de la CVRS. El cuestionario PSO-LIFE mostró una mayor tendencia para discriminar entre ambos grupos de pacientes (PsoA y PsoE) que el DLQI y PDI. La localización de las lesiones y la gravedad, según el PASI, se correlacionaron de forma significativa con las puntuaciones del cuestionario PSO-LIFE.
Psoriasis is a common chronic systemic inflammatory disease with a clinical course marked by exacerbations, remissions, and relapses. The prevalence in the general Spanish population is estimated to be between 1.17% and 1.43%.1,2
Although plaque psoriasis is the most common clinical form, there are others, including guttate, pustular, and erythrodermic types. Psoriatic arthritis is found in around 14% of patients with psoriasis.3
A great many drugs are available for the treatment of psoriasis and current thinking favors an individually tailored approach to management.4,5 Topical agents, which comprise the first-line of therapy, are the medications most often prescribed singly or in combination regimens.6 Systemic drugs, which are used when psoriasis is extensive or severe (erythrodermic or pustular forms, for example), comprise the second-line of treatment. The biologic agents that have become available in recent years have efficacy and safety profiles that are superior to traditional systemic drugs.7
Health related quality of life (HRQOL) is a particularly important concept in this disease. A study by the National Psoriasis Foundation in the United States found that up to 75% of patients with the disease reported a negative impact on daily living,8 and patients with moderate to severe psoriasis reported greater impact than those with mild forms.9
A variety of HRQOL questionnaires have been used to study the effect of psoriasis. Among them are the Dermatology Life Quality Index (DLQI), the Skindex-29, the Psoriasis Disability Index (PDI), the Impact of Psoriasis Questionnaire, the 12-Item Psoriasis Quality of Life Questionnaire, and more. The main constraint on the use of these instruments is that some are relatively insensitive to clinical changes.10
Using standard procedures, w e developed a new Psoriasis Quality of Life (PSO-LIFE) questionnaire, a new, psoriasis-specific instrument to enable more sensitive measurement of HRQOL with an easy-to-interpret tool.11 The PSO-LIFE instrument was subsequently shown to be valid, reliable, and sensitive to change.12 In this study we used this validated questionnaire to compare HRQOL in patients with active and stable psoriasis.
Material and MethodsIn this prospective observational multicenter study in Spain 39 dermatologists studied patients between October 2008 and May 2009. The study was approved by the clinical research ethics committee of Hospital General Universitario de Valencia.
The patients were adults with plaque psoriasis diagnosed by a dermatologist at least 6 months before inclusion; all participants gave their written informed consent. Each physician selected 5 patients with active psoriasis (individuals who were experiencing an exacerbation or who were recovering from an acute flare-up but were not yet stable) and 4 patients with stable psoriasis (individuals who had no lesions or no new lesions while others remained the same size, even if the affected surface area was extensive13,14). In this observational, naturalistic study, lesion stability was defined according to the clinical judgment of each physician. Patients attended 3 visits: at baseline (enrollment), 7 days after enrollment, and 12 weeks after enrollment. Disease was managed according to the treating physician's routine practice, including choice of treatment and route of administration or modality (topical, oral, or parenteral treatments; or phototherapy).
Sociodemographic, clinical, and patient-centered variables were recorded. Sociodemographic variables were age, sex, educational level, and employment status. Clinical variables were weight, time since diagnosis of psoriasis, duration of the last flare-up, treatment, Psoriasis Area and Severity Index (PASI) at baseline, changes in treatment and PASI at the follow-up visits (7 days and last visit).
Patient-centered variables were the results of the self-administered DLQI, PDI, and PSO-LIFE questionnaires at all visits.
The DLQI is a generic dermatology HRQOL questionnaire with a time frame of 7 days. Each of 10 items is measured on a scale of 0 (not at all) to 3 (very much). The dimensions are symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment. The overall score ranges from 0 (no effect on HRQOL) to 30 (extremely large effect).15,16
The PDI questionnaire is specific to psoriasis and has been validated in a Spanish population.17 The time frame is 4 weeks. Fifteen items are answered on a scale from 0 (no effect) to 3 (great effect). The 4 dimensions of the PDI are daily activities, work or school or alternatives, personal relationships, leisure, and treatment. The overall score ranges from 0 to 45, with higher scores reflecting greater impact on HRQOL.
The PSO-LIFE is a psoriasis-specific HRQOL questionnaire with a time frame of 7 days. Responses to all 20 items are given on a Likert-type scale (always to never), and the overall score ranges from 0 to 100 points. Higher scores reflect a higher level of HRQOL (less impact of disease).
Statistical AnalysisStatistics were compiled using the SPSS software package, version 15.0 for Windows. In all statistical tests the level of significance was set at a value of P less than .05.
Descriptive statistics were compiled for sociodemographic and clinical variables. Group comparability was confirmed on the basis of sociodemographic characteristics of patients with active and stable disease, using the Mann-Whitney U test to compare continuous variables (e.g., age, years since diagnosis, and PASI) and the χ2 test for categorical variables (e.g., sex, educational level, employment status, concomitant diseases, treatment, and duration of the last flare-up). The Wilcoxon test was used to analyze changes in PASI during follow-up.
The effect size of each of the 3 questionnaires was used to compare their sensitivity to change. The standardized mean differences in HRQOL scores between groups were calculated as the difference in scores divided by the pooled SDs. The Wilcoxon test was also used to analyze changes in HRQOL between visits.
The PSO-LIFE scores were compared according to lesion site using analysis of variance and the Scheffe test for multiple comparisons. The Pearson correlation coefficient was used to analyze associations between the PSO-LIFE and PASI scores. The association was considered weak if the coefficient was –0.3 or less, moderate if between –0.3 and –0.5, and high if greater than –0.5. The negative coefficients indicate the inverse correlation between the 2 variables.
ResultsA total of 317 patients were enrolled. Thirteen were excluded from the final analysis: 3 patients with active psoriasis and 1 patient with stable psoriasis had been diagnosed less than 6 months earlier, and information was missing for 9 patients (4 with active disease and 5 with stable disease). The final dataset for analysis, therefore, included 304 patients, 182 with active disease and 122 with stable disease.
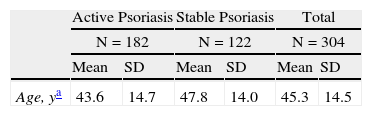
Men comprised 56.3% of the population, and the mean (SD) age was 45.3 (14.5) years overall. Patients with active psoriasis were significantly younger than those with stable disease (43.6 vs 47.8 years, respectively, P<.05). A primary or secondary school education had been completed by 69% and nearly half (48%) were employed workers. Forty percent had concomitant diseases (Table 1).
Sociodemographic Characteristics of Patients at Baseline.
| Active Psoriasis | Stable Psoriasis | Total | ||||
| N=182 | N=122 | N=304 | ||||
| Mean | SD | Mean | SD | Mean | SD | |
| Age, ya | 43.6 | 14.7 | 47.8 | 14.0 | 45.3 | 14.5 |
| n | % | n | % | n | % | |
| Sex | ||||||
| Male | 101 | 55.5 | 70 | 57.4 | 171 | 56.3 |
| Female | 81 | 44.5 | 52 | 42.6 | 133 | 43.8 |
| Educational level | 179 | 59.7 | 121 | 40.3 | 300 | 100.0 |
| No formal schooling | 5 | 1.7 | 4 | 1.3 | 9 | 3.0 |
| Primary school | 66 | 22.0 | 46 | 15.3 | 112 | 37.3 |
| Secondary school | 62 | 20.7 | 45 | 15.0 | 107 | 35.7 |
| Higher education | 46 | 15.3 | 26 | 8.7 | 72 | 24.0 |
| Work situation | 182 | 59.9 | 122 | 40.1 | 304 | 100.0 |
| Unemployed | 19 | 6.3 | 12 | 3.9 | 31 | 10.2 |
| Working, self-employed | 26 | 8.6 | 11 | 3.6 | 37 | 12.2 |
| Working, employed | 87 | 28.6 | 59 | 19.4 | 146 | 48.0 |
| Disabled | 1 | 0.3 | 4 | 1.3 | 5 | 1.6 |
| Retired/pensioner | 19 | 6.3 | 25 | 8.2 | 44 | 14.5 |
| Housewife | 22 | 7.2 | 9 | 3.0 | 31 | 10.2 |
| Student | 9 | 3.0 | 5 | 1.6 | 14 | 4.6 |
| Concomitant diseasesb | 70 | 38.5 | 52 | 42.6 | 122 | 40.1 |
| Blood or hematopoietic tissues | 1 | 0.5 | 2 | 1.6 | 3 | 1.0 |
| Digestive tract | 2 | 1.1 | 2 | 1.6 | 4 | 1.3 |
| Genitourinary system | 4 | 2.2 | 3 | 2.5 | 7 | 2.3 |
| Infectious or parasitic | 2 | 1.1 | 2 | 1.6 | 4 | 1.3 |
| Respiratory tract | 5 | 2.7 | 4 | 3.3 | 9 | 3.0 |
| Circulatory system | 25 | 13.7 | 20 | 16.4 | 45 | 14.8 |
| Musculoskeletal. joints, and connective tissues | 3 | 1.6 | 2 | 1.6 | 5 | 1.6 |
| Endocrine, metabolic, and nutritional | 48 | 26.4 | 27 | 22.1 | 75 | 24.7 |
| Mental or psychiatric disorder | 1 | 0.5 | 4 | 3.3 | 5 | 1.6 |
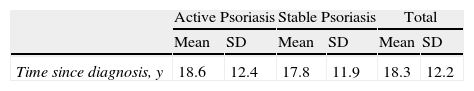
The mean time elapsed since diagnosis of psoriasis was 18.3 (12.2) years (range, 6 months to 54 years). The last psoriasis flare-up lasted more than 6 weeks in 72.5% of the patients with active disease and in 62.8% of those with stable disease (P<.01); 92.4% were under treatment at the time of the baseline visit. The most frequently reported type of treatment was a systemic agent (51%), followed by topical treatment (44.1%), and biologic agents (38.2%). In all treatment categories, there were instances of monotherapy and combined treatment. At the end of the study some change in treatment had been prescribed for 30.5% of the patients (32.9% of those with active disease and 26.8% of those with stable disease). Treatment had been discontinued for 6 patients (2.1%), all in the active psoriasis group (Table 2).
Clinical Characteristics of the Patients With Psoriasis.
| Active Psoriasis | Stable Psoriasis | Total | ||||
| Mean | SD | Mean | SD | Mean | SD | |
| Time since diagnosis, y | 18.6 | 12.4 | 17.8 | 11.9 | 18.3 | 12.2 |
| n | % | n | % | n | % | |
| Psoriasis treatmenta | 177 | 97.3 | 104 | 85.2 | 281 | 92.4 |
| Topicalb | 90 | 49.5 | 44 | 36.1 | 134 | 44.1 |
| Psoralen plus UV-A/UV-B phototherapy | 25 | 13.7 | 10 | 8.2 | 35 | 11.5 |
| Conventional systemic drug | 101 | 55.5 | 54 | 44.3 | 155 | 51.0 |
| Biologic agenta | 57 | 31.3 | 59 | 48.4 | 116 | 38.2 |
| Other | 32 | 17.6 | 9 | 7.4 | 41 | 13.5 |
| Duration of last psoriasis flare-up | 182 | 100.0 | 113 | 100.0 | 295 | 100.0 |
| <2 wk | 3 | 1.6 | 24 | 21.2 | 27 | 9.2 |
| 2-4 wk | 20 | 11.0 | 7 | 6.2 | 27 | 9.2 |
| 4-6 wk | 27 | 14.8 | 11 | 9.7 | 38 | 12.9 |
| >6 wka | 132 | 72.5 | 71 | 62.8 | 203 | 68.8 |
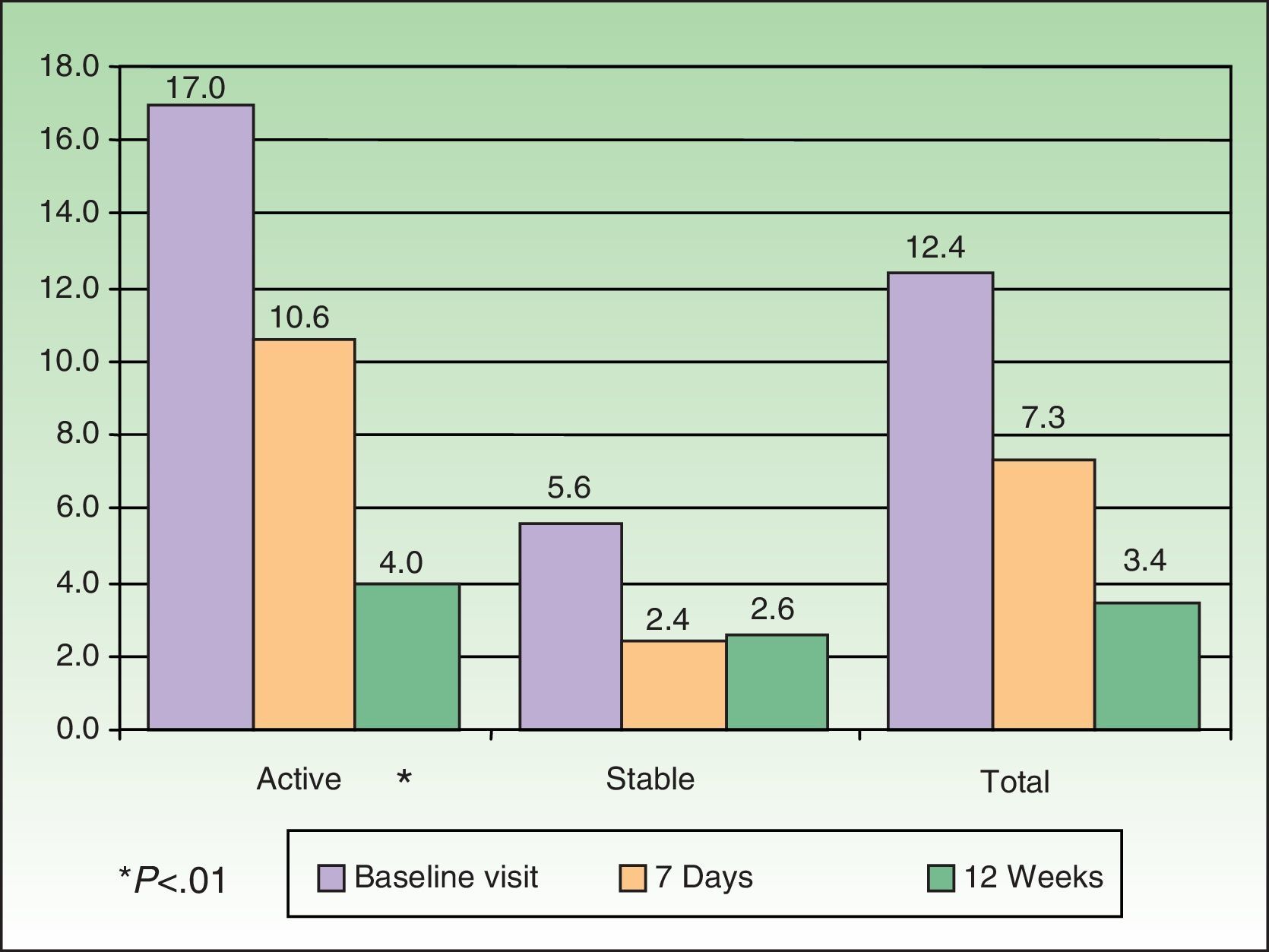
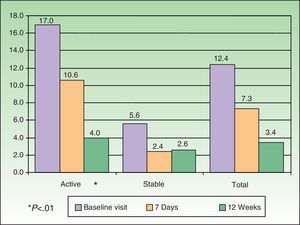
The mean PASI at baseline was significantly higher in the group with active disease (17.0 [7.4] vs 5.6 [5.3] for the stable disease group) (P<.01). Significant differences were also found on comparing the baseline and final-visit PASI scores of patients with active psoriasis (P<.01), whereas the scores of patients with stable disease did not differ significantly between baseline and 12 weeks (Fig. 1).
At the last visit, the flare-up had not yet resolved in 46% of patients with active psoriasis at baseline. No change was observed during follow-up in 80.5% of the patients with stable disease.
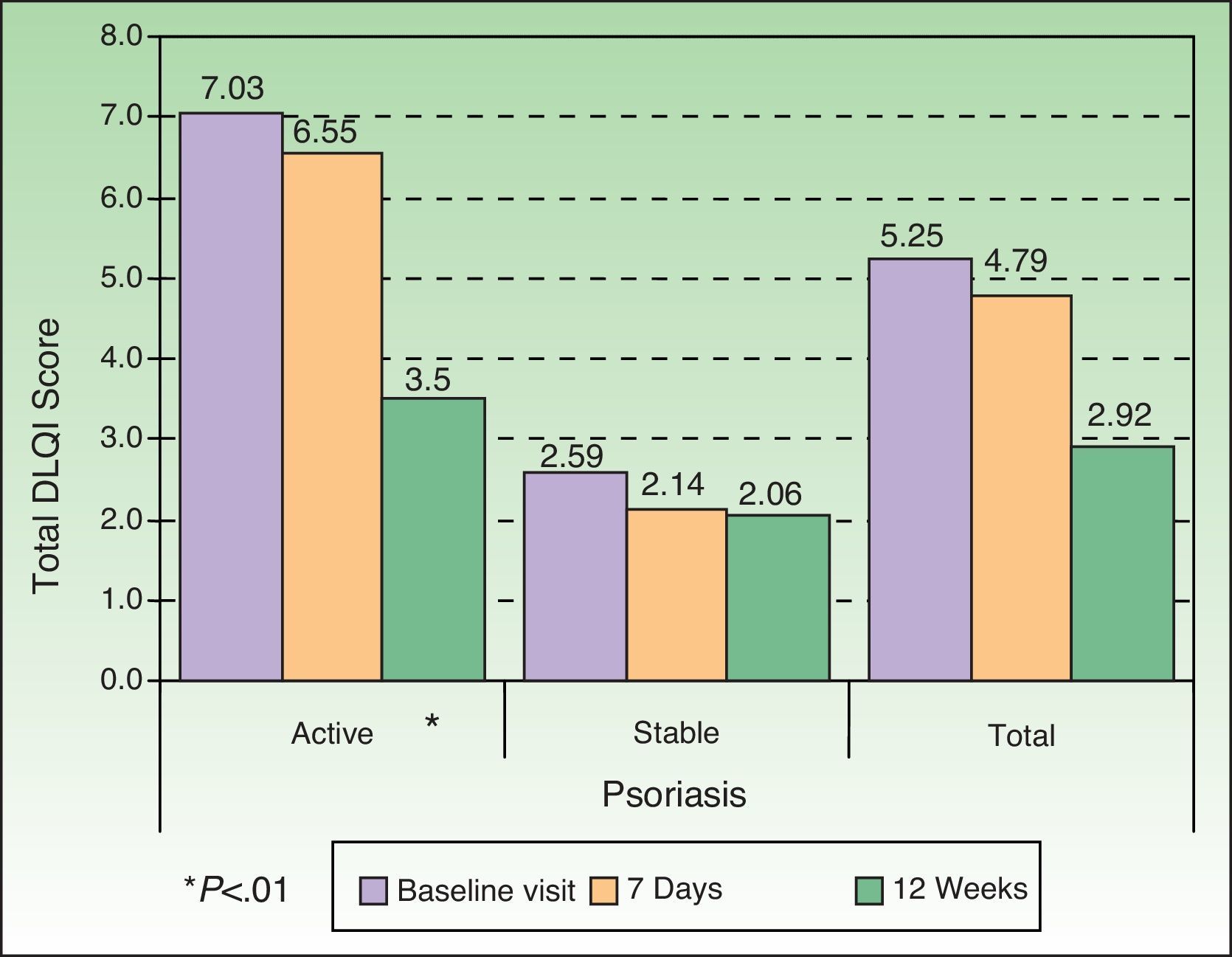
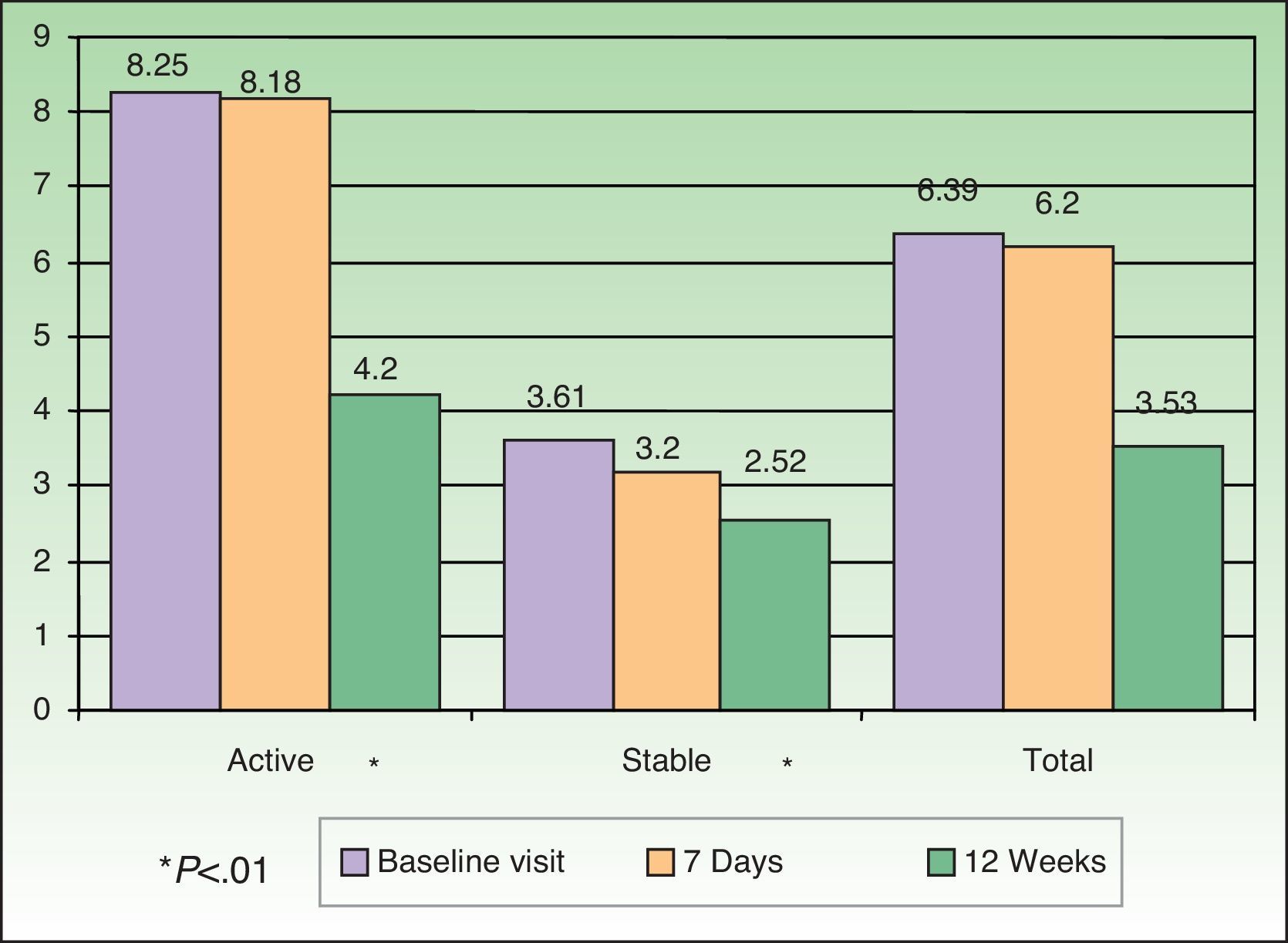
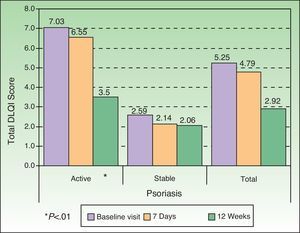
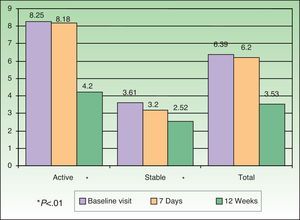
HRQOL AssessmentHRQOL was assessed by means of the DLQI, PDI, and PSO-LIFE questionnaires. The mean baseline scores on the 3 instruments were significantly different between the active and stable disease groups (all comparisons, P<.01): DLQI, 7.0 (5.9) vs 2.6 (3.6); PDI, 8.3 (8.1) vs 3.6 (5.5); and PSO-LIFE, 57.4 (20.4) vs 76.4 (20.6). Mean scores on all questionnaires had changed significantly (P<.01) between baseline and the last visit on all 3 questionnaires for patients with active disease: DLQI, difference of 3.5 (4.6); PDI, 4.2 (5.9); and PSO-LIFE, 15.3 (17.0). Significant change was detected for patients with stable disease only with the PDI (2.5 [5.2], P<.01) and the PSO-LIFE (5.0 [16.5], P<.01) (Figs. 2–4).
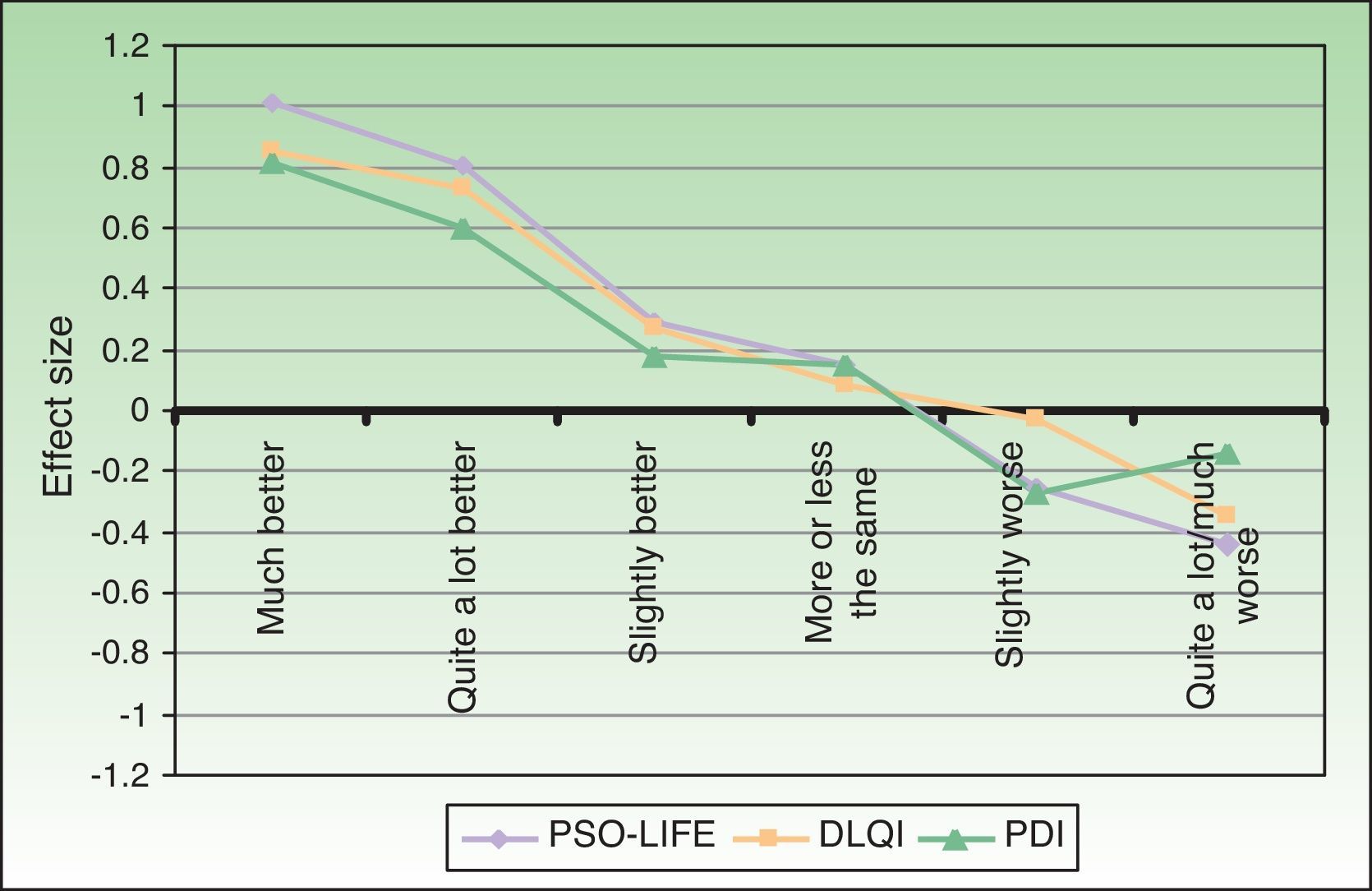
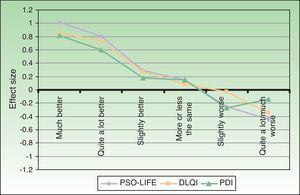
The effect size was greater for the PSO-LIFE, indicating that this instrument is more sensitive to change in a patient's perception of health than the other questionnaires used in this study (Fig. 5).
The nonsignificant differences in the standardized mean scores of patients with active disease and those with stable disease were 0.79 for the DLQI, 0.62 for the PDI, and 0.85 for the PSO-LIFE.
The PSO-LIFE also detected differences related to lesion site. Patients with visible lesions (such as those on the head or arms) had a mean score of 63 (22) points whereas patients with less visible lesions (on the trunk or legs, for example) had a higher score on this instrument (mean, 74.8 [23.9] points), indicating less impact of disease on HRQOL; for patients with no lesions at the baseline visit the score was 78.5 (21.6) points (P<.01).
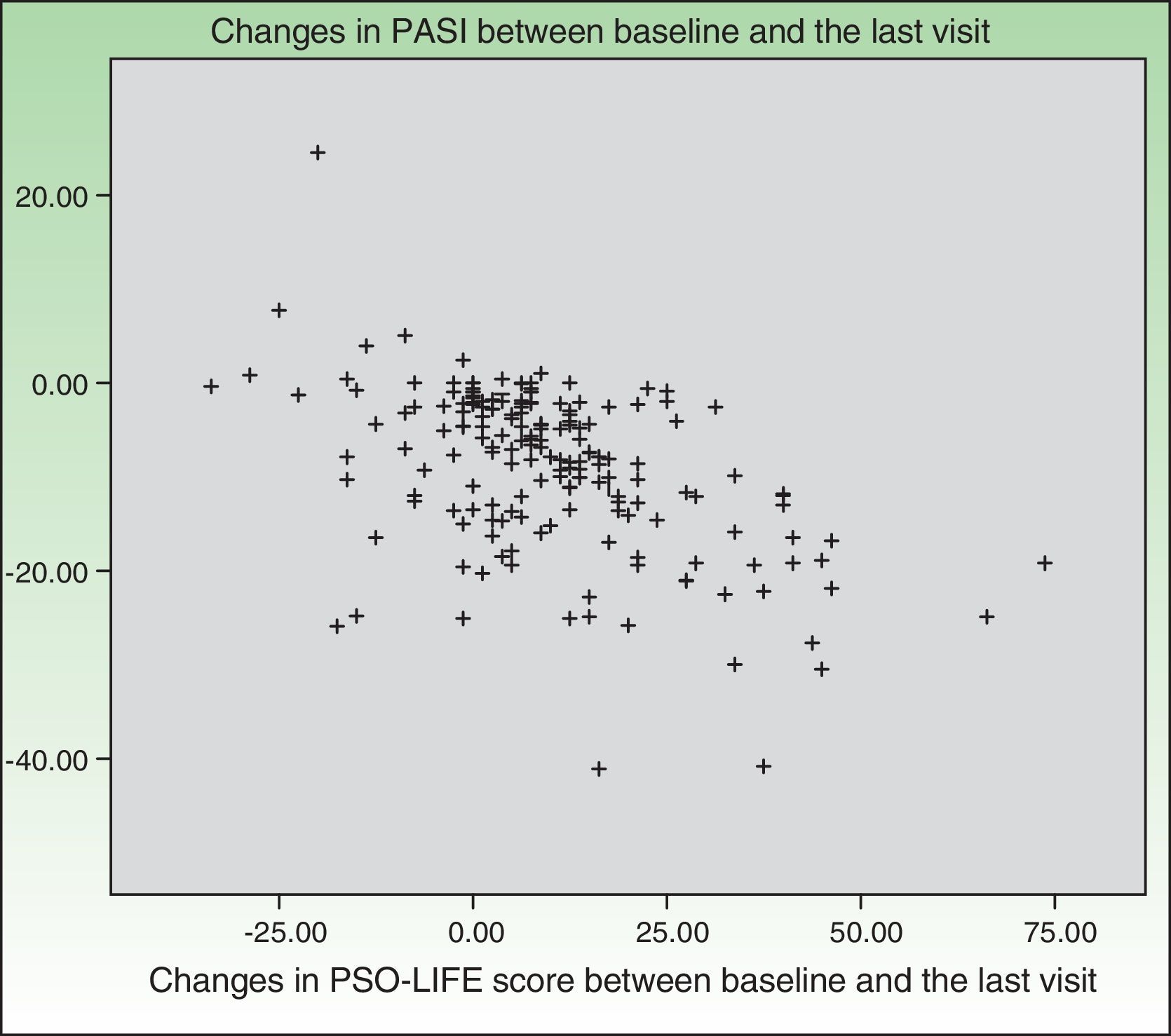
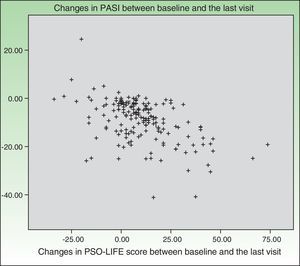
Changes in PSO-LIFE score were moderately correlated with PASI (r=–0.4) (Fig. 6). The DLQI and PDI questionnaires were also moderately correlated with PASI (r=0.5 and 0.4, respectively).
The PSO-LIFE detected no differences between patients whose treatment was changed during the follow-up period and those who remained on the same treatment.
DiscussionThis study shows that psoriasis affects HRQOL, and that while all 3 instruments detected that effect, the PSO-LIFE tended to be better able to differentiate between active and stable disease according to our analysis of standardized mean scores.
The sociodemographic characteristics of our patients were similar to those enrolled in similar studies in Spain,13,15,18 suggesting the sample we studied was representative of psoriasis patients who seek care from dermatologists in this country.
All 3 questionnaires detected significant differences between patients with active and stable disease at baseline, a finding that seems logical. Holm et al.19 demonstrated that the DLQI was able to distinguish between patients with atopic dermatitis of differing degrees of severity. Ferrándiz et al.18 showed that the PDI was correlated with the severity of psoriasis lesions, although the correlation was weak. Finally, we also found a moderate correlation between the PASI and scores on all 3 HRQOL instruments.
Also to be expected was the HRQOL improvement evident at the end of the study compared to baseline, particularly for patients with active disease given that most of these patients were on therapy during follow-up. This improvement is attributable to the clinical change reflected in their PASI scores.
The DLQI-measured effect of psoriasis on HRQOL was similar to that reported by other authors who have studied patients with this same disease10 and also similar to the effect reported for other dermatologic conditions such as vitiligo,20 confirming that this questionnaire is not specific enough to detect HRQOL differences in relation to different dermatologic diseases. The DLQI and the PDI were less sensitive to clinical change (reflected by PASI score) than the Skindex-29, according to the findings of a recent study.21
The PSO-LIFE is demonstrably more sensitive to clinical change, whether improvement or worsening. This questionnaire is thus useful for routine follow-up in the management of psoriasis.
Our study shows that the PSO-LIFE is not only able to detect HRQOL difference between patients with active and stable disease but also measures change over time as shown by the correlation with PASI variation. However, additional studies should be done to establish the clinical significance of PSO-LIFE scores in relation to different therapeutic approaches. Such information would be useful for interpreting scores in an individualized manner rather than in a limited or general manner.22
The effect of disease perceived by patients with lesions on visible areas of the body (e.g., on the head) is greater than the effect reported by patients with less visible lesions. However, we did not find a difference between complete absence of lesions and the less visible lesions (on the legs and/or trunk). Nor did we find a difference on comparing the responses of patients with lesions on the head to responses of those with lesions on the extremities and/or trunk, although the absence of differences in this last comparison is probably due to the small number of patients with only extremity and/or trunk lesions in our sample. Our results are consistent with the National Psoriasis Foundation's finding that 41% of patients changed the way they dressed to hide psoriasis lesions.23 A recent population-based study also demonstrated that even patients with hand eczema noticed an effect on HRQOL regardless of age.24 Both those studies support our finding that lesion site is relevant to degree of impact on HRQOL.
A limitation of our study is the approach to enrolling patients based on lesion activity. Even though the inclusion criteria were well defined in the protocol, no objective test was used to identify whether disease was active or inactive. Classification was left to the physician's judgment, possibly introducing selection bias if patients were incorrectly classified. We attempted to attenuate the effect of this limitation by recruiting dermatologists who were experienced with the clinical and therapeutic management of psoriasis. However, it is possible that because these physicians were experienced, the psoriasis patients they enrolled may reflect a population with more severe disease. One indicator of a bias toward severity would be the percentage of patients on a biologic treatment in this study.
Another limitation was that the effects of a change in therapy or an adverse event that might have occurred at some point might not have been reflected in the HRQOL score on the PSO-LIFE given the instrument's time frame of 7 days.
The question of whether the PSO-LIFE can discriminate between patients with mild, moderate or severe psoriasis as well as between active and stable disease would be a useful one for future researchers to study.
ConclusionsPatients with active psoriasis experience greater impact on HRQOL than patients with stable disease. The standardized mean difference in scores between the active-disease and stable-disease groups was greater for the PSO-LIFE than for the DLQI (a generic dermatology instrument) or the PDI (a psoriasis-specific instrument). Thus, the PSO-LIFE was better able to discriminate between active and stable disease, as has been found previously,21 and this new questionnaire was also more sensitive to change than the instruments routinely used to date.
The site of psoriasis lesions and level of severity (PASI) were significantly correlated with the PSO-LIFE findings for HRQOL.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their hospitals concerning the publication of patient data, and that all the patients included in this study were appropriately informed and gave their written informed consent.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
FundingThis study was funded by Schering-Plough.
Conflicts of InterestDr Daudén is or has been a member of advisory boards or has received research grants or fees for consulting, speaking or participating in clinical trials from the following companies: Abbott, Amgen, Astellas, Biogen, Centocor Ortho Biotech Inc, Galderma, Glaxo, Janssen-Cilag, Leo Pharma, MSD, Pfizer, Novartis, Stiefel, and Celgene.
Dr J. L. Sanchez has received grants or fees for various activities (serving on advisory boards, consultancy, research/study, participation in clinical trials, and speaking engagements) from the following companies: Abbott, Janssen-Cilag, Leo Pharma, MSD, Pfizer, and Novartis.
The other authors declare that they have no conflicts of interest.
Please cite this article as: Daudén E, et al. Impacto en la calidad de vida relacionada con la salud de pacientes con psoriasis activa y estable. Estudio PSO-LIFE. Actas Dermosifiliogr. 2013;104:685–93.